Forms of Nitrogen and Phosphorus in Suspended Solids: A Case Study of Lihu Lake, China
Abstract
:1. Introduction
2. Materials and Methods
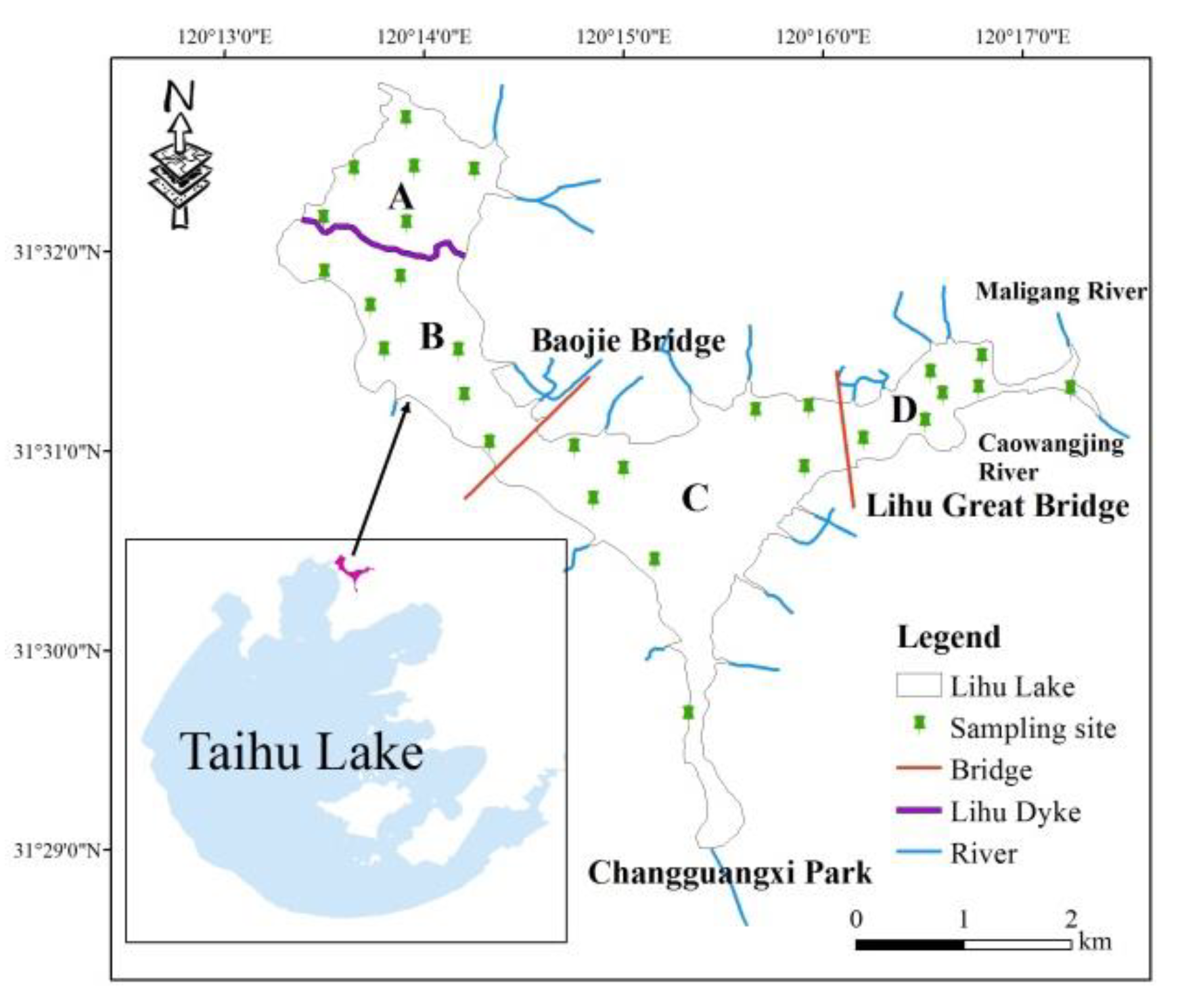
2.1. Study Area
2.2. Sampling and Sample Preparation
2.3. Sample Analysis
2.3.1. Nitrogen Forms
2.3.2. Phosphorus Forms
2.4. Quality Assurance and Quality Control (QA/QC)
3. Results and Discussion
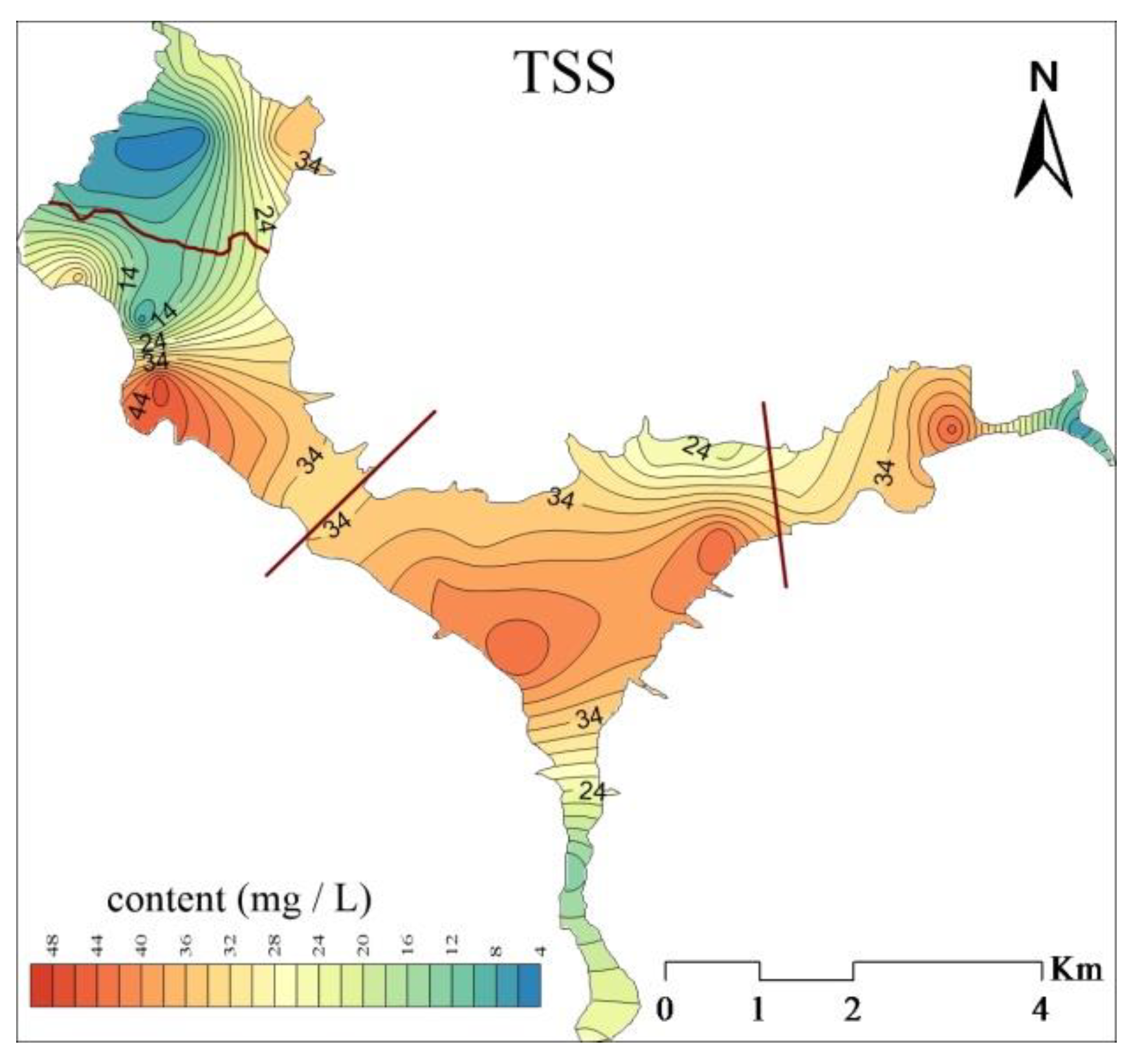
3.1. Spatial Distribution of Total Suspended Solids
3.2. Spatial Distribution of Nitrogen Forms in Suspended Solids
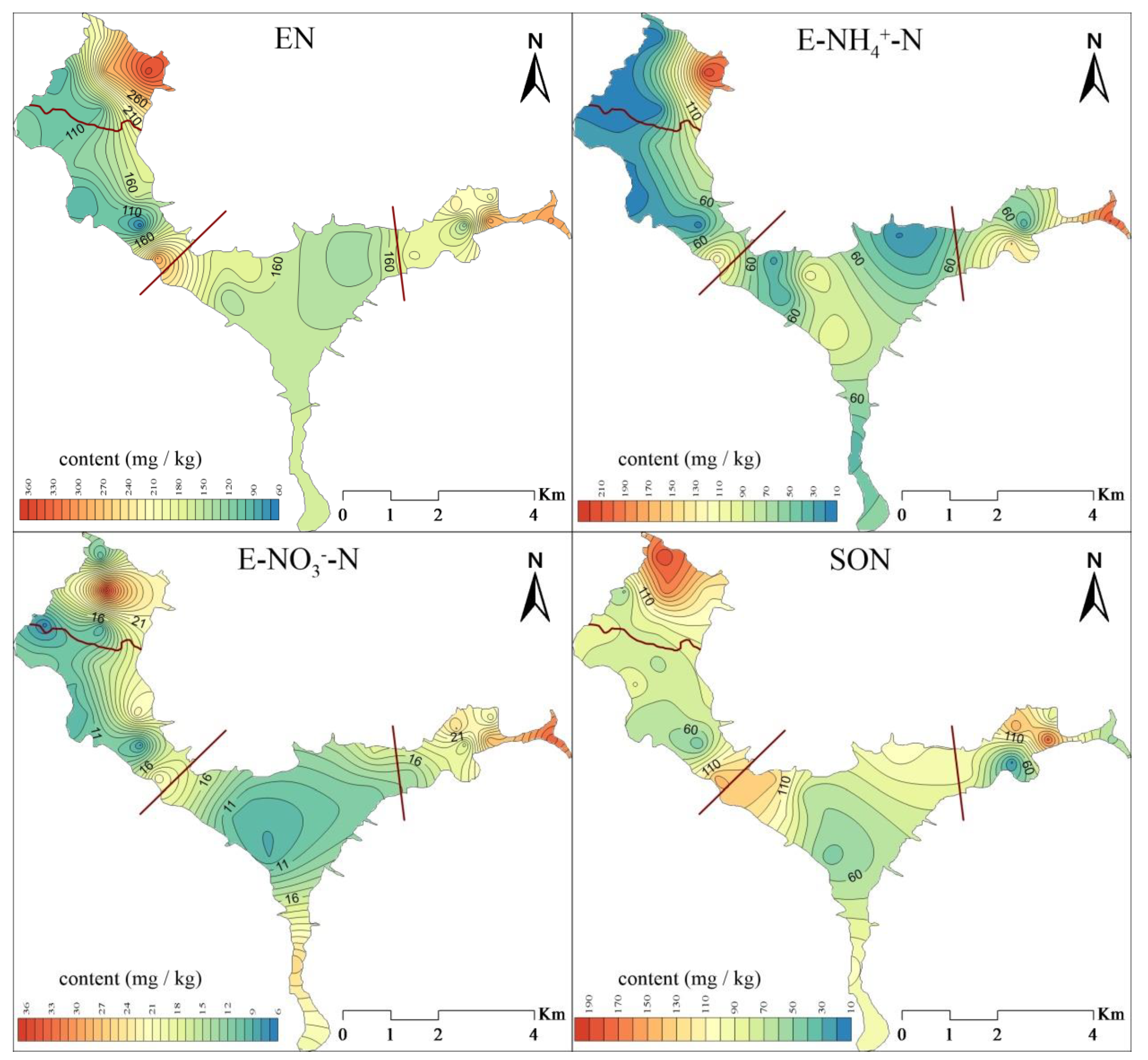
3.2.1. Spatial Distribution of Exchangeable Nitrogen (EN) in Suspended Solids
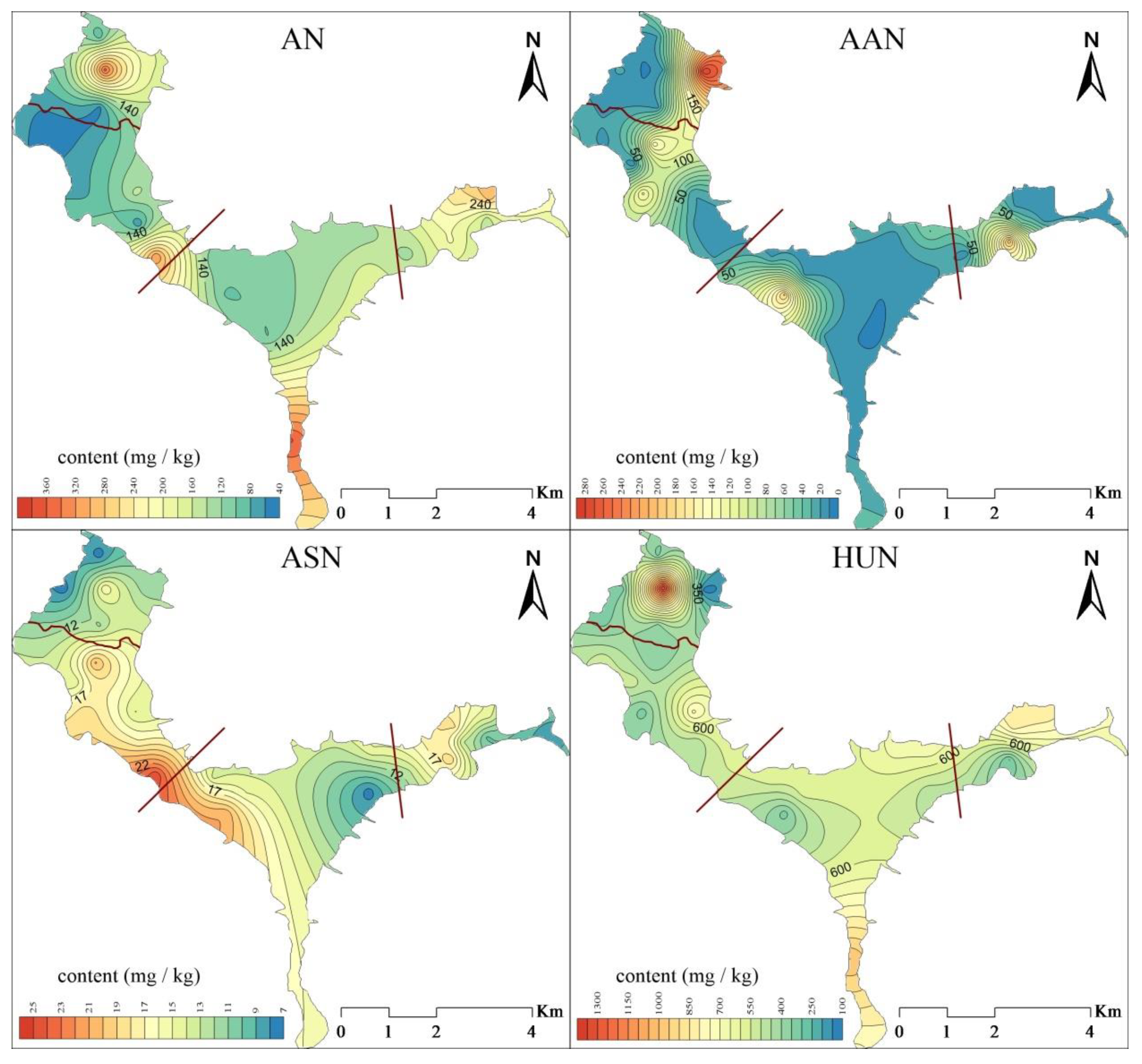
3.2.2. Spatial Distribution of Acid-Hydrolyzable Nitrogen (HN) in Suspended Solids
3.2.3. Spatial Distribution of Residual Nitrogen (RN) and Total Nitrogen (TN) in Suspended Solids
3.3. Spatial Distribution of Phosphorus Forms in Suspended Solids
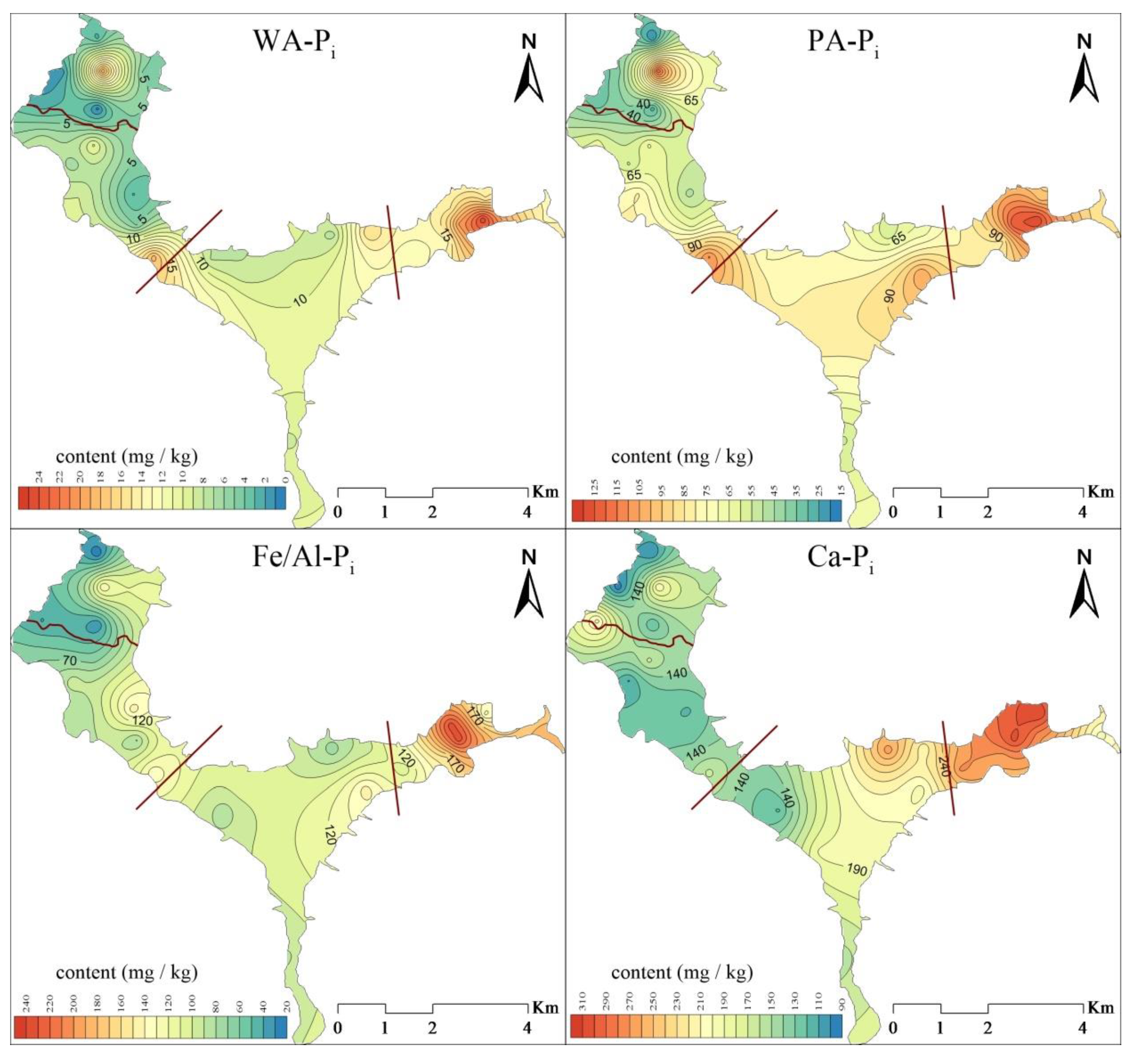
3.3.1. Spatial Distribution of Inorganic Phosphorus in Suspended Solids
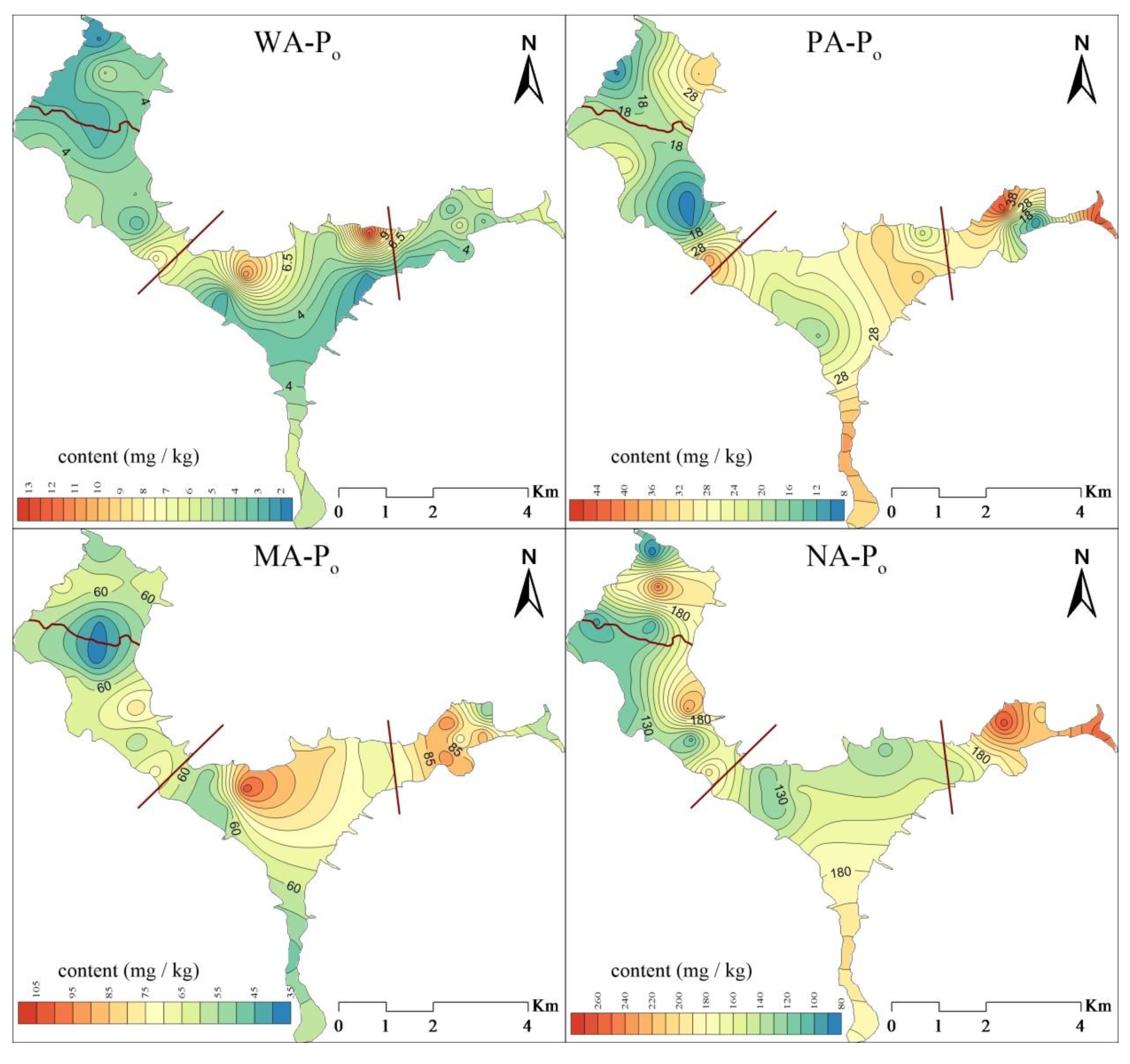
3.3.2. Spatial Distribution of Organic Phosphorus in Suspended Solids
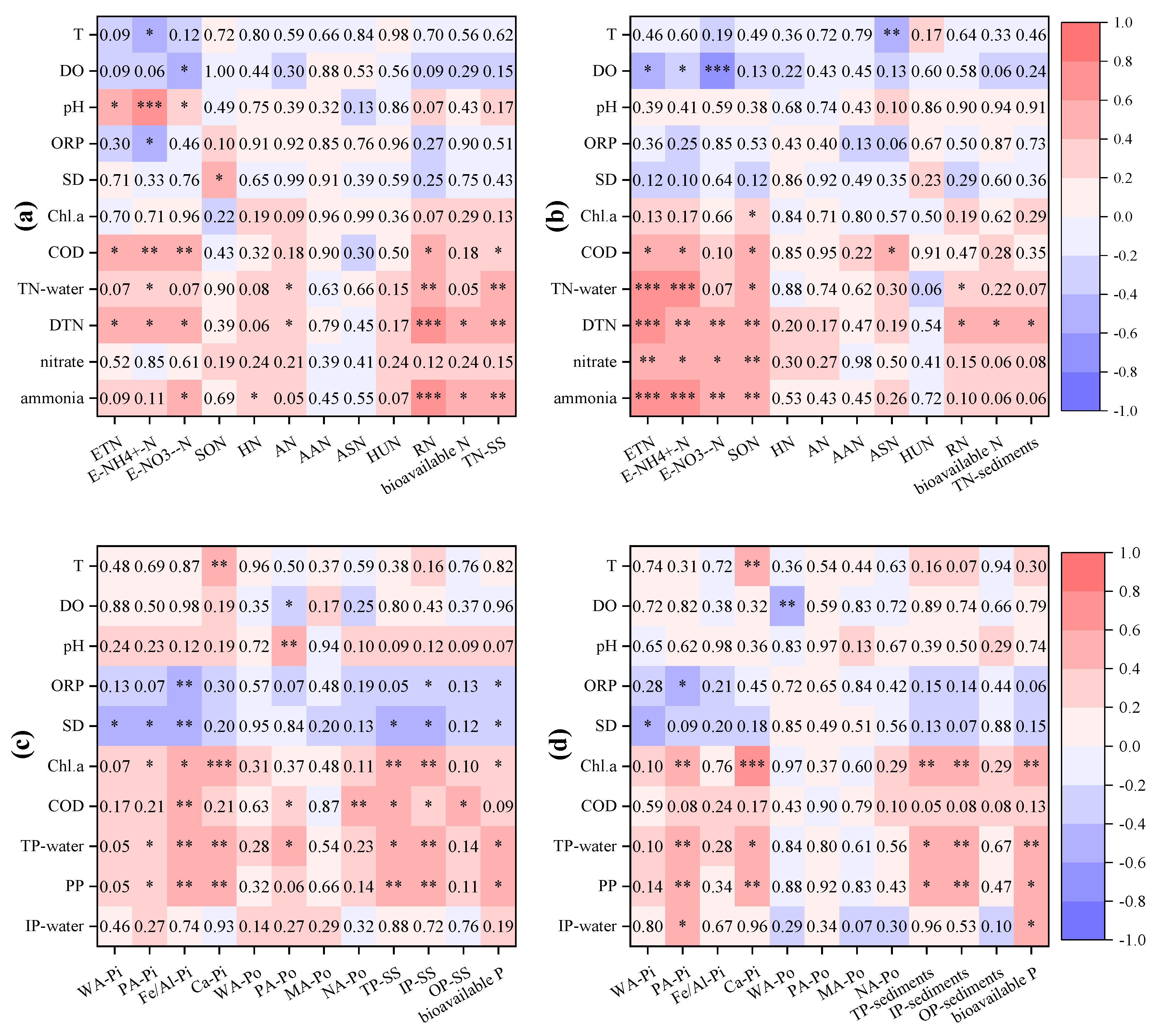
3.4. Correlation Analysis Indicative of Nutrient Sources
3.5. Analysis of Influencing Factors
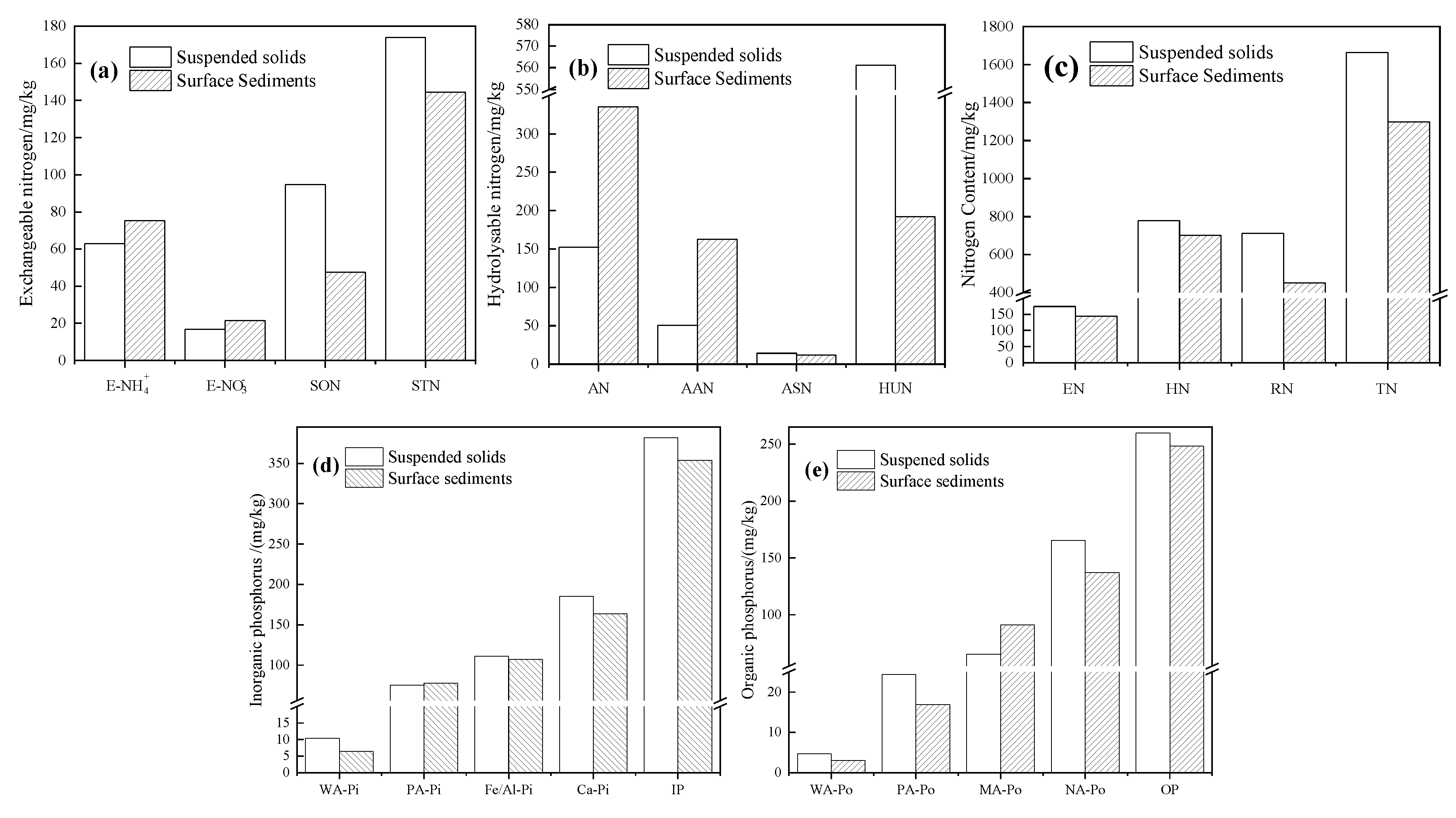
3.6. Comparison Analysis between Suspended Solids and Surface Sediments
4. Conclusions
- (1)
- The content of TN in the suspended solids of Lihu Lake was 758.9–3098.1 mg/kg and showed a decreasing trend from east to west. The proportions of various N forms in the suspended solids of the study areas were ranked as follows: Hydrolyzable nitrogen (HN) > residual nitrogen (RN) > exchangeable nitrogen (EN). The total phosphorus (TP) ranged from 294.8 to 1066.4 mg/kg, the contents of IP and OP in suspended solids were 381.9 and 259.8 mg/kg, respectively, and Ca-Pi and NA-Po were the major components of IP and OP. Almost all P forms exhibited higher contents in the west than in the east. This is because several environmental dredging and ecological restoration projects have been carried out in western Lihu Lake.
- (2)
- The correlation analysis of various forms of nitrogen and phosphorus in suspended solids indicated that there were different sources of suspended nitrogen for Lihu Lake, and TN in suspended solids was mainly affected by RN. Inorganic phosphorus in suspended solids likely originated from a common source, whereas organic phosphorus came from different sources. Phosphorus in suspended solids was controlled by both IP and OP, but was mainly affected by IP.
- (3)
- The nitrogen in sediments was more bioavailable than that in suspended solids. In particular, once the physical and biochemical conditions of the sediment change, the EN in the sediment can be released and can diffuse into the overlying water, affecting the contents of NH4+-N and TN in water. The nitrogen forms in SS change continuously through rapid transformations when water quality conditions change. The contents of phosphorus in water were affected both by suspended solids and sediment. The bioavailable phosphorus content in suspended solids was higher than that in sediments, and the suspended solids influenced the ρ(TP) by affecting particulate phosphorus. Suspended solids act as a medium that continually carries phosphorus from sediments to water and provides a substrate that enables phosphorus to remain in the water column for long periods.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Abbreviation | Meaning |
|---|---|
| SS | suspended solids |
| TSS | total suspended solids |
| OM | organic matter |
| TN | total nitrogen |
| DTN | dissolved total nitrogen |
| PN | particulate nitrogen |
| TP | total phosphorus |
| DTP | dissolved total phosphorus |
| PP | particulate phosphorus |
| IP | inorganic phosphorus |
| OP | organic phosphorus |
| EN | exchangeable nitrogen |
| E-NH4+-N | exchangeable ammonium nitrogen |
| E-NO3−-N | exchangeable nitrate nitrogen |
| SON | exchangeable soluble organic nitrogen |
| HN | acid-hydrolyzable nitrogen |
| AAN | amino acid nitrogen |
| ASN | amino sugar nitrogen |
| AN | acid-hydrolyzable ammonium nitrogen |
| HUN | acid-hydrolyzable unknown nitrogen |
| RN | residual nitrogen |
| WA-Pi | weakly adsorbed inorganic phosphorus |
| PA-Pi | potentially active inorganic phosphorus |
| Fe/Al-Pi | iron (Fe)- or aluminum (Al)-bound inorganic phosphorus |
| Ca-Pi | calcium (Ca)-bound inorganic phosphorus |
| WA-Po | weakly absorbed organic phosphorus |
| PA-Po | potentially active organic phosphorus |
| MA-Po | medium active organic phosphorus |
| NA-Po | non-active organic phosphorus |
| R-Po | residual phosphorus |
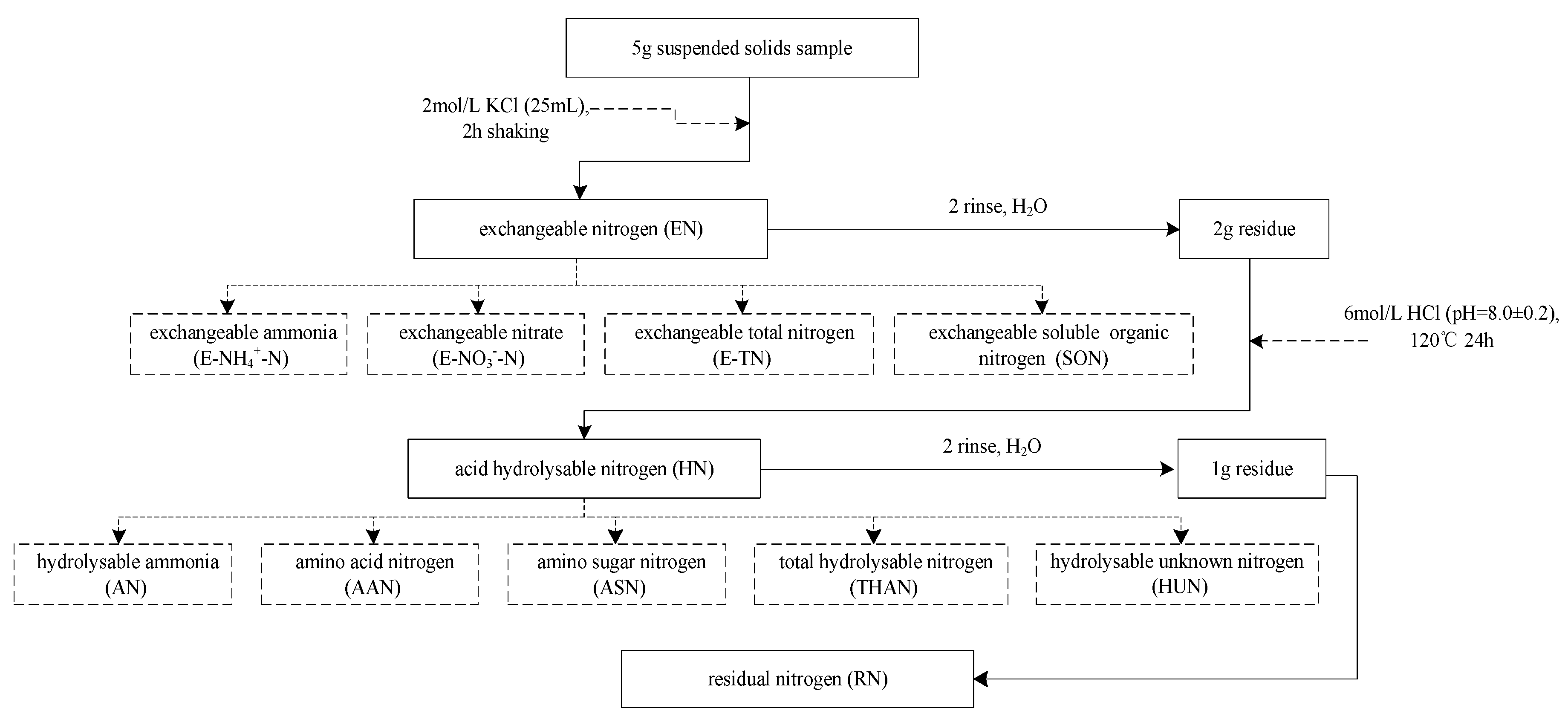
| Index | Determination Method | Reference |
|---|---|---|
| EN, HN | Alkaline potassium persulfate digestion UV spectrophotometric method | HJ 636-2012 from Ministry of Ecology and Environment, PRC |
| E-NH4+-N, AN | Nessler’s reagent spectrophotometry | HJ 535-2009 from Ministry of Ecology and Environment, PRC |
| E-NO3 NO3−-N | Ultraviolet spectrophotometry | HJ/T 346-2007 from Ministry of Ecology and Environment, PRC |
| SON | SON = EN- E-NH4+-N- E-NO3−-N | |
| AAN | Ninhydrin colorimetric |
|
| ASN | Elson–Morgen colorimetric method | Bremner J.M. Organic forms of nitrogen. Madison: American Society of Agronomy, 1965: 1148–1178. |
| HUN | HUN = TH-AN-AAN-ASN | |
| RN, TN | Modified Kjeldahl method | HJ 717 - 2014 from Ministry of Ecology and Environment, PRC |
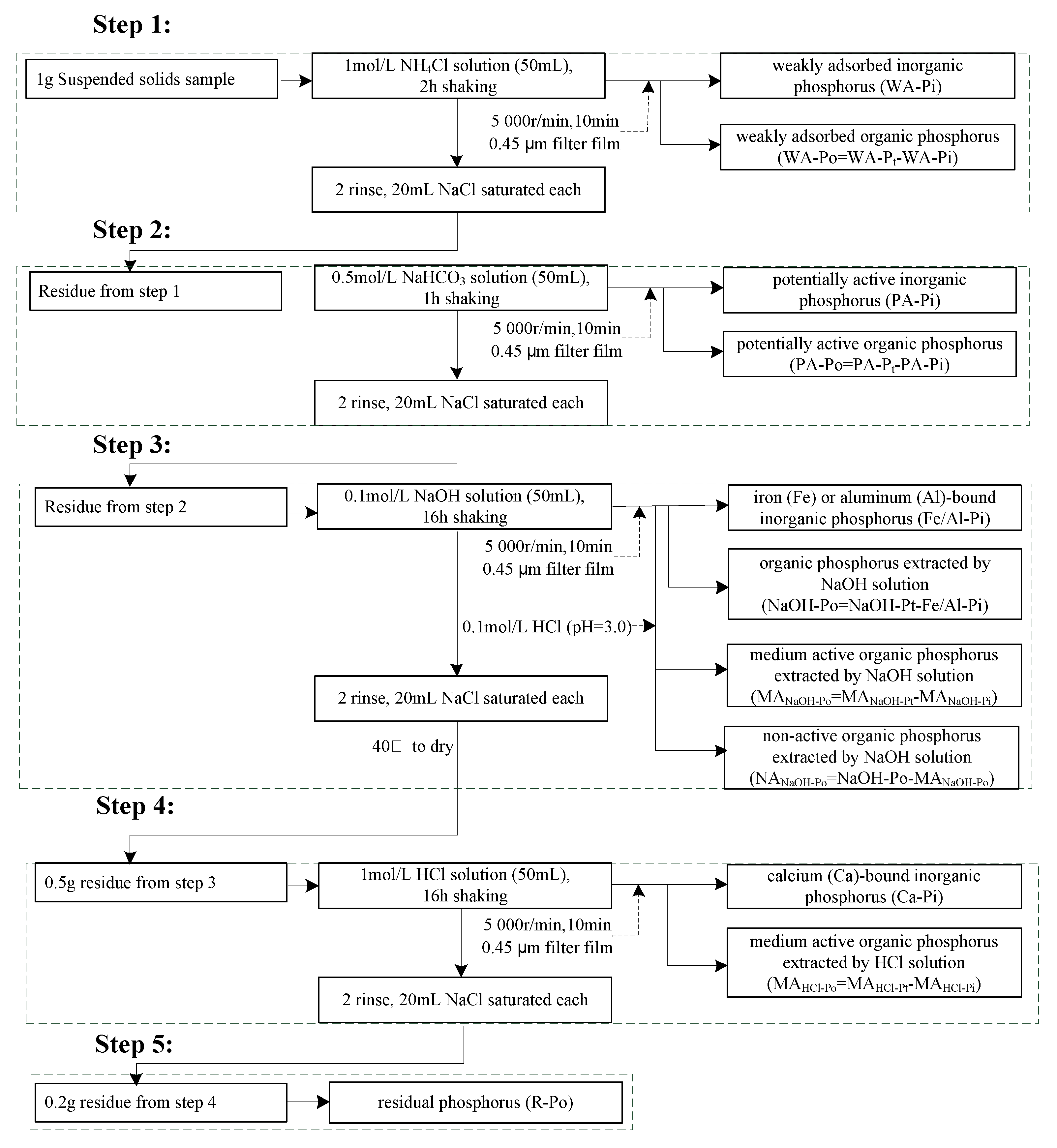
| Index | Measurement | Reference |
|---|---|---|
| inorganic phosphorus | phosphomolybdenum blue spectrophotometric method | HJ 593-2010 from Ministry of Ecology and Environment, PRC |
| TP | ammonium molybdate spectrophotometric method | GB 11893-89 from Ministry of Ecology and Environment, PRC |
| organic phosphorus | OP = TP − IP |

| Index | Unit | Min | Max | Average | SD 1 |
|---|---|---|---|---|---|
| T | °C | 18.50 | 19.30 | 18.88 | 0.22 |
| pH | / | 7.84 | 8.37 | 8.15 | 0.09 |
| DO | mg/L | 3.60 | 8.50 | 7.05 | 0.96 |
| ORP | mV | 166.90 | 247.20 | 197.72 | 22.59 |
| COD | mg/L | 3.42 | 8.06 | 4.41 | 0.82 |
| Chl.a | mg/m3 | 6.30 | 50.54 | 16.20 | 11.11 |
| TSS | mg/L | 4 | 47 | 27 | 13.69 |
| TN | mg/L | 0.26 | 0.90 | 0.53 | 0.20 |
| DTN | mg/L | 0.04 | 0.41 | 0.18 | 0.11 |
| NH4+-N | mg/L | 0.03 | 0.25 | 0.11 | 0.05 |
| NO3−-N | mg/L | 0.008 | 0.069 | 0.048 | 0.007 |
| TP | mg/L | 0.05 | 0.23 | 0.12 | 0.04 |
| DTP | mg/L | 0.004 | 0.114 | 0.032 | 0.031 |
| IP | mg/L | 0.0006 | 0.0205 | 0.0087 | 0.006 |
| Index | Min | Max | Average | SD |
|---|---|---|---|---|
| ETN | 33.42 | 431.19 | 144.50 | 95.27 |
| E-NH4+-N | 3.12 | 222.66 | 75.31 | 56.92 |
| E-NO3−-N | 4.99 | 85.45 | 21.59 | 15.89 |
| SON | 17.76 | 126.22 | 47.60 | 30.42 |
| HN | 477.91 | 1156.33 | 701.96 | 158.99 |
| AN | 208.28 | 698.38 | 335.19 | 108.17 |
| AAN | 10.10 | 383.07 | 162.91 | 94.27 |
| ASN | 3.49 | 29.17 | 11.79 | 5.81 |
| HUN | 62.29 | 281.15 | 192.08 | 61.17 |
| RN | 0 | 1163.30 | 451.17 | 315.24 |
| TN | 516.34 | 2551.14 | 1297.64 | 497.75 |
| Index | Min | Max | Average | SD |
|---|---|---|---|---|
| WA-Pi | 1.40 | 20.50 | 6.37 | 4.50 |
| PA-Pi | 10.34 | 107.15 | 53.85 | 24.84 |
| Fe/Al-Pi | 40.93 | 243.13 | 106.93 | 51.85 |
| Ca-Pi | 62.10 | 348.53 | 163.43 | 80.71 |
| WA-Po | 0.67 | 10.69 | 3.01 | 1.92 |
| PA-Po | 0.03 | 54.00 | 9.52 | 10.11 |
| MA-Po | 50.97 | 142.15 | 91.29 | 18.85 |
| NA-Po | 76.93 | 244.99 | 137.07 | 45.83 |
References
- Morgane, L.M.; Chantal, G.O.; Alain, M.; Yves, S. Eutrophication: A new wine in an old bottle? Sci. Total Environ. 2019, 651, 1–11. [Google Scholar]
- Brigitte, V.L.; Celine, C. Modelling eutrophication in lake ecosystems: A review. Sci. Total Environ. 2019, 651, 2985–3001. [Google Scholar]
- Bhagowati, B.; Ahamad, K.U. A review on lake eutrophication dynamics and recent developments in lake modelling. Ecohydrol. Hydrobil. 2016, 175, 1–12. [Google Scholar]
- Schindler, D.W.; Carpenter, S.R.; Chapra, S.C.; Hecky, R.E.; Orihel, D.M. Reducing phosphorus to Curb Lake eutrophication is a success. Environ. Sci. Technol. 2016, 50, 8923–8929. [Google Scholar] [CrossRef] [PubMed]
- McCrakin, M.; Jones, H.P.; Jones, P.C.; Mateos, D.M. Recovery of lakes and coastal marine ecosystems from eutrophication: A global meta-analysis. Limnol. Oceanogr. 2017, 62, 57–518. [Google Scholar] [CrossRef]
- Cook, S.C.; Housley, L.; Back, J.A.; King, R.S. Freshwater eutrophication drives sharp reductions in temporal beta diversity. Ecology 2018, 99, 47–56. [Google Scholar] [CrossRef]
- O’Hare, M.T.; Pedersen, A.B.; Baumgarte, I.; Freeman, A.; Gunn, I.D.M. Responses of aquatic plants to eutrophication in rivers: A revised conceptual model. Front. Plant. Sci. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Glibert, P.M. Harmful algae at the complex nexus of eutrophication and climate change. Harmful Algae 2019, in press. [Google Scholar] [CrossRef]
- Zeng, J.; Yang, L.Y.; Xiao, L. Biogeochemical cycling of nitrogen in lakes and the role of microorganisms in conversion of nitrogen compounds. J. Lake Sci. 2007, 19, 382–389. [Google Scholar]
- Schindler, D.W.; Hecky, R.E.; Findla, Y. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37 year whole ecosystem experiment. PNAS 2008, 105, 11254–11258. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.H.; Fan, C.X.; Zhong, J.C.; Zhang, Y.L. Evaluation of in situ simulated dredging to reduce internal nitrogen flux across the sediment-water interface in Lake Taihu, China. J. Environ. Pollut. 2016, 214, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Y.; Jin, X.; Tang, W.Z.; Meng, X.; Shan, B.Q. Comprehensive analysis of nitrogen distributions and ammonia nitrogen release fluxes in the sediments of Baiyangdian Lake, China. J. Environ. Sci. 2019, 76, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wang, Y.Q.; He, J.; Luo, X.Z.; Zheng, Z. Phosphorus mobility among sediments, water and cyanobacteria enhanced by cyanobacteria blooms in eutrophic Lake Dianchi. Environ. Pollut. 2016, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yuan, X.Y.; Marip, J.B.; Yu, H.H.; Zhang, Q.; Tang, D.D. Spatial distributions of transferable nitrogen forms and influencing factors in sediments from inflow rivers in different lake basins. Environ. Sci. 2018, 39, 1122–1128. [Google Scholar]
- Cavalcante, H.; Araújo, F.; Noyma, N.P.; Becker, V. Phosphorus fraction in sediments of tropical semiarid reservoirs. Sci. Total Environ. 2018, 619–620, 1022–1029. [Google Scholar] [CrossRef]
- Brezonik, P.; Bouchard, W.B., Jr.; Finlay, J.C.; Griffin, C.G.; Olmanson, L.G.; Anderson, J.P. Color, chlorophyll a, and suspended solids effects on Secchi depth in lakes: Implications for trophic state assessment. Ecol. Appl. 2019, 29, e01871. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, W.N.; Huang, Y.X.; Gao, X.P. Analysing the correlations of long-term seasonal water quality parameters, suspended solids and total dissolved solids in a shallow reservoir with meteorological factors. Environ. Sci. Pollut. Res. 2017, 24, 6746–6756. [Google Scholar] [CrossRef]
- Nasrabadi, T.; Ruegner, H.; Sirdari, Z.Z.; Schiwientek, M.; Grathwohl, P. Using total suspended solids (TSS) and turbidity as proxies for evaluation of metal transport in river water. Appl. Geochem. 2016, 68, 1–9. [Google Scholar] [CrossRef]
- Sandström, S.; Futter, M.N.; Kyllmar, K.; Bishop, K.; O’Connell, D.W.; Djodjic, F. Particulate phosphorus and suspended solids losses from small agricultural catchments: Links to stream and catchment characteristics. Sci. Total Environ. 2020, 711, 1–12. [Google Scholar] [CrossRef]
- Arantes, R.; Schveitzer, R.; Magnotti, C.; Lapa, K.R.; Vinatea, L. A comparison between water exchange and settling tank as a method for suspended solids management in intensive biofloc technology systems: Effects on shrimp (Litopenaeus vannamei) performance, water quality and water use. Aquac. Res. 2017, 48, 1478–1490. [Google Scholar] [CrossRef]
- Qi, C.; Wang, G.X.; Wu, X.T.; Xu, X.G.; Han, R.M.; Wu, S.J. Deposition Characteristics of Suspended Solids and the Response of Dissolved Nutrients in spring in the Western Lakeside of Taihu Lake. Environ. Sci. 2017, 38, 95–103. [Google Scholar]
- Niu, Y.; Jiang, X.; Wang, K.; Xia, J.D.; Jiao, W.; Niu, Y.; Yu, H. Meta analysis of heavy metal pollution and sources in surface sediments of Lake Taihu, China. Sci. Total Environ. 2020, 700, 134509. [Google Scholar] [CrossRef]
- Ti, C.P.; Gao, B.; Luo, Y.X.; Wang, S.W.; Chang, S.X.; Yan, X.Y. Dry deposition of N has a major impact on surface water quality in the Taihu Lake region in southeast China. Atmos. Environ. 2018, 190, 1–9. [Google Scholar] [CrossRef]
- Wang, S.H.; Wang, W.W.; Jiang, X. Spatial-temporal dynamic changes of nitrogen and phosphorus and different analysis in water body of Lihu Lake. China Environ. Sci. 2014, 34, 1268–1276. [Google Scholar]
- Wang, W.W.; Wang, S.H.; Jiang, X.; Zheng, B.H. Occurrence characteristics and release potential of nitrogen fractions in sediments of Lihu Lake. China Environ. Sci. 2017, 37, 292–301. [Google Scholar]
- Wang, S.H.; Zhao, L.; Jiang, X. Forms, occurrence characteristics and biological of phosphorus in surface sediments of Lihu Lake. Res. Environ. Sci. 2015, 28, 1741–1748. [Google Scholar]
- Gu, G.; Lu, G.F. On the integrated control of water environment of Wuli Lake, Lake Taihu. J. Lake Sci. 2004, 16, 56–60. [Google Scholar]
- Li, W.C. Biological and environmental succession in Wuli Bay of Taihu Lake. J. Lake Sci. 1996, 8, 37–45. [Google Scholar]
- Morris, D.P.; Zagarese, H.; Williamson, C.E.; Balseiro, E.G.; Hargreaves, B.R. The attenuation of solar UV radiation in lakes and the role of dissolved organic carbon. Limmol. Oceanogr. 1995, 40, 1381–1391. [Google Scholar] [CrossRef] [Green Version]
- Nõges, P.; Nõges, T.; Tuvikene, L.; Smal, H.; Ligeza, S.; Kornijów, R. Factors controlling hydrochemical and trophic state variables in 86 shallow lakes in Europe. Hydrobiologia 2003, 506–509, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Jeppsen, E.; Jensen, J.P.; Sødergaard, M.; Hansen, K.S. Does resuspension prevent a shift to a clear state in shallow lakes during reoligotrophication? Limmol. Oceanogr. 2003, 48, 1913–1919. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.L.; Qin, B.Q.; Zhu, G.W.; Huang, Q.F.; Gu, X.H. The light condition and affect factors in Hangzhou West Lake. Resour. Environ. Yangtze Basin 2005, 14, 744–749. [Google Scholar]
- Zhang, Y.L.; Qin, B.Q.; Chen, W.M.; Luo, L.C. A study on total suspended matter in Lake Taihu. Resour. Environ. Yangtze Basin 2004, 13, 266–271. [Google Scholar]
- Liu, Z.H.; Li, Y.M.; Tan, J.; Guo, Y.L.; Zhou, L.; Liu, G. Construction of semi-analytical model for inversing total suspended matter in Lake Taihu and Chaohu and assessment of its applicability. Environ. Sci. 2012, 33, 3000–3008. [Google Scholar]
- Lange, G.J. Distribution of exchangeable, fixed, organic and total nitrogen in interbedded turbiditic pelagic sediments of the Maderia abyssal-plain, eastern north-Atlantic. Mar. Geol. 1992, 109, 95–114. [Google Scholar] [CrossRef]
- Wang, S.R.; Jiao, L.X.; Jin, X.C. Distribution of total, exchangeable and fixed nitrogen in the sediments from shallow lakes in the middle and lower reaches of the Yangtze River. Acta Sci. Circumstantiae 2008, 28, 37–43. [Google Scholar]
- Jin, J.; Yuan, X.Y.; Chen, S.W.; Li, Z.Y.; Ye, H.M.; Xu, H.Y. Distribution of nitrogen forms in suspended sediments and surface sediments of East Tiaoxi River, upper reaches of Taihu Basin and their influence factors. J. Lake Sci. 2017, 29, 594–603. [Google Scholar]
- Zehr, J.P.; Paulsen, S.G.; Axler, R.P. Dynamics of dissolved organic nitrogen in subalpine Castle Lake, California. Hydrobiologia 1988, 157, 33–45. [Google Scholar] [CrossRef]
- Bremner, J.M. Organic Forms of Soil Nitrogen; Bremner, J.M., Black, C.A., Eds.; Methods of soil analysis; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1148–1178. [Google Scholar]
- Mengel, K.; Schmeer, H. Effect of straw, cellulose and lignin on the turnover and availability of labelled ammonium nitrate. Biol. Fertil. Soils 1985, 1, 175–181. [Google Scholar] [CrossRef]
- Wang, S.R.; Jiao, L.X.; Jin, X.C. Characteristics of organic nitrogen fractions in sediments of shallow lakes in the middle and lower reaches of the Yangtze River area in the China. Water Environ. J. 2012, 26, 473–481. [Google Scholar] [CrossRef]
- Zhong, L.X.; Wang, S.H.; Jiang, X.; Jin, X.C. Speciation characteristics of different combined nitrogen in the spring sediments of Chaohu Lake by sequential extraction methods. J. Agro-Environ. Sci. 2009, 28, 2132–2137. [Google Scholar]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; John Wiley&Sons: New York, NY, USA, 1994; pp. 55–119. [Google Scholar]
- Schulten, H.R.; Schnitzer, M. The chemistry of soil organic nitrogen: A review. Biol. Fertil. Soils 1997, 26, 1–15. [Google Scholar] [CrossRef]
- Jones, D.L.; Shannon, D.; Junvee-Fortune, T.; Farrar, J.F. Plant capture of free amino acids is maximized under high soil amino acid concentrations. Soil Biol. Biochem. 2005, 37, 179–181. [Google Scholar] [CrossRef]
- Ruban, V.; Brigault, S.; Demare, D.; Phillippe, A.M. An investigation of the origin and mobility of phosphorus in freshwater sediments from Bort-Les-Orgues Reservoir, France. J. Environ. Monit. 1999, 1, 403–407. [Google Scholar] [CrossRef]
- Sun, S.J.; Huang, S.L.; Sun, X.M.; Wen, W. Phosphorus fractions and its release in the sediments of Haihe River, China. J. Environ. Sci. 2009, 21, 291–295. [Google Scholar] [CrossRef]
- Fabre, A.; Qotbi, A.; Dauta, A.; Baldy, V. Relation between algal available phosphate in the sediments of the River Garonne and chemically-determined phosphate fractions. Hydrobiologia 1996, 335, 43–48. [Google Scholar] [CrossRef]
- William, J.D.H.; Shear, H.; Thomas, R.L. Availability to Scenedesmus, quadrcauda of different forms of phosphorus in sedimentary materials from the Great Lakes. Limnol. Oceanogr. 1980, 25, 1–11. [Google Scholar] [CrossRef]
- Kaiserli, A.; Voutsa, D.; Samara, C. Phosphorus fractionation in lake sediments-Lakes Volvi and Koronia, N. Greece. Chemosphere 2002, 46, 1147–1155. [Google Scholar] [CrossRef]
- He, Z.Q.; Griffin, T.S.; Honeycutt, C.W. Evaluation of soil phosphorus transformations by sequential fraction and phosphatase hydrolysis. Soil Sci. 2004, 169, 515–527. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, S.L.; Zhao, H.D.; Deng, L.; Wang, C. The phosphorus speciation in the sediments up and down-stream of cascade dams along the middle Lancang River. Chemosphere 2015, 120, 653–659. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Wu, F.C.; Liu, C.Q.; Fu, P.Q.; Li, W. Characteristics of organic phosphorus fractions in different trophic sediments of lakes from the middle and lower reaches of Yangtze River region and Southwestern Plateau, China. Environ. Pollut. 2008, 152, 366–372. [Google Scholar] [CrossRef]
- Sun, J.; Wang, S.R.; Zeng, Q.R. Distribution characteristics of organic phosphorus fractions and influencing factors in surface sediments of Lake Erhai. Res. J. Environ. Sci. 2011, 24, 1226–1232. [Google Scholar]
- Ivanoff, D.B.; Reddy, K.R.; Robinson, S. Chemical fractionation of organic phosphorus in selected histosols. Soil Sci. 1998, 163, 36–45. [Google Scholar] [CrossRef]
- Chen, H.L.; Yuan, X.Y.; Wang, H.; Li, Z.Y.; Xu, H.Y. Distributions of phosphorus fractions in suspended sediments and surface sediments of Tiaoxi mainstreams and cause analysis. Environ. Sci. 2015, 36, 464–470. [Google Scholar]
- Huang, J.C.; Gao, J.F.; Jiang, Y. Source, distribution and export coefficient of phosphorus in lowland polders of Lake Taihu Basin, China. Environ. Pollut. 2017, 231, 1274–1283. [Google Scholar] [CrossRef]
- He, Z.Q.; Senwo, Z.N.; Mankolo, R.N.; Honeycutt, C.W. Phosphorus fractions in poultry litter characterized by sequential fractionation coupled with phosphatase hydrolysis. J. Food Agric. Environ. 2006, 4, 304–312. [Google Scholar]
- Huang, W.L.; Cai, W.; Huang, H.; Lei, Z.F.; Zhang, Z.Y.; Tay, J.H.; Lee, D.J. Identification of inorganic and organic species of phosphorus and its bio-availability in nitrifying aerobic granular sludge. Water Res. 2015, 68, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Ding, S.M.; Li, B.; Bai, X.L.; Fan, C.X.; Zhang, C.S. Speciation of organic phosphorus in a sediment profile of Lake Taihu: I chemical forms and their transformation. J. Environ. Sci. 2013, 25, 637–644. [Google Scholar] [CrossRef]
- Zan, F.Y.; Huo, A.L.; Xi, B.D.; Li, Q.Q.; Liao, H.Q.; Zhang, J.T. Phosphorus distribution in the sediments of a shallow eutrophic lake, Lake Chaohu, China. Environ. Earth Sci. 2011, 62, 1643–1653. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, L.L.; Yan, S.W.; Li, H.L.; Xu, J. Atmospheric wet deposition characteristics of nitrogen and phosphorus nutrients in Taihu Lake and contributions to the lake. Res. Environ. Sci. 2011, 24, 1210–1219. [Google Scholar]
- Liu, T.; Yang, L.Y.; Hu, Z.X.; Sun, Y.N. Spatial-temporal features of atmospheric deposition of nitrogen and phosphorus to the Lake Taihu. ATEM 2012, 24, 20–22. [Google Scholar]
- Wang, Q.L.; Liang, T. Distribution patterns and dynamics of phosphorus forms in the overlying water and sediment of Dongting Lake. J. Great Lake Res. 2016, 42, 565–570. [Google Scholar] [CrossRef]
- Monchamp, M.E.; Pick, F.R.; Beisner, B.E.; Maranger, R. Nitrogen forms influence microcystin concentration and composition via changes in cyanobacterial community structure. PLoS ONE 2014, 9, 1–10. [Google Scholar] [CrossRef]
- Horst, G.P.; Sarnelle, O.; White, J.D.; Hamilton, S.K.; Kaul, R.B.; Bressie, J.D. Nitrogen availability increases the toxin quota of a harmful cyanobacterium, Microcystic aeruginosa. Water Res. 2014, 54, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Tang, C.Y.; Li, X.; Jiang, T.; Shi, Y.P.; Cao, Y.J. Reconstructing the historical pollution levels and ecological risks over the past sixty years in sediments of the Beijiang River, South China. Sci. Total Environ. 2019, 649, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Lindström, M.; Håkanson, L.; Abrahamsson, O.; Johansson, H. An empirical model for prediction of lake water suspended particulate matter. Ecol. Model. 1999, 121, 185–198. [Google Scholar] [CrossRef]
- Liu, X.J.; Li, Z.B.; Li, P.; Zhu, B.B.; Long, F.F.; Cheng, Y.T. Changes in carbon and nitrogen with particle size in bottom sediments in the Dan River, China. Quatern. Int. 2015, 380–381, 305–313. [Google Scholar] [CrossRef]
- Sulce, S.; Lopez, D.P.; Jacquin, F.; Vong, P.C.; Guiraud, G. Study of immobilization and remobilization of nitrogen fertilizer in cultivated soils by hydrolytic fractionation. Eur. J. Soil Sci. 1996, 47, 249–255. [Google Scholar] [CrossRef]
- Löfgren, S.; Boström, B. Interstitial water concentrations of phosphorus, iron and manganese in a shallow, eutrophic Swedish lake—Implications for phosphorus cycling. Wat. Res. 1989, 23, 1115–1125. [Google Scholar] [CrossRef]
- Sødergaard, M.; Kristensen, P.; Jeppesen, E. Phosphorus release from resuspended sediment in the shallow and wind-exposed Lake Arresø, Denmark. Hydrobiologia 1992, 228, 91–99. [Google Scholar]
- Froelich, P.N.; Bender, M.L.; Heath, G.R. Phosphorus accumulation rates in metalliferous sediments on the east pacific rise. Earth Planet. Sci. Lett. 1977, 34, 351–359. [Google Scholar] [CrossRef]

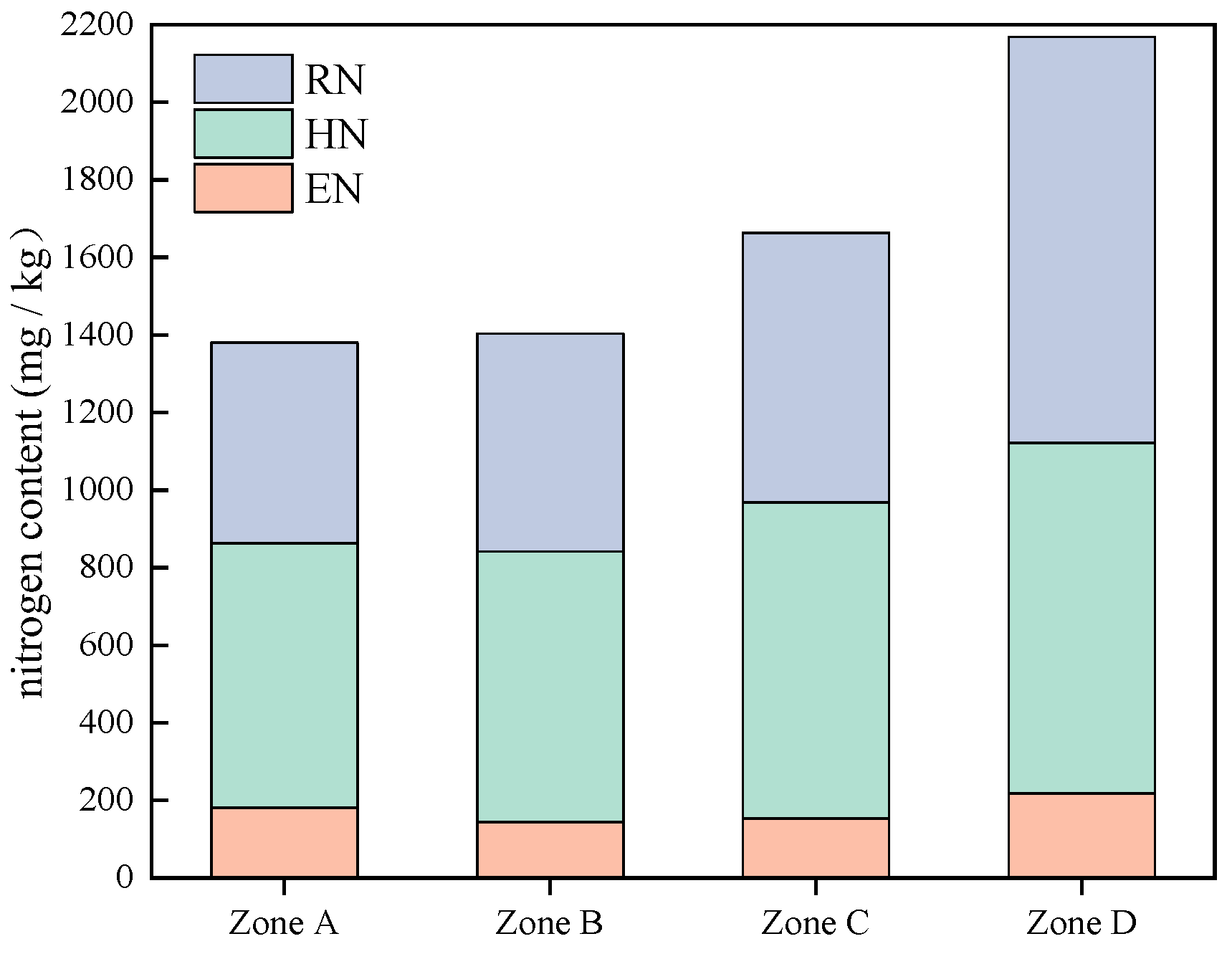
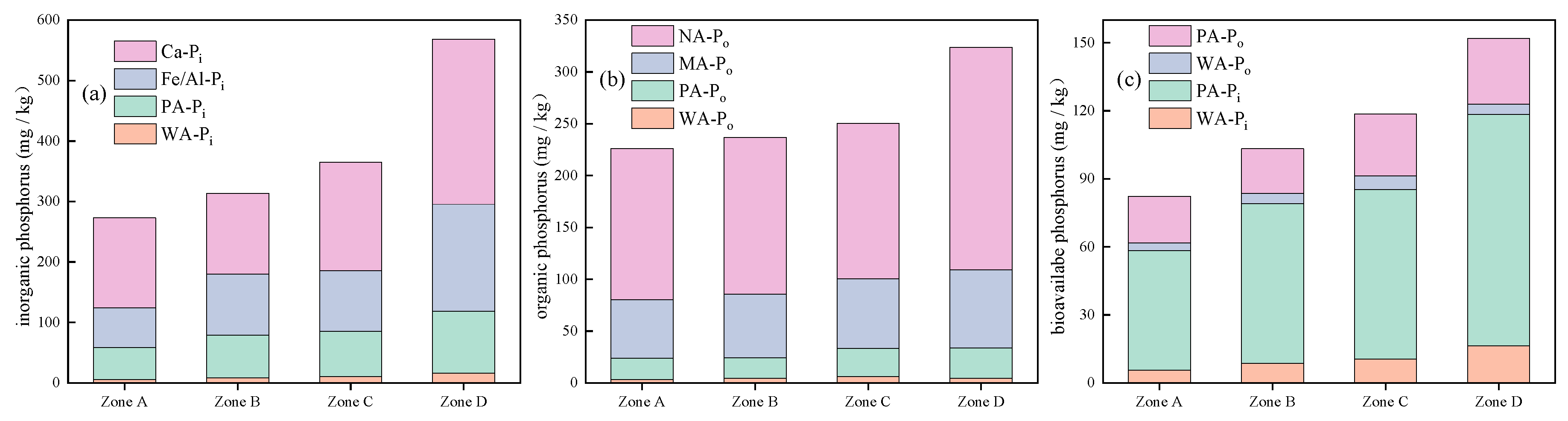
| ETN | E-NH4+-N | E-NO3−-N | SON | HN | AN | AAN | ASN | HUN | RN | TN | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ETN | 1 | ||||||||||
| E-NH4+-N | 0.794 ** | 1 | |||||||||
| E-NO3 NO3−-N | 0.736 ** | 0.549 ** | 1 | ||||||||
| SON | 0.539 ** | −0.078 | 0.359 | 1 | |||||||
| HN | 0.440 * | 0.198 | 0.759 ** | 0.365 | 1 | ||||||
| AN | 0.577 ** | 0.403 * | 0.745 ** | 0.325 | 0.854 ** | 1 | |||||
| AAN | 0.178 | 0.374 | −0.004 | −0.201 | −0.216 | −0.050 | 1 | ||||
| ASN | −0.045 | 0.030 | −0.039 | −0.115 | 0.218 | 0.234 | 0.301 | 1 | |||
| HUN | 0.265 | −0.014 | 0.630 ** | 0.371 | 0.929 ** | 0.659 ** | −0.517 ** | 0.073 | 1 | ||
| RN | 0.643 ** | 0.558 ** | 0.814 ** | 0.216 | 0.769 ** | 0.816 ** | 0.072 | 0.089 | 0.593 ** | 1 | |
| TN | 0.656 ** | 0.481 ** | 0.862 ** | 0.338 | 0.911 ** | 0.888 ** | −0.036 | 0.140 | 0.764 ** | 0.959 ** | 1 |
 represents an extremely significant positive correlation;
represents an extremely significant positive correlation;  represents a significant positive correlation;
represents a significant positive correlation;  represents a positive correlation;
represents a positive correlation;  represents an extremely significant negative correlation;
represents an extremely significant negative correlation;  represents a negative correlation.
represents a negative correlation.| WA-Pi | PA-Pi | Fe/Al-Pi | Ca-Pi | WA-Po | PA-Po | MA-Po | NA-Po | IP | OP | TP | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WA-Pi | 1 | ||||||||||
| PA-Pi | 0.910 ** | 1 | |||||||||
| Fe/Al-Pi | 0.728 ** | 0.817 ** | 1 | ||||||||
| Ca-Pi | 0.670 ** | 0.616 ** | 0.626 ** | 1 | |||||||
| WA-Po | 0.344 | 0.234 | 0.201 | 0.142 | 1 | ||||||
| PA-Po | 0.256 | 0.293 | 0.374 | 0.332 | 0.209 | 1 | |||||
| MA-Po | 0.346 | 0.492 * | 0.563 ** | 0.419 * | 0.294 | 0.159 | 1 | ||||
| NA-Po | 0.568 ** | 0.634 ** | 0.822 ** | 0.467 * | 0.181 | 0.377 * | 0.414 * | 1 | |||
| IP | 0.844 ** | 0.868 ** | 0.899 ** | 0.887 ** | 0.212 | 0.380 * | 0.529 ** | 0.707 ** | 1 | ||
| OP | 0.588 ** | 0.663 ** | 0.856 ** | 0.530 ** | 0.285 | 0.499 ** | 0.612 ** | 0.960 ** | 0.757 ** | 1 | |
| TP | 0.805 ** | 0.848 ** | 0.936 ** | 0.816 ** | 0.249 | 0.443 * | 0.588 ** | 0.834 ** | 0.975 ** | 0.884 ** | 1 |
 represents an extremely significant positive correlation;
represents an extremely significant positive correlation;  represents a significant positive correlation;
represents a significant positive correlation;  represents a positive correlation.
represents a positive correlation.© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zuo, Q. Forms of Nitrogen and Phosphorus in Suspended Solids: A Case Study of Lihu Lake, China. Sustainability 2020, 12, 5026. https://doi.org/10.3390/su12125026
Li J, Zuo Q. Forms of Nitrogen and Phosphorus in Suspended Solids: A Case Study of Lihu Lake, China. Sustainability. 2020; 12(12):5026. https://doi.org/10.3390/su12125026
Chicago/Turabian StyleLi, Jialu, and Qiting Zuo. 2020. "Forms of Nitrogen and Phosphorus in Suspended Solids: A Case Study of Lihu Lake, China" Sustainability 12, no. 12: 5026. https://doi.org/10.3390/su12125026
APA StyleLi, J., & Zuo, Q. (2020). Forms of Nitrogen and Phosphorus in Suspended Solids: A Case Study of Lihu Lake, China. Sustainability, 12(12), 5026. https://doi.org/10.3390/su12125026





