Performance Assessment of a Hybrid System with Hydrogen Storage and Fuel Cell for Cogeneration in Buildings
Abstract
:1. Introduction
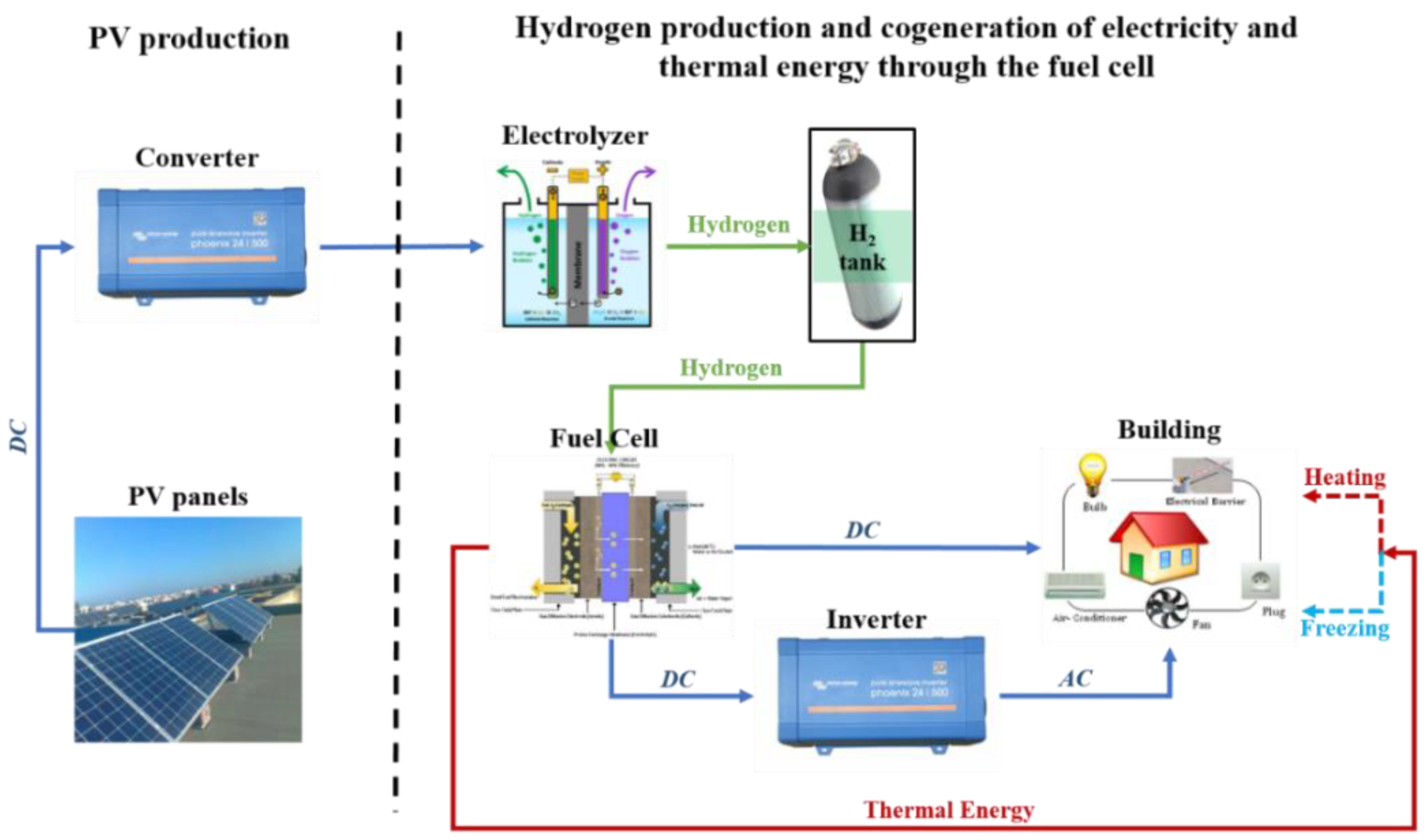
2. Hybrid System’s Description
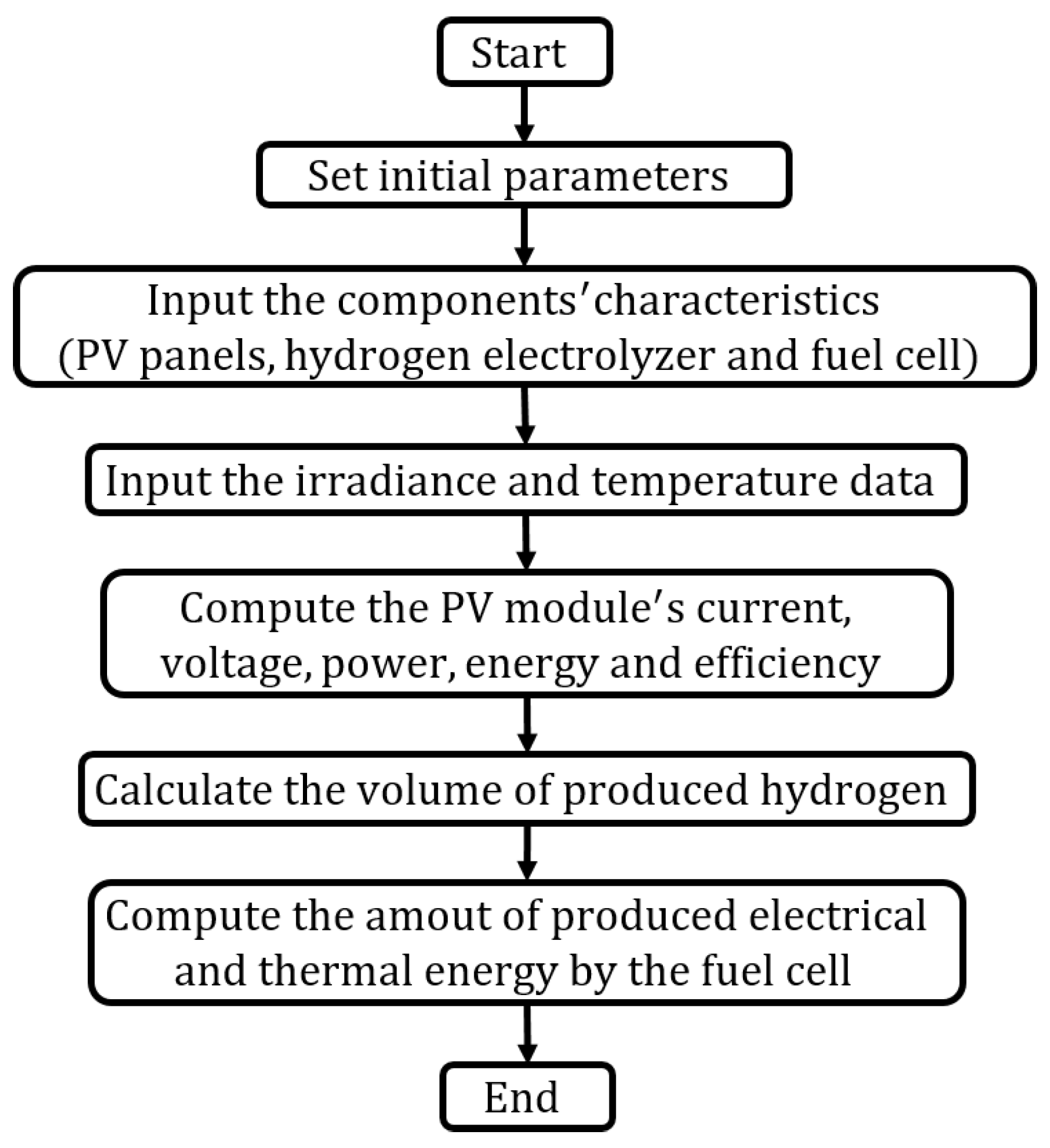
3. System’s Modeling
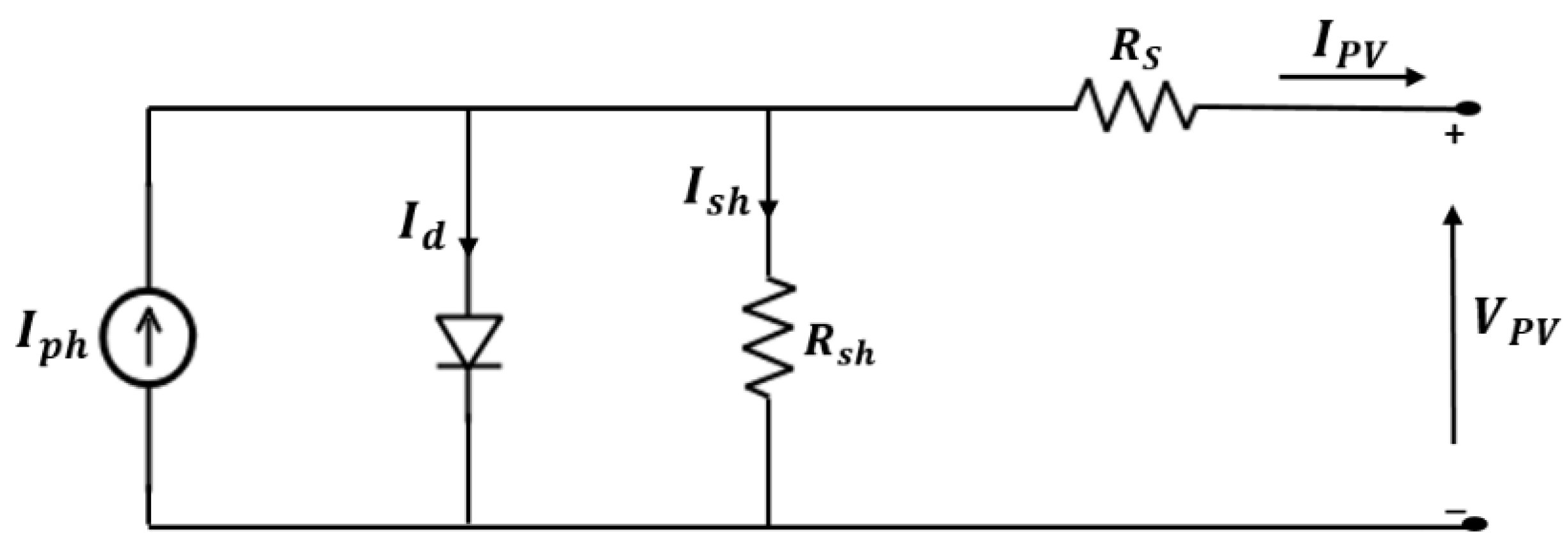
3.1. PV System’s Model
3.2. Hydrogen Electrolyzer Model
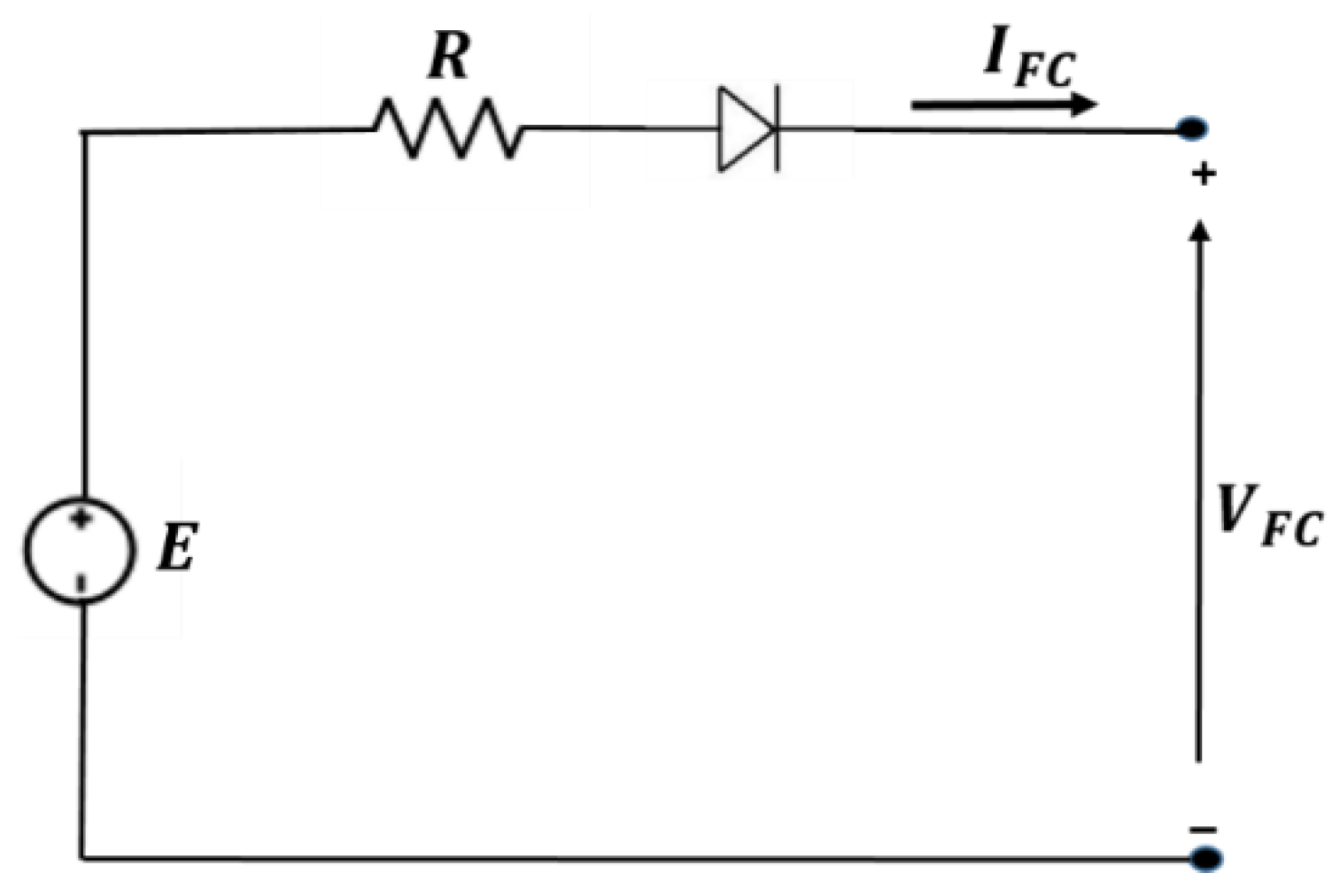
3.3. Fuel Cell’s Model
4. Simulation and Experimental Results
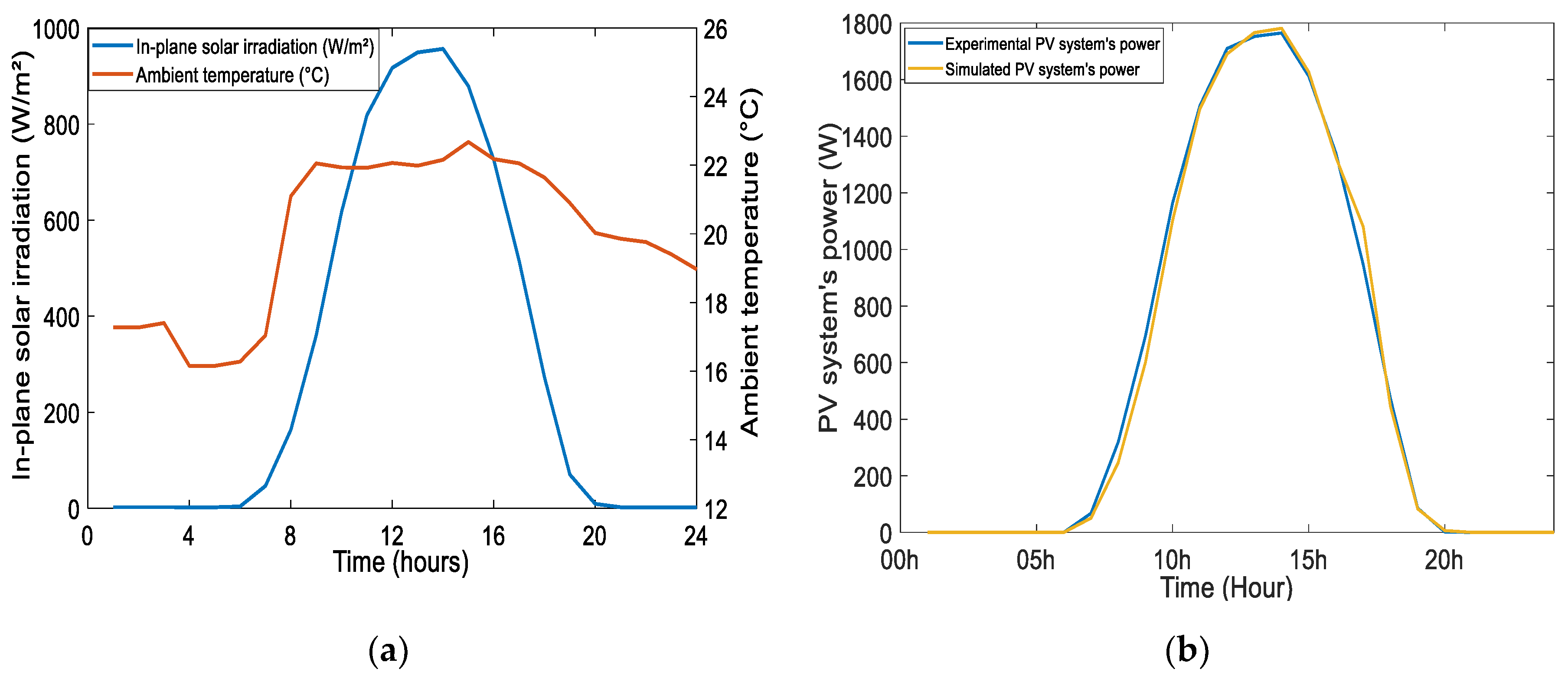
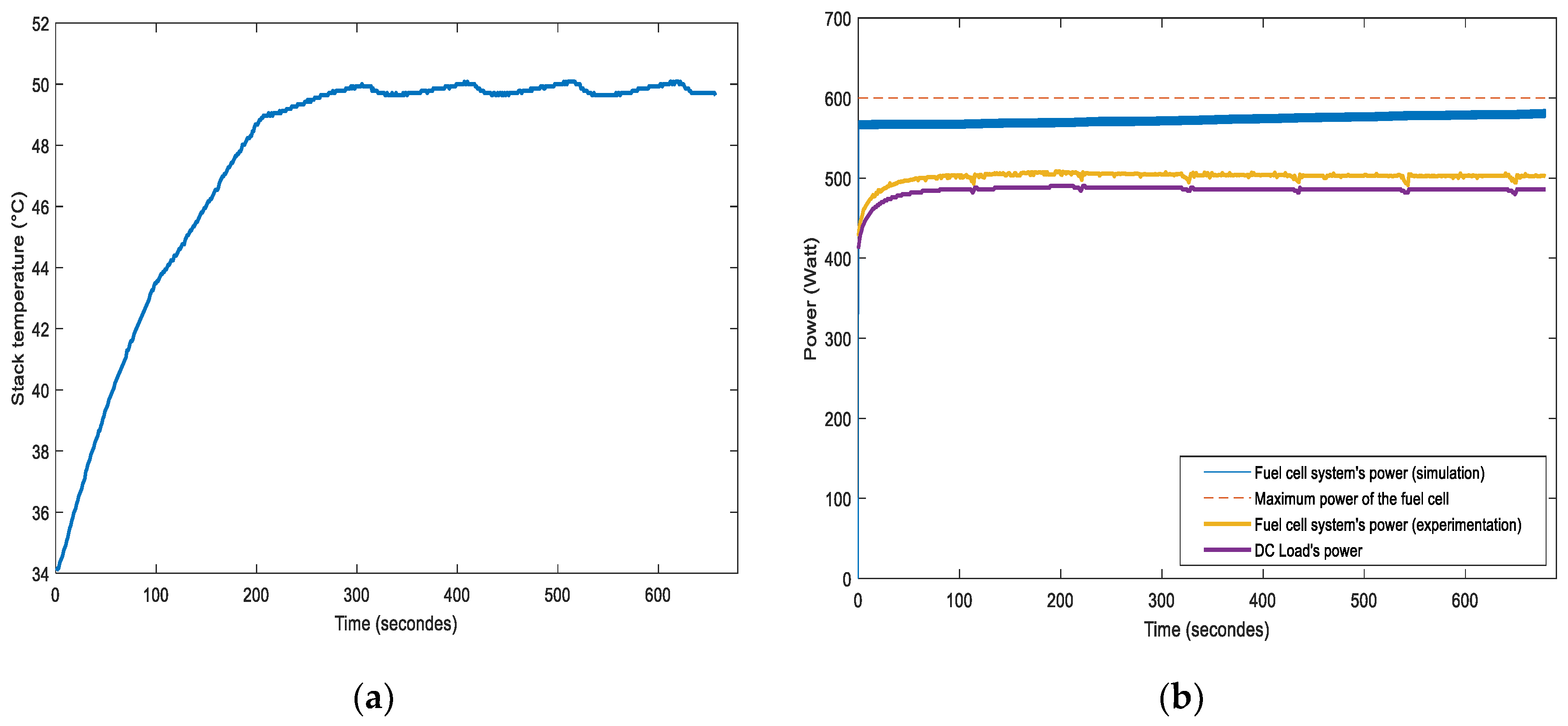
4.1. Results of Short-Term Simulations and Experimentations of the Hybrid System
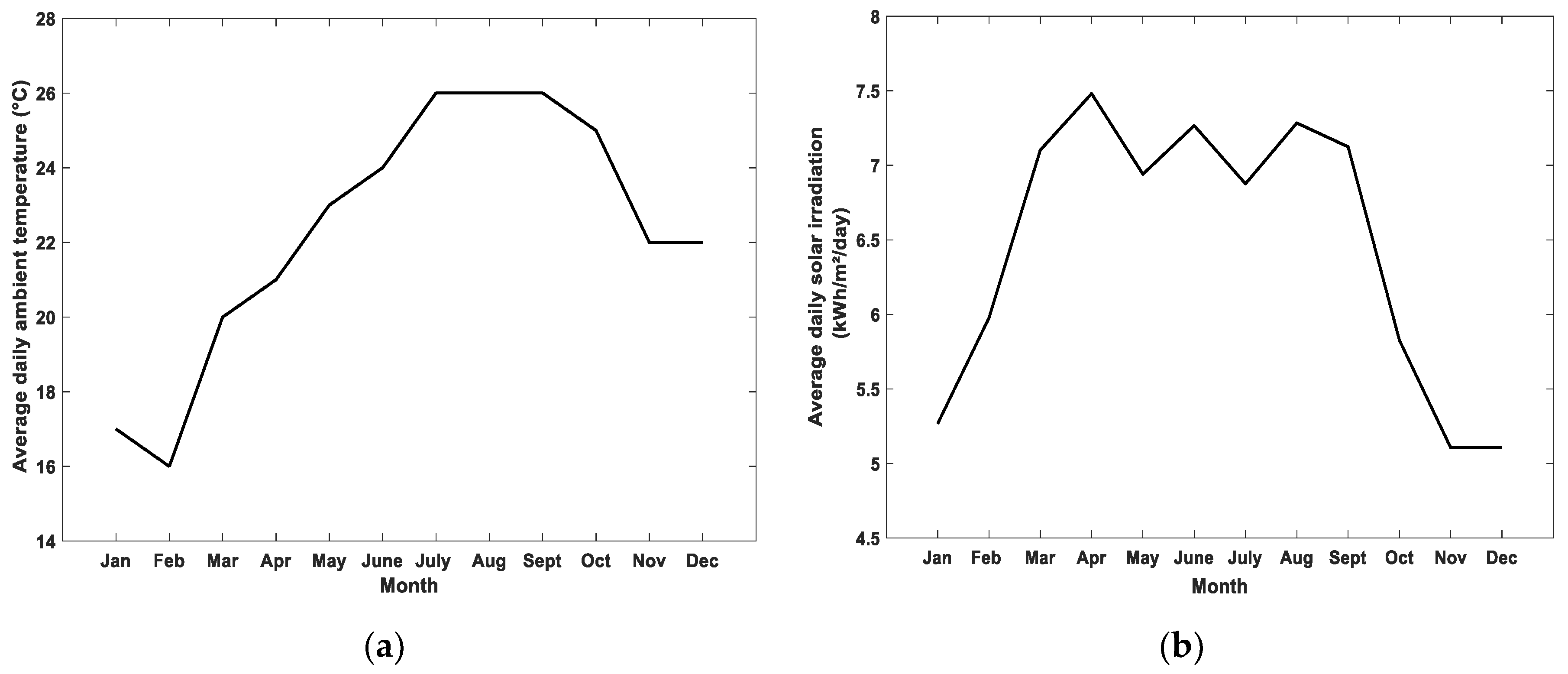
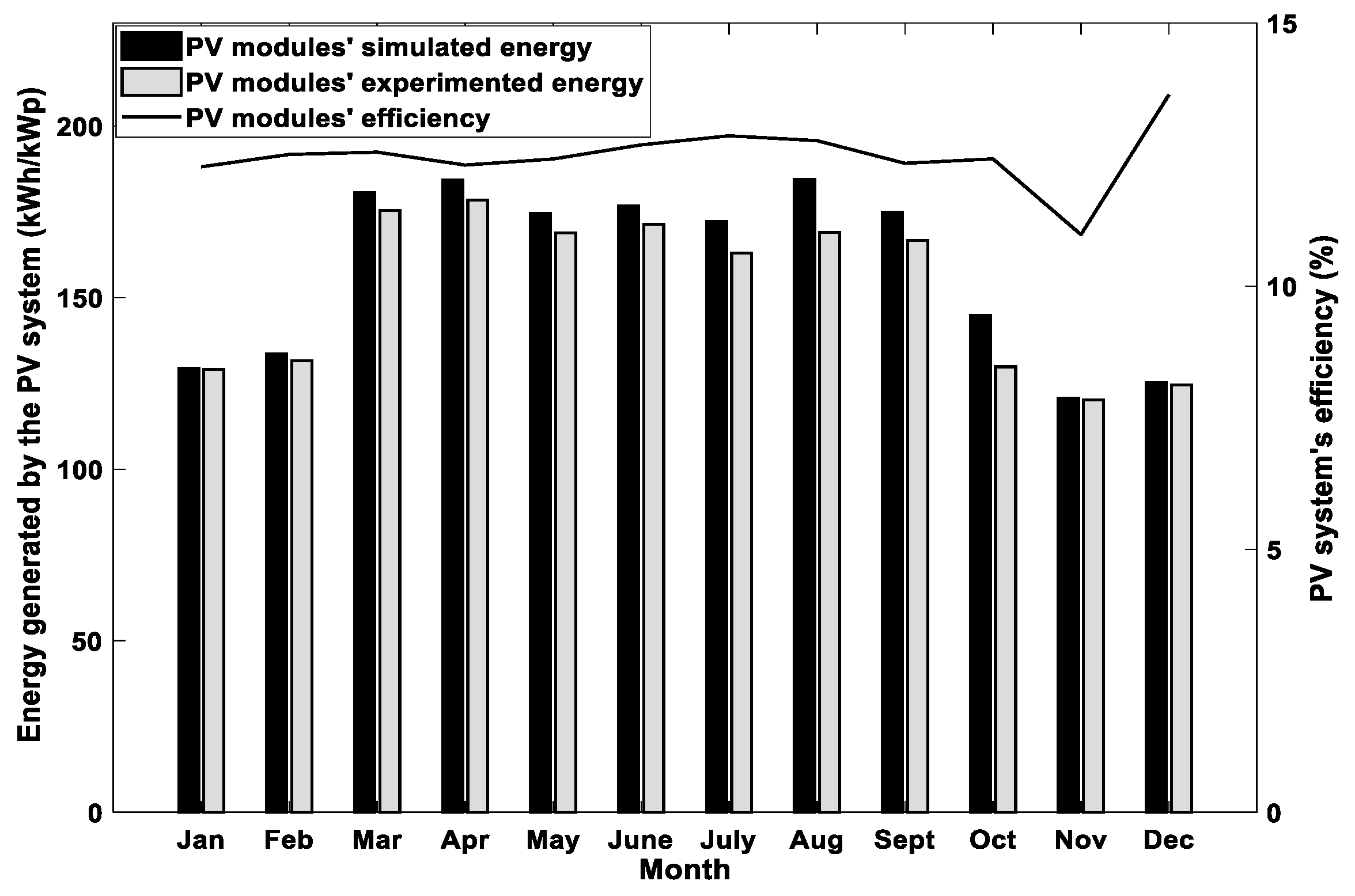
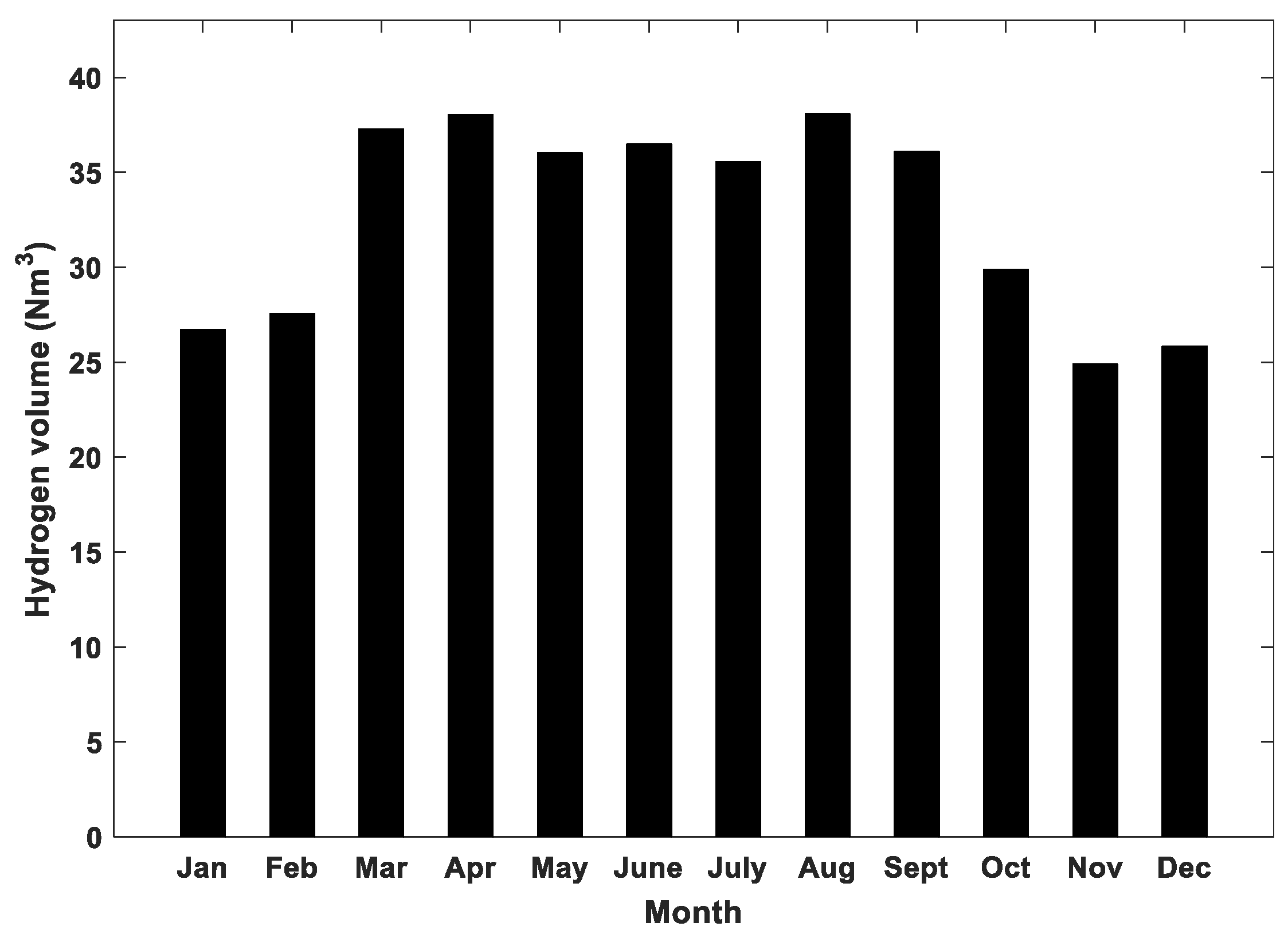
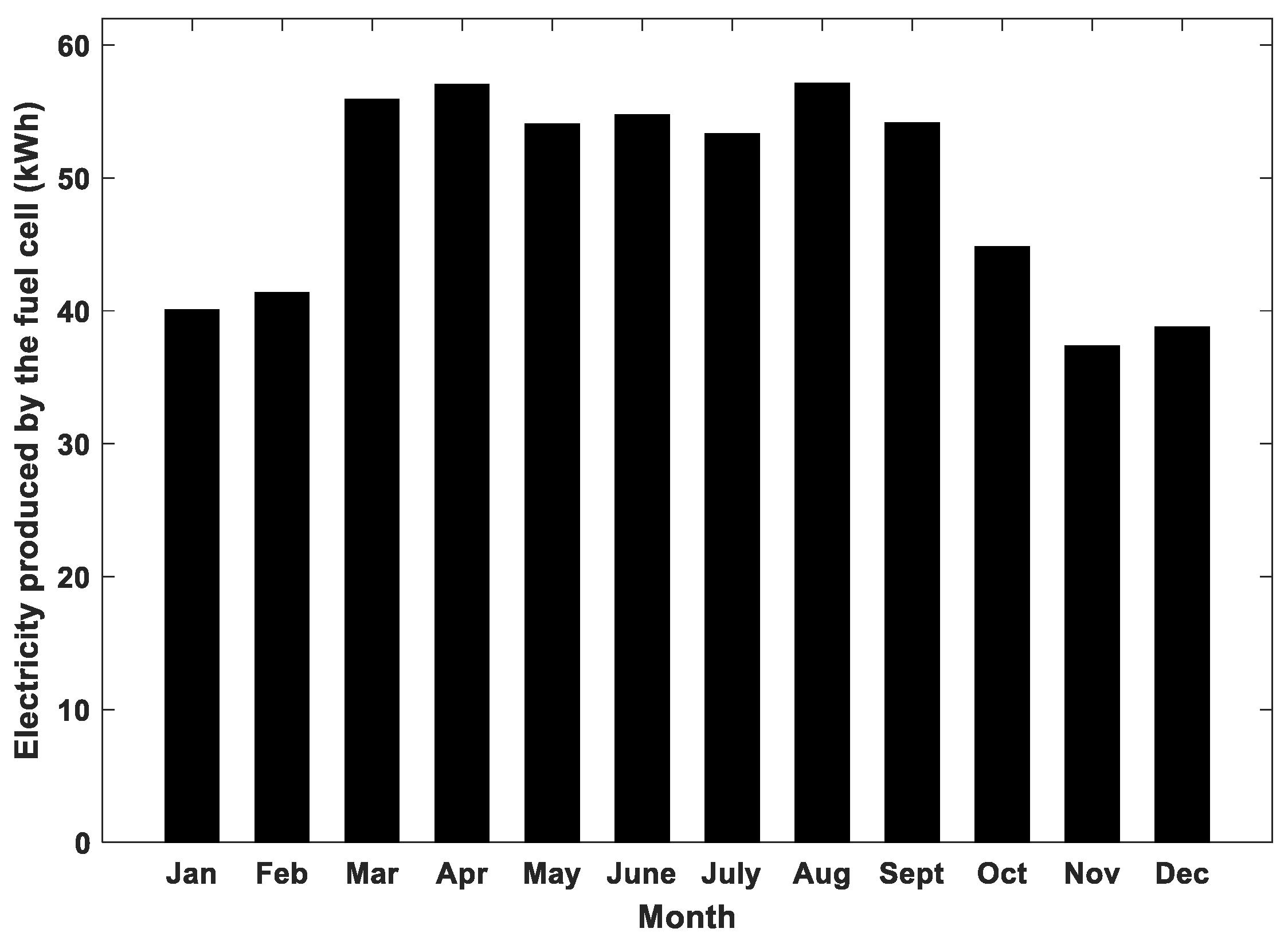
4.2. Results of Long-Term Simulations of the Hybrid System
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Feron, S. Sustainability of Off-Grid Photovoltaic Systems for Rural Electrification in Developing Countries: A Review. Sustainability 2016, 8, 1326. [Google Scholar] [CrossRef] [Green Version]
- Jaramillo-Nieves, L.; Del Río, P. Contribution of Renewable Energy Sources to the Sustainable Development of Islands: An Overview of the Literature and a Research Agenda. Sustainability 2010, 2, 783–811. [Google Scholar] [CrossRef] [Green Version]
- Schirone, L.; Pellitteri, F. Energy Policies and Sustainable Management of Energy Sources. Sustainability 2017, 9, 2321. [Google Scholar] [CrossRef] [Green Version]
- Boulmrharj, S.; NaitMalek, Y.; Elmouatamid, A.; Bakhouya, M.; Ouladsine, R.; Zine-Dine, K.; Khaidar, M.; Siniti, M. Battery Characterization and Dimensioning Approaches for Micro-Grid Systems. Energies 2019, 12, 1305. [Google Scholar] [CrossRef] [Green Version]
- El Ibrahimi, M.; Khay, I.; El Maakoul, A.; Moussa, M.O.; Barkaoui, A.; Bakhouya, M. Anaerobic co-digestion in a liquid recirculation pilot-scale reactor: Thermal and hydraulic study. Energy Rep. 2020, 6, 496–502. [Google Scholar] [CrossRef]
- Fossil Fuel Energy Consumption (% of Total). Available online: https://data.worldbank.org/indicator/eg.use.comm.fo.zs?end=2015&start=1960 (accessed on 15 February 2020).
- Othieno, H.; Awange, J. Energy Resources in Africa, Distribution, Opportunities and Challenges; Springer: New York, NY, USA, 2016. [Google Scholar]
- Sahbani, S.; Mahmoudi, H.; Hasnaoui, A.; Kchikach, M. Development Prospect of Smart Grid in Morocco. Procedia Comput. Sci. 2016, 83, 1313–1320. [Google Scholar] [CrossRef] [Green Version]
- Aqachmar, Z.; Allouhi, A.; Jamil, A.; Gagouch, B.; Kousksou, T. Parabolic trough solar thermal power plant Noor I in Morocco. Energy 2019, 178, 572–584. [Google Scholar] [CrossRef]
- Samus, T.; Lang, B.; Rohn, H. Assessing the natural resource use and the resource efficiency potential of the Desertec concept. Sol. Energy 2013, 87, 176–183. [Google Scholar] [CrossRef] [Green Version]
- Bajracharya, Q. Dynamic Modeling, Monitoring and Control of Energy Storage System. Master’s Thesis, Karlstad University, Karlstad, Sweden, 2013. [Google Scholar]
- Amrouche, S.O.; Rekioua, D.; Rekioua, T.; Bacha, S. Overview of energy storage in renewable energy systems. Int. J. Hydrog. Energy 2016, 41, 20914–20927. [Google Scholar] [CrossRef]
- Onovwiona, H.I.; Ugursal, V.I. Residential cogeneration systems: Review of the current technology. Renew. Sustain. Energy Rev. 2006, 10, 389–431. [Google Scholar] [CrossRef]
- Züttel, A. Hydrogen storage methods. Naturwissenschaften 2004, 91, 157–172. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; von Colbe, J.B.; Blanchard, D.; Bowman, R.C., Jr.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P.; et al. Materials for hydrogen-based energy storage–past, recent progress and future outlook. J. Alloy. Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Yartys, V.A.; Lototskyy, M.V.; Akiba, E.; Albert, R.; Antonov, V.E.; Ares, J.R.; Baricco, M.; Bourgeois, N.; Buckley, C.E.; von Colbe, J.B.; et al. Magnesium based materials for hydrogen based energy storage: Past, present and future. Int. J. Hydrog. Energy 2019, 44, 7809–7859. [Google Scholar] [CrossRef]
- Abe, J.O.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrog. Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Moradi, R.; Groth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrog. Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Von Colbe, J.B.; Ares, J.R.; Barale, J.; Baricco, M.; Buckley, C.; Capurso, G.; Gallandat, N.; Grant, D.M.; Guzik, M.N.; Jacob, I.; et al. Application of hydrides in hydrogen storage and compression: Achievements, outlook and perspectives. Int. J. Hydrog. Energy 2019, 44, 7780–7808. [Google Scholar] [CrossRef]
- Andersson, J.; Grönkvist, S. Large-scale storage of hydrogen. Int. J. Hydrog. Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Ball, M.; Wietschel, M. The Hydrogen Economy: Opportunities and Challenges; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Boulmrharj, S.; Khaidar, M.; Siniti, M.; Bakhouya, M.; Zine-dine, K. Towards performance assessment of fuel cell integration into buildings. Energy Rep. 2020, 6, 288–293. [Google Scholar] [CrossRef]
- Dutta, S. A review on production, storage of hydrogen and its utilization as an energy resource. J. Ind. Eng. Chem. 2014, 20, 1148–1156. [Google Scholar] [CrossRef]
- Mohammadi, A.; Mehrpooya, M. A comprehensive review on coupling different types of electrolyzer to renewable energy sources. Energy 2018, 158, 632–655. [Google Scholar] [CrossRef]
- Stojić, D.L.; Grozdić, T.D.; Umićević, B.; Maksić, A.D. A comparison of alkaline and proton exchange membrane electrolyzers. Russ. J. Phys. Chem. A Focus Chem. 2008, 82, 1958–1960. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrog. Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Kumar, S.S.; Himabindu, V. Hydrogen production by PEM water electrolysis–A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Rashid, M.M.; Al Mesfer, M.K.; Naseem, H.; Danish, M. Hydrogen production by water electrolysis: A review of alkaline water electrolysis, PEM water electrolysis and high temperature water electrolysis. Int. J. Eng. Adv. Technol 2015, 4, 2249–8958. [Google Scholar]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrog. Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Alanne, K.; Saari, A. Sustainable small-scale CHP technologies for buildings: The basis for multi-perspective decision-making. Renew. Sustain. Energy Rev. 2004, 8, 401–431. [Google Scholar] [CrossRef]
- Dodds, P.E.; Staffell, I.; Hawkes, A.D.; Li, F.; Grünewald, P.; McDowall, W.; Ekins, P. Hydrogen and fuel cell technologies for heating: A review. Int. J. Hydrog. Energy 2015, 40, 2065–2083. [Google Scholar] [CrossRef] [Green Version]
- Hamelin, J.; Agbossou, K.; Laperriere, A.; Laurencelle, F.; Bose, T.K. Dynamic behavior of a PEM fuel cell stack for stationary applications. Int. J. Hydrog. Energy 2001, 26, 625–629. [Google Scholar] [CrossRef]
- Sinha, S.; Chandel, S.S. Review of software tools for hybrid renewable energy systems. Renew. Sustain. Energy Rev. 2014, 32, 192–205. [Google Scholar] [CrossRef]
- Singh, G.K. Solar power generation by PV (photovoltaic) technology: A review. Energy 2013, 53, 1–13. [Google Scholar] [CrossRef]
- Gow, J.A.; Manning, C.D. Development of a photovoltaic array model for use in power-electronics simulation studies. IEE Proc. Electr. Power Appl. 1999, 146, 193–200. [Google Scholar] [CrossRef]
- Elibrahimi, M.; Elmouatamid, A.; Bakhouya, M.; Feddi, K.; Ouladsine, R. Performance Evaluation of Fixed and Sun Tracking Photovoltaic Systems. In Proceedings of the 2018 6th International Renewable and Sustainable Energy Conference (IRSEC), Rabat, Morocco, 5–8 December 2018; pp. 1–6. [Google Scholar] [CrossRef]
- Chin, V.J.; Salam, Z.; Ishaque, K. Cell modelling and model parameters estimation techniques for photovoltaic simulator application: A review. Appl. Energy 2015, 154, 500–519. [Google Scholar] [CrossRef]
- Jordehi, A.R. Parameter estimation of solar photovoltaic (PV) cells: A review. Renew. Sustain. Energy Rev. 2016, 61, 354–371. [Google Scholar] [CrossRef]
- Boulmrharj, S.; Rabeh, R.; Felix, V.; Ouladsine, R.; Bakhouya, M.; Zine-Dine, K.; Khaidar, M.; Siniti, M.; Abid, M.R. Modeling and dimensioning of grid-connected photovoltaic systems. In Proceedings of the 2017 International Renewable and Sustainable Energy Conference (IRSEC), Tangier, Morocco, 4–7 December 2017; pp. 1–6. [Google Scholar] [CrossRef]
- Rahim, A.A.; Tijani, A.S.; Kamarudin, S.K.; Hanapi, S. An overview of polymer electrolyte membrane electrolyzer for hydrogen production: Modeling and mass transport. J. Power Sources 2016, 309, 56–65. [Google Scholar] [CrossRef]
- Han, B.; Steen, S.M., III; Mo, J.; Zhang, F.Y. Electrochemical performance modeling of a proton exchange membrane electrolyzer cell for hydrogen energy. Int. J. Hydrog. Energy 2015, 40, 7006–7016. [Google Scholar] [CrossRef] [Green Version]
- Ni, M.; Leung, M.K.; Leung, D.Y. Mathematical modeling of the coupled transport and electrochemical reactions in solid oxide steam electrolyzer for hydrogen production. Electrochim. Acta 2007, 52, 6707–6718. [Google Scholar] [CrossRef]
- Ni, M. Computational fluid dynamics modeling of a solid oxide electrolyzer cell for hydrogen production. Int. J. Hydrog. Energy 2009, 34, 7795–7806. [Google Scholar] [CrossRef]
- Zhou, T.; Francois, B. Modeling and control design of hydrogen production process for an active hydrogen/wind hybrid power system. Int. J. Hydrog. Energy 2009, 34, 21–30. [Google Scholar] [CrossRef]
- Sarrias-Mena, R.; Fernández-Ramírez, L.M.; García-Vázquez, C.A.; Jurado, F. Electrolyzer models for hydrogen production from wind energy systems. Int. J. Hydrog. Energy 2015, 40, 2927–2938. [Google Scholar] [CrossRef]
- Ulleberg, Ø. Modeling of advanced alkaline electrolyzers: A system simulation approach. Int. J. Hydrog. Energy 2003, 28, 21–33. [Google Scholar] [CrossRef]
- Dursun, E.; Acarkan, B.; Kilic, O. Modeling of hydrogen production with a stand-alone renewable hybrid power system. Int. J. Hydrog. Energy 2012, 37, 3098–3107. [Google Scholar] [CrossRef]
- Deshmukh, S.S.; Boehm, R.F. Review of modeling details related to renewably powered hydrogen systems. Renew. Sustain. Energy Rev. 2008, 12, 2301–2330. [Google Scholar] [CrossRef]
- Gandia, L.M.; Arzamedi, G.; Diéguez, P.M. Renewable Hydrogen Technologies: Production, Purification, Storage, Applications and Safety. Newnes; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Trifkovic, M.; Sheikhzadeh, M.; Nigim, K.; Daoutidis, P. Modeling and control of a renewable hybrid energy system with hydrogen storage. IEEE Trans. Control Syst. Technol. 2013, 22, 169–179. [Google Scholar] [CrossRef]
- Lajnef, T.; Abid, S.; Ammous, A. Modeling, control, and simulation of a solar hydrogen/fuel cell hybrid energy system for grid-connected applications. Adv. Power Electron. 2013, 2013, 352765. [Google Scholar] [CrossRef] [Green Version]
- Cheddie, D.; Munroe, N. Review and comparison of approaches to proton exchange membrane fuel cell modeling. J. Power Sources 2005, 147, 72–84. [Google Scholar] [CrossRef]
- Amphlett, J.C.; Baumert, R.M.; Mann, R.F.; Peppley, B.A.; Roberge, P.R.; Harris, T.J. Performance modeling of the Ballard Mark IV solid polymer electrolyte fuel cell I. Mechanistic model development. J. Electrochem. Soc. 1995, 142, 1–8. [Google Scholar] [CrossRef]
- Amphlett, J.C.; Baumert, R.M.; Mann, R.F.; Peppley, B.A.; Roberge, P.R.; Rodrigues, A. Parametric modelling of the performance of a 5-kW proton-exchange membrane fuel cell stack. J. Power Sources 1994, 49, 349–356. [Google Scholar] [CrossRef]
- Amphlett, J.C.; Baumert, R.M.; Mann, R.F.; Peppley, B.A.; Roberge, P.R.; Harris, T.J. Performance modeling of the Ballard Mark IV solid polymer electrolyte fuel cell II. Empirical model development. J. Electrochem. Soc. 1995, 142, 9–15. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.M.; Srinivasan, S.; Chamberlin, C.E. Modeling of proton exchange membrane fuel cell performance with an empirical equation. J. Electrochem. Soc. 1995, 142, 2670–2674. [Google Scholar] [CrossRef]
- Souleman, N.M.; Tremblay, O.; Dessaint, L.A. A generic fuel cell model for the simulation of fuel cell power systems. In Proceedings of the 2009 IEEE Power & Energy Society General Meeting, Calgary, AB, Canada, 26–30 July 2009. [Google Scholar]
- Bennouna, A.; Aarich, N.; Erraissi, N.; Akhsassi, M.; Asselman, A.; Barhdadi, A.; Boukhattem, L.; Cherkaoui, A.; Darmane, Y.; Doudou, A.; et al. Energy performance of 3 silicon-based PV module technologies in 20 sites of Morocco. Energy Sustain. Dev. 2019, 53, 30–56. [Google Scholar] [CrossRef]
- Lotfi, H.; Bennouna, A.; Izbaim, D.; Neffa, M. Performance Analysis of Amorphous Photovoltaic Module Technology in Laâyoune –Morocco. J. Electr. Electron. Eng. 2018, 13, 35–42. [Google Scholar] [CrossRef]
- Aarich, N.; Erraissi, N.; Akhsassi, M.; Bennouna, A.; Asselman, A.; Barhdadi, A.; Boukhattem, L.; Cherkaoui, A.; Darmane, Y.; Doudou, A.; et al. Photovoltaic DC yield maps for all Morocco validated with ground measurements. Energy Sustain. Dev. 2018, 47, 158–169. [Google Scholar] [CrossRef]
- Bakhouya, M.; NaitMalek, Y.; Elmouatamid, A.; Lachhab, F.; Berouine, A.; Boulmrharj, S.; Ouladsine, R.; Felix, V.; Zine-dine, K.; Khaidar, M.; et al. Towards a Data-Driven Platform using IoT and Big Data Technologies for Energy Efficient Buildings. In Proceedings of the 2017 3rd International Conference of Cloud Computing Technologies and Applications (CloudTech), Rabat, Morocco, 24–26 October 2017. [Google Scholar] [CrossRef]
- Boulmrharj, S.; NaitMalek, Y.; Elmouatamid, A.; Bakhouya, M.; Ouladsine, R.; Zine-dine, K.; Khaidar, M.; Abid, R. Approach for Dimensioning Stand-alone Photovoltaic Systems. Energy Procedia 2018, 153, 56–61. [Google Scholar] [CrossRef]
- Boulmrharj, S.; El Ibrahimi, M.; Louardi, A.; Aarich, N. Modeling and Performance Analysis of a Grid-connected Polycrystalline Silicon Photovoltaic System under the Maritime Climate of El Jadida in Morocco. Accept. Int. J. Renew. Energy Res. 2020, in press. [Google Scholar]
- Erraissi, N.; Raoufi, M.; Aarich, N.; Akhsassi, M.; Bennouna, A. Implementation of a low-cost data acquisition system for “PROPRE.MA” project. Measurement 2008, 117, 21–40. [Google Scholar] [CrossRef]
- Larminie, J.; Dicks, A. Fuel Cell Systems Explained, 2nd ed.; John Wiley & Sons, Inc.: Chichester, UK, 2003. [Google Scholar]
- Ham, S.W.; Jo, S.Y.; Dong, H.W.; Jeong, J.W. A simplified PEM fuel cell model for building cogeneration applications. Energy Build. 2015, 107, 213–225. [Google Scholar] [CrossRef]
- Durbin, D.J.; Malardier-Jugroot, C. Review of hydrogen storage techniques for on board vehicle applications. Int. J. Hydrog. Energy 2013, 38, 14595–14617. [Google Scholar] [CrossRef]
- Hong, Y.Y.; Chang, W.C.; Chang, Y.R.; Lee, Y.D.; Ouyang, D.C. Optimal sizing of renewable energy generations in a community microgrid using Markov model. Energy 2017, 135, 68–74. [Google Scholar] [CrossRef]
- Jacobson, M.Z. Reducing T&D Losses Allows Faster Retirement of Fossil Plants. In 100% Clean, Renewable Energy and Storage for Everything; Cambridge University Press: Cambridge, UK, 2019; Available online: https://web.stanford.edu/group/efmh/jacobson/Articles/I/TransmisDistrib.pdf (accessed on 21 May 2020).
- Gahleitner, G. Hydrogen from renewable electricity: An international review of power-to-gas pilot plants for stationary applications. Int. J. Hydrog. Energy 2013, 38, 2039–2061. [Google Scholar] [CrossRef]
- Schiro, F.; Stoppato, A.; Benato, A. Potentialities of hydrogen enriched natural gas for residential heating decarbonization and impact analysis on premixed boilers. E3S Web Conf. 2019, 116, 00072. [Google Scholar] [CrossRef]


| PV Module’s Characteristics | Values |
|---|---|
| Solar cell number | 60 cells |
| Maximum Power Point at STC (Pmpp) | 255 W |
| Maximum Power Point Voltage (Vmpp) | 30.5 V |
| Maximum Power Point Current (Impp) | 8.27 A |
| Open Circuit Voltage (Voc) | 37.6 V |
| Short Circuit Current (Isc) | 8.81 A |
| Dimensions | 1676 1001 31 mm3 |
| Weight | 18 kg |
| Electrolyzer Characteristics | Values |
|---|---|
| Max hydrogen flow rate | 100 nl/h |
| Max outlet pressure | 10 bar |
| Purity of hydrogen | 99.999% @ 30 bar |
| Operative power consumption | 800 W |
| Max power consumption | 900 W |
| Operating temperature | 45 °C |
| Water tank volume | 2.5 l |
| Max water consumption | 0.1 l/h |
| Dimensions | 325 560 500 mm3 |
| Weight | 50 kg |
| Fuel Cell’s Characteristics | Values |
|---|---|
| Rated output | 1200 W @ 5…25 °C |
| Output voltage | 20…36 VDC |
| Rated current | 65 ADC max |
| Fuel | Hydrogen gas |
| Hydrogen consumption at 1200 W | 15 nl/min |
| Oxidant | Atmospheric oxygen |
| Oxidant volume flow rate | Max 335 m3/h |
| Dimensions | 220 400 550 mm3 |
| Weight | 22 kg |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boulmrharj, S.; Khaidar, M.; Bakhouya, M.; Ouladsine, R.; Siniti, M.; Zine-dine, K. Performance Assessment of a Hybrid System with Hydrogen Storage and Fuel Cell for Cogeneration in Buildings. Sustainability 2020, 12, 4832. https://doi.org/10.3390/su12124832
Boulmrharj S, Khaidar M, Bakhouya M, Ouladsine R, Siniti M, Zine-dine K. Performance Assessment of a Hybrid System with Hydrogen Storage and Fuel Cell for Cogeneration in Buildings. Sustainability. 2020; 12(12):4832. https://doi.org/10.3390/su12124832
Chicago/Turabian StyleBoulmrharj, Sofia, Mohammed Khaidar, Mohamed Bakhouya, Radouane Ouladsine, Mostapha Siniti, and Khalid Zine-dine. 2020. "Performance Assessment of a Hybrid System with Hydrogen Storage and Fuel Cell for Cogeneration in Buildings" Sustainability 12, no. 12: 4832. https://doi.org/10.3390/su12124832
APA StyleBoulmrharj, S., Khaidar, M., Bakhouya, M., Ouladsine, R., Siniti, M., & Zine-dine, K. (2020). Performance Assessment of a Hybrid System with Hydrogen Storage and Fuel Cell for Cogeneration in Buildings. Sustainability, 12(12), 4832. https://doi.org/10.3390/su12124832





