Soil Physicochemical Properties, Metal Deposition, and Ultrastructural Midgut Changes in Ground Beetles, Calosoma chlorostictum, under Agricultural Pollution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Sampling Procedure
2.2. The Insect Dissection
2.3. Physicochemical Parameters of Soil Samples
2.4. Analysis of Heavy Metal Concentrations in Soil Samples
2.5. Analysis of Heavy Metal Concentrations in the Midgut of C. chlorostictum Samples
2.6. Transmission Electron Microscopy (TEM) Examinations of the Midgut of C. chlorostictum Samples
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Parameters of the Soil Samples
3.2. Heavy Metal Concentrations in the Soil Samples
3.3. Heavy Metal Concentrations in the Midgut of C. chlorostictum
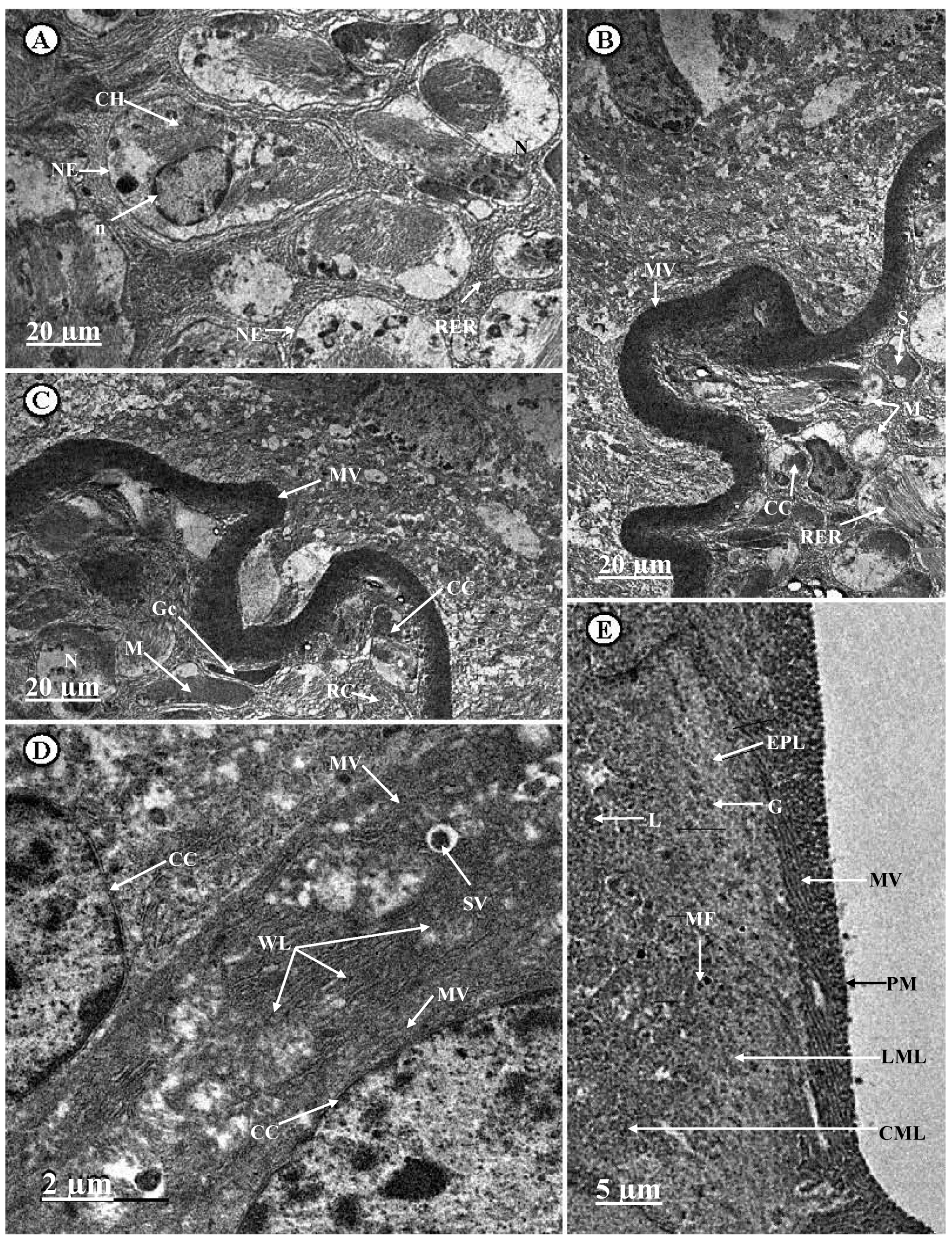
3.4. Ultrastructural Changes in the Midgut of C. chlorostictum Samples
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Collison, E.; Hird, H.; Cresswell, J.; Tyler, C. Interactive effects of pesticide exposure and pathogen infection on bee health—A critical analysis. Biol. Rev. 2016, 91, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Bayo, F.; Goulson, D.; Pennacchio, F.; Nazzi, F.; Goka, K.; Desneux, N. Are bee diseases linked to pesticides?—A brief review. Environ. Int. 2016, 89, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Niklinska, M.; Laskowski, R.; Maryanski, M. Effect of heavy metals and storage time on two types of forest litter: Basal respiration rate and exchangeable metals. Ecotox Environ. Saf. 1998, 41, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Siddig, A.A.; Ellison, A.M.; Ochs, A.N.; Villar, L.C.; Lau, M.K. How do ecologists select and use indicator species to monitor ecological change? Insights from 14 years of publication in Ecological Indicators. Ecol. Indic. 2016, 60, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Hamelink, J.L.; Landrum, P.F.; Bergman, L.H.; Benson, W.H. Bioavailability: Physical, Chemical and Biological Interactions; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Hodkinson, I.D.; Jackson, J.K. Terrestrial and aquatic invertebrates as bioindicators for environmental monitoring, with particular reference to mountain ecosystems. Environ. Manag. 2005, 35, 649–666. [Google Scholar] [CrossRef] [PubMed]
- Skaldina, O.; Sorvari, J. Ecotoxicological effects of heavy metal pollution on economically important terrestrial insects. In Networking of Mutagenes in Terrestrial Ecotoxicology; Kesari, K., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 137–144. [Google Scholar]
- Kremen, C.N.; Colwell, R.K.; Erwin, T.L.; Murphy, D.D.; Noss, R.F.; Sanjayan, M.A. Terrestrial arthropod assemblages: Their use in conservation planning. Conserv. Biol. 1993, 7, 796–808. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.B.; Zheng, Y.M.; Lei, M.; Huang, Z.C.; Wu, H.T.; Chen, H. Assessment of heavy metal pollution in surface soils of urban parks in Beijing, China. Chemosphere 2005, 60, 542–551. [Google Scholar] [CrossRef]
- Stork, N.E.; McBroom, A.J.; Claire, G.I.; Andrew, J.H. New approaches narrow global species estimates for beetles, insects, and terrestrial arthropods. Proc. Natl. Acad. Sci. USA 2015, 112, 7519–7523. [Google Scholar] [CrossRef] [Green Version]
- Zodl, B.; Wittmann, J. Effects of sampling, preparation and defecation on metal concentrations in selected invertebrates at urban sites. Chemosphere 2003, 52, 1095–1103. [Google Scholar] [CrossRef]
- Desender, K.; Maelfait, J.; Baert, L. Carabid beetles as ecological indicators in dune management. Coleotera Carabidae Elytron Suppl. 1991, 5, 239–247. [Google Scholar]
- Lorenz, W. Systematic List of Extant ground Beetles of the World (Insecta Coleoptera “Geadephaga”: Trachypachidae and Carabidae Incl. Paussinae, Cicindelinae, Rhysodinae), 2nd ed.; Wolfgang Lorenz: Tutzing, Germany, 2005; p. 530. [Google Scholar]
- Hopkin, S.P. Ecophysiology of Metals in Invertebrates; Elsevier, Applied Science: New York, NY, USA, 1989. [Google Scholar]
- Agnieszka, J.; Bednarska, A.; Laskowski, R.; Elżbieta, P.; Danuta, S.; Zuzanna, S.; Olga, W. Metal toxicokinetics and metal driven damage to the gut of the ground beetle. Pterostichus Oblongopunctatus Environ. Sci. Pollut. Res. 2016, 23, 22047–22058. [Google Scholar]
- Rawi, S.M.; Bakry, F.A.; Al Hazmi, M.A. Biochemical and histopathological effect of crude extracts on Spodoptera littoralis larvae. J. Evol. Biol. Res. 2011, 3, 67–78. [Google Scholar]
- Rainbow, R.B. Trace metal bioaccumulation: Models, metabolic availability and toxicity. Environ. Entomol. 2007, 33, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Rowell, D.L. Soil sciences: methods and applications; John Wiley and Sons Inc.: New York, NY, USA, 1994. [Google Scholar]
- Gupta, P.K. Methods in environmental analysis water, soil and air. Agrobios 2000, 5, 1–400. [Google Scholar]
- Allen, S.; Grimshaw, L.; Parkinson, A.; Quarmby, C. Chemical Analysis of Ecological Materials; Blackwell Scientific Publications: Oxford, UK, 1974. [Google Scholar]
- Akubugwo, I.E.; Ofoegbu, C.J.; Ukwuoma, C.U. Physicochemical studies on Uburu Salt Lake Ebonyi State-Nigeria. Pak. J. Biol. Sci. 2007, 10, 3170–3174. [Google Scholar]
- Abd El-Shafee, M.E. Assessment of Some Trace Elements in Bladder Cancer Patients Associated with Bilharzia. M. Sc. Thesis, Faculty of Science, Zagazig University, Zagazig, Egypt, 2003. [Google Scholar]
- Karnovsky, M.J. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 1965, 27, 137–138. [Google Scholar]
- Reynolds, E.S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [Green Version]
- Sokal, R.; Rohlf, F. Biometry: The Principles and Practice of Statistics in Biological Research, 2nd ed.; WH Freeman and Company: San Francisco, CA, USA, 1981. [Google Scholar]
- Norusis, M.J. SPSS 13.0 Statistical Procedures Companion; Prentice-Hall: Upper Saddle River, NJ, USA, 2005. [Google Scholar]
- Adviento-Borbe, M.A.; Doran, J.W.; Drijber, R.A.; Dobermann, A. Soil electrical conductivity and water content affect nitrous oxide and carbon dioxide emissions in intensively managed soils. J. Environ. Qual. 2006, 35, 1999–2010. [Google Scholar] [CrossRef] [Green Version]
- Allowary, B.J. Heavy Metals in Soils. Blackie Academic and Professional; Chapman and Hall: London, UK, 1995; p. 368. [Google Scholar]
- Soni, M. Analysis of soil samples for its physicochemical parameters from Abohar city. Pharma Innov. J. 2016, 5, 37–39. [Google Scholar]
- Arias, M.E.; Gonzalez-Perez, J.A.; Gonzalez-Villa, F.J.; Ball, A.S. Soil health: A new challenge for microbiologists and chemists. Int. Microbiol. 2005, 8, 13–21. [Google Scholar] [PubMed]
- Kekane, S.S.; Chavan, R.P.; Shinde, D.N.; Patil, C.L.; Sagar, S.S. A review on physicochemical properties of soil. Intern. J. Chem. Stud. 2015, 3, 29–32. [Google Scholar]
- Osuji, L.C.; Nwoye, I.M. An appraisal of the impact of petroleum hydrocarbons on soil fertility: The Owaza experience. Afr. J. Agric. Res. 2007, 2, 318–324. [Google Scholar]
- Edori, O.S.; Iyama, W.A. Assessment of Physicochemical Parameters of Soils from Selected Abattoirs in Port Harcourt Rivers State Nigeria. J. Environ. Anal. Chem. 2017, 4, 1–5. [Google Scholar]
- Schulte, E.E. Soil and Applied Chlorine; College of Agriculture and Life Sciences, University of Wisconsin-Madison; University of Wisconsin Extension, Cooperative Extension: Madison, WI, USA, 1999. [Google Scholar]
- Chukwu, U.J.; Anuchi, S.O. Impact of Abattoir Wastes on the Physicochemical Properties of Soils within Port Harcourt Metropolis. Int. J. Eng. Sci. 2016, 5, 17–21. [Google Scholar]
- Ayulungit, N.; Balwant, S.; Edith, L. Evaluation of Cadmium Toxicity to Collembola Proisotoma minuta Using Electron Microscopy. In Proceedings of the Australian New Zealand Soils Conference, Sydney, Australia, 5–9 December 2004. [Google Scholar]
- Abu-hashim, M.; Elsayed, M.; Belal, A.E. Effect of land-use changes and site variables on surface soil organic carbon pool at Mediterranean Region. J. Afr. Earth Sci. 2016, 114, 78–84. [Google Scholar] [CrossRef]
- Steevens, J.A.; Benson, W.A. Toxicokinetic interactions and survival of Hyalella azteca exposed to binary mixtures of chlorpyrifos, dieldrin and methyl mercury. Aquat. Toxicol. 2001, 51, 377–388. [Google Scholar] [CrossRef]
- Adriano, D.C. Trace elements in the Terrestrial Environments, 2nd ed.; Springer: New York, NY, USA, 2001. [Google Scholar]
- Food Agriculture Organization (FAO); International Soil reference Information Centre (ISRIC). Guiding Principles for the Quantitative Assessment of Soil Degradation with a Focus on Salinization, Nutrient Decline and Soil Pollution; FAO: Rome, Italy; ISRIC: Wageningen, The Netherlands, 2004. [Google Scholar]
- Wenzel, W.W. Arsenic. In Heavy Metals in Soils. Trace Metals and Metalloids in Soils and Their Bioavailability; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 241–282. [Google Scholar]
- Kabata, P.A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- United Nations Environment Programme. Final Review of Scientific Information on Cadmium—Version of December 2010; United Nations Geneva: Genève, Switzerland, 2010. [Google Scholar]
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Allowary, B.J. Heavy Metals in Soils, 3rd ed.; Environmental Pollution Series 22; Springer: Dordrecht, The Netherlands, 2013; p. 615. [Google Scholar]
- Moriarty, M.M.; Koch, I.N.; Gordon, R.A.; Reimer, K.J. Arsenic speciation of terrestrial invertebrates. Environ. Sci. Technol. 2009, 43, 4818–4823. [Google Scholar] [CrossRef]
- Diener, S.; Zurbrugg, C.; Tockner, K. Bioaccumulation of heavy metals in the black soldier fly, Hermetia illucens and effects on its life cycle. J. Ins. Food Feed 2015, 1, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Ge, S.; Haihua, W.; Ying, X.; Meiling, Y.; Enbo, M.; Yaping, G. Accumulation and distribution of cadmium in Oxya chinensis after feeding on wheat seedlings contaminated with cadmium. J. Agro. Environ. Sci. 2009, 28, 1812–1817. [Google Scholar]
- Bohac, J. Accumulation of heavy metals in the bodies of staphylinid beetles Coleoptera, Staphylinidae. In Proceedings of the 5th Internat Confer On Bioindicators in Deteriorisating Regions, Ceske Budejovice, Czech Republic, 23–27 May 1988; pp. 319–321. [Google Scholar]
- Menta, C.; Parisi, V. Metal concentrations in Helix pomatia, Helix aspersa and Arion rufus: A comparative study. Environ. Poll 2001, 115, 205–208. [Google Scholar] [CrossRef]
- He, Z.L.; Yang, X.E.; Stoffell, P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Heikens, A.; Peijnenburg, W.; Hendriks, J. Bioaccumulation of heavy metals in terrestrial invertebrates. Environ. Pollu. 2001, 113, 385–393. [Google Scholar] [CrossRef]
- Van Straalen, N.M.; Butovsky, R.O.; Pokarzhevskii, A.D.; Zaitsev, A.S.; Verhoef, C.S. Metal concentrations in soil and in invertebrates in the vicinity of a metallurgical factory near Tula (Russia). Pedobiol. Inter. J. Soil Biol. 2001, 45, 451–466. [Google Scholar] [CrossRef]
- Jelaska, S.; Blanusa, M.; Durbesic, P.; Jelaska, D. Heavy metal concentrations in ground beetles, leaf litter, and soil of a forest ecosystem. Ecotoxicol. Environ. Saf. 2007, 66, 74–81. [Google Scholar] [CrossRef]
- Chapman, R.F. The Insects: Structure and Function, 4th ed.; Cambridge, UK University Press: Cambridge, UK, 1998; pp. 38–66. [Google Scholar]
- Micchelli, C.A.; Perrimon, N.A. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 2006, 439, 475–479. [Google Scholar] [CrossRef]
- Billingsley, P.F.; Lehane, M.J. Structure and Ultrastructure of the Insect Midgut. Biology of the Insect Midgut; Springer: Dordrecht, The Netherlands, 1996; Volume 9, pp. 3–30. [Google Scholar]
- Geneser, F. Histologia: Sobre Bases Biomoleculare, 3rd ed.; Medica Panamericana: Mexico City, Mexico, 2000; p. 813. [Google Scholar]
- Rost Roszkowska, M.M.; Jansta, P.; Vilimova, J. Fine structure of the midgut epithelium in two Archaeognatha, Lepismachilis notata and Machilis hrabei (Insecta), in relation to its degeneration and regeneration. Protoplasma 2010, 247, 91–101. [Google Scholar] [CrossRef]
- Olivares, A.S.; Diaz, E.; Shibayama, M.; Tsutsumi, V.; Cisneros, R.; Zuniga, G. Ultrastructural study of the midgut and hindgut in eight species of the genus. Dendroctonus Erichson Coleopt. Scolytidae Ann. Entomol. Soc. Am. 2014, 96, 883–900. [Google Scholar] [CrossRef] [Green Version]
- Yingmei, Z.; Simonetta, L.; Mauro, F.; Carlo, G.; Aldo, G.; Ugo, L. Mortality and tissue damage by heavy metal contamination in the German cockroach. Bl. Ger. Bl. Bl. Ital J. Zool 2001, 68, 137–145. [Google Scholar]
- Polidori, C.; Pastorm, A.; Jorge, A.; Pertusa, J. Ultrastructural alterations of midgut epithelium, but not greater wing fluctuating asymmetry, in paper wasps Polistes dominula from urban environments. Microsc. Microanal 2018, 24, 183–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, A.; Cunha, L.; Amaral, A.; Medeiros, J.; Garcia, P. Bioavailability of heavy metals and their effects on the midgut cells of a phytophagous insect inhabiting volcanic environments. Sci. Total Environ. 2008, 406, 116–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabata, P.A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin, Germany, 2007. [Google Scholar]
- Bednarska, A.J.; Opyd, M.; Żurawicz, E.; Laskowski, R. Regulation of body metal concentrations: Toxicokinetics of cadmium and zinc in crickets. Ecotoxicol. Environ. Saf. 2015, 119, 9–14. [Google Scholar] [CrossRef]
- Khan, I.; Qamar, A.; Mehdi, S.; Shahid, M. Histopathological effects of Datura alba leaf extract on the midgut of Periplaneta Americana. Biol. Med. 2011, 3, 260–264. [Google Scholar]
- Kohler, H.R. Localization of metals in cells of saprophagous soil arthropods Isopoda, Diplopoda, Collembola. Microsc. Res. Tech. 2002, 56, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, Z.; Lockshin, A. Cell death during development. J. Immunol. Methods 2002, 265, 3–20. [Google Scholar] [CrossRef]
- Schonthal, A.H. Endoplasmic reticulum stress: Its role in disease and novel prospects for therapy. Scientifica 2012. [Google Scholar] [CrossRef] [Green Version]
- Meyer, J.N.; Leung, M.C.; Rooney, J.P.; Sendoel, A.L.; Hengartner, M.O.; Kisby, G.E.; Bess, A.S. Mitochondria as a target of environmental toxicants. Toxicol. Sci. 2013, 134, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Vaidyanathan, R.; Scott, T.W. Apoptosis in mosquito midgut epithelia associated with West Nile virus infection. Apoptosis 2006, 11, 1643–1651. [Google Scholar] [CrossRef]
- Lockshin, R.A.; Zakeri, Z. Apoptosis, autophagy and more. Int. J. Biochem. Cell Biol. 2004, 36, 2405–2419. [Google Scholar] [CrossRef] [PubMed]
- Loeb, M.J.; Hakim, R.S.; Martin, P.L.; Narang, N.R.; Goto, S.L.; Takeda, M.W. Apoptosis in cultured midgut cells from Heliothis virescens larvae exposed to various conditions. Arch. Insect. Biochem. Physiol. 2000, 45, 12–23. [Google Scholar] [CrossRef]




| Parameters | Soil Samples | Independent T-test | ||
|---|---|---|---|---|
| Reference Site | Polluted Agri. Site | T-Statistic | p-Value | |
| Conductivity (ds/m) | 6.271 ± 0.832 | 9.019 ± 1.007 | 7.288 * | ≤0.05 |
| pH value | 8.355 ± 0.770 | 7.515 ± 0.683 | 2.827 * | ≤0.05 |
| Moisture (%) | 1.956 ± 0.078 | 2.512 ± 0.096 | 15.571 * | ≤0.01 |
| Chloride content (%) | 0.015 ± 0.081 | 0.018 ± 0.057 | 0.105 | >0.05 |
| Total CaCO3 (%) | 2.891 ± 0.379 | 3.254 ± 0.402 | 2.276 * | ≤0.05 |
| Organic matter (%) | 1.951 ± 0.208 | 1.034 ± 0.208 | 10.799 * | ≤0.01 |
| Alkalinity (%) | 6.024 ± 0.735 | 4.825 ± 0.358 | 5.080 * | ≤0.05 |
| Heavy Metals | Soil Samples (µg/g−1) | Independent T-Test | ||
|---|---|---|---|---|
| Reference Site | Polluted Agri. Site | T-Statistic | p-Value | |
| Arsenic (As) | 9.075 ± 0.808 | 13.235 ± 1.014 | 11.115 * | ≤0.01 |
| Mercury (Hg) | 0.808 ± 0.044 | 1.178 ± 0.113 | 10.570 * | ≤0.05 |
| Aluminium (Al) | 2.780 ± 0.097 | 6.918 ± 0.475 | 29.568 * | ≤0.001 |
| Cadmium (Cd) | 0.322 ± 0.046 | 0.905 ± 0.089 | 20.158 * | ≤0.01 |
| Lead (Pb) | 0.918 ± 0.071 | 1.218 ± 0.095 | 8.762 * | ≤0.05 |
| Selenium (Se) | 0.386 ± 0.043 | 0.512 ± 0.067 | 5.483 * | ≤0.05 |
| Heavy Metals | Beetle Samples (µg/g−1) | Independent T-Test | ||
|---|---|---|---|---|
| Reference Site | Polluted Agri. Site | T-Statistic | p-Value | |
| Arsenic (As) | 6.903 ± 0.628 | 11.858 ± 0.912 | 21.460 * | ≤0.01 |
| Mercury (Hg) | 0.209 ± 0.036 | 0.630 ± 0.077 | 23.753 * | ≤0.01 |
| Aluminium (Al) | 0.993 ± 0.077 | 2.768 ± 0.199 | 39.895 * | ≤0.001 |
| Cadmium (Cd) | 0.278 ± 0.031 | 0.765 ± 0.069 | 30.876 * | ≤0.001 |
| Lead (Pb) | 0.525 ± 0.045 | 0.849 ± 0.057 | 21.396 * | ≤0.01 |
| Selenium (Se) | 0.201 ± 0.019 | 0.293 ± 0.036 | 10.839 * | ≤0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasr, E.E.; Khater, Z.Z.; Zelenakova, M.; Vranayova, Z.; Abu-Hashim, M. Soil Physicochemical Properties, Metal Deposition, and Ultrastructural Midgut Changes in Ground Beetles, Calosoma chlorostictum, under Agricultural Pollution. Sustainability 2020, 12, 4805. https://doi.org/10.3390/su12124805
Nasr EE, Khater ZZ, Zelenakova M, Vranayova Z, Abu-Hashim M. Soil Physicochemical Properties, Metal Deposition, and Ultrastructural Midgut Changes in Ground Beetles, Calosoma chlorostictum, under Agricultural Pollution. Sustainability. 2020; 12(12):4805. https://doi.org/10.3390/su12124805
Chicago/Turabian StyleNasr, Enas E., Zeinab Z. Khater, Martina Zelenakova, Zuzana Vranayova, and Mohamed Abu-Hashim. 2020. "Soil Physicochemical Properties, Metal Deposition, and Ultrastructural Midgut Changes in Ground Beetles, Calosoma chlorostictum, under Agricultural Pollution" Sustainability 12, no. 12: 4805. https://doi.org/10.3390/su12124805







