Soil Yeasts in the Vicinity of Syowa Station, East Antarctica: Their Diversity and Extracellular Enzymes, Cold Adaptation Strategies, and Secondary Metabolites
Abstract
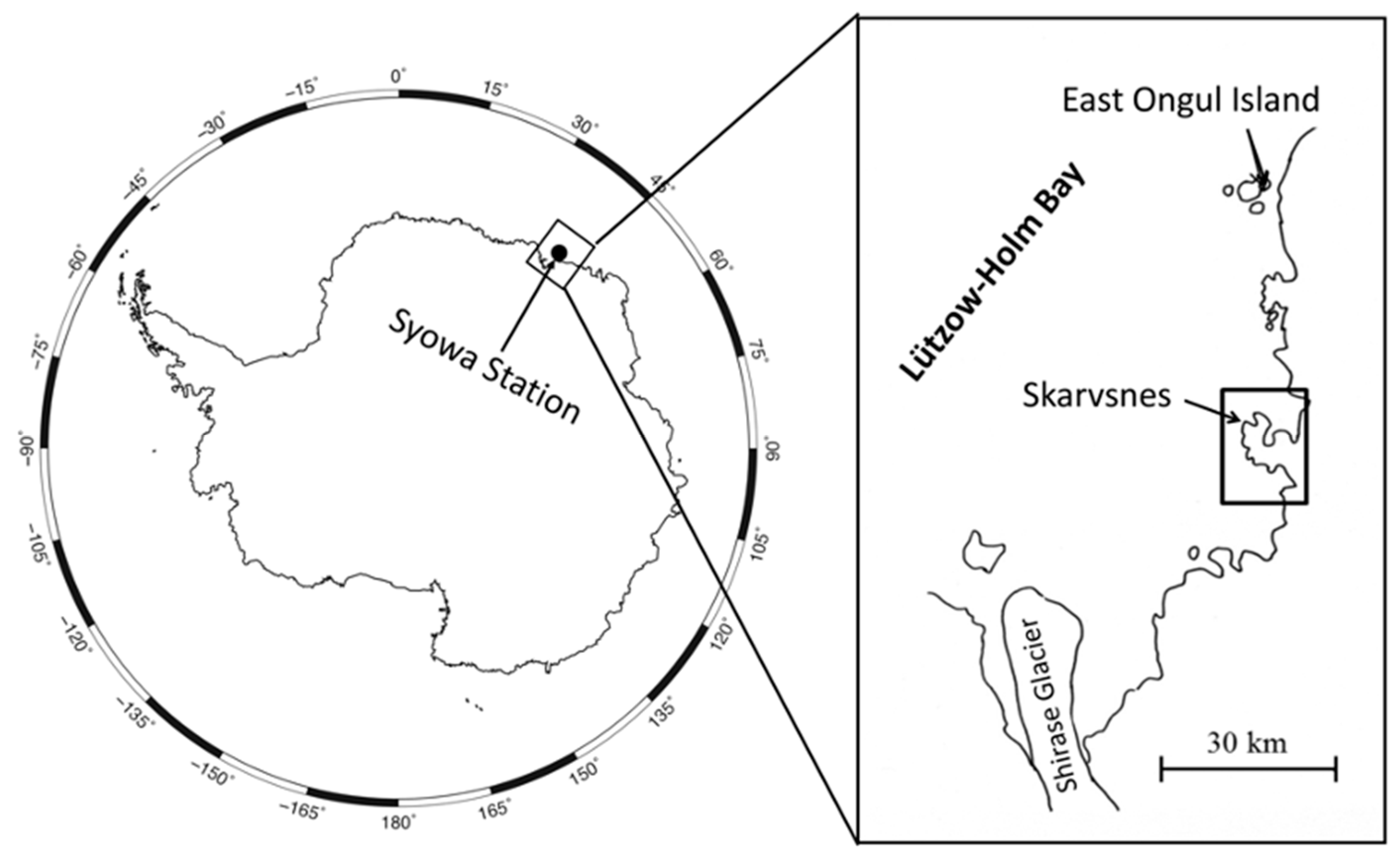
1. Introduction
2. Soil Yeast Diversity and Their Ecological Role in East Ongul Island
3. Soil Yeast Diversity and Their Cold Adaptation Strategies in the Skarvsnes Ice-Free Area
4. Secondary Metabolites Induced by Cold Stress
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Feller, G.; Gerday, C. Psychrophilic enzymes: Hot topics in cold adaptation. Nat. Rev. Microbiol. 2003, 1, 200–208. [Google Scholar] [CrossRef]
- Gerday, C.; Aittaleb, M.; Bentahir, M.; Chessa, J.P.; Claverie, P.; Collins, T.; D’Amico, S.; Dumont, J.; Garsoux, G.; Georlette, D.; et al. Cold-adapted enzymes: From fundamentals to biotechnology. Trends Biotechnol. 2000, 18, 103–107. [Google Scholar] [CrossRef]
- Welander, U. Microbial degradation of organic pollutants in soil in a cold climate. Soil Sediment. Contam. 2005, 14, 281–291. [Google Scholar] [CrossRef]
- Margesin, R.; Neuner, G.; Storey, K.B. Cold-loving microbes, plants, and animals—fundamental and applied aspects. Naturwissenschaften 2007, 94, 77–99. [Google Scholar] [CrossRef]
- Ravindra, R.; Chaturvedi, A. Antarctica. In Encyclopedia of Snow, Ice and Glaciers; Singh, V.P., Singh, P., Haritashya, U.K., Eds.; Springer: Berlin, Germany, 2011; pp. 45–53. [Google Scholar]
- Onofri, S.; Zucconi, L.; Tosi, S. Continental Antarctic Fungi; IHW Verlag: München, Germany, 2007. [Google Scholar]
- Bridge, P.D.; Spooner, B.M. Non-lichenized Antarctic fungi: Transient visitors or members of a cryptic ecosystem? Fungal Ecol. 2012, 5, 381–394. [Google Scholar] [CrossRef]
- Tsuji, M. A catalog of fungi recorded from the vicinity of Syowa Station. Mycoscience 2018, 59, 319–324. [Google Scholar] [CrossRef]
- Tsuji, M. An index of non-lichenized fungi recorded in the vicinity of Syowa Station, East Antarctica. In Fungi in Polar Regions; Tsuji, M., Hoshino, T., Eds.; CRC Press: Oxford, UK, 2019; pp. 1–16. [Google Scholar]
- Tsuji, M. Genetic diversity of yeasts from East Ongul Island, East Antarctica and their extracellular enzymes secretion. Polar Biol. 2018, 41, 249–258. [Google Scholar] [CrossRef]
- Fell, J.W.; Mrakia, Y. Yamada & Komagata (1987). In The Yeast, a Taxonomic Study, 5th ed.; Kurtzman, C.P., Fell, J.W., Boeckhout, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1503–1510. [Google Scholar]
- Fonseca, Á.; Boekhout, T.; Fell, J.W. Cryptococcus Vuillemin (1901). In The Yeast, a Taxonomic Study, 5th ed.; Kurtzman, C.P., Fell, J.W., Boeckhout, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1661–1737. [Google Scholar]
- Sampaio, J.P. Rhodotorula Harrison (1928). In The Yeast, a Taxonomic Study, 5th ed.; Kurtzman, C.P., Fell, J.W., Boeckhout, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1873–1927. [Google Scholar]
- Takashima, M.; Nakase, T. Udeniomyces Nakase & Takematsu (1992). In The Yeast, a Taxonomic Study, 5th ed.; Kurtzman, C.P., Fell, J.W., Boeckhout, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 2063–2068. [Google Scholar]
- Tsuji, M.; Tsujimoto, M.; Imura, S. Cystobasidium tubakii and Cystobasidium ongulense, new basidiomycetous yeast species isolated from East Ongul Island, East Antarctica. Mycoscience 2017, 58, 103–107. [Google Scholar] [CrossRef]
- Robinson, C.H. Cold adaptation in Arctic and Antarctic fungi. New Phytol. 2001, 151, 341–353. [Google Scholar] [CrossRef]
- Franks, F. Protein destabilization at low temperatures. Adv. Protein Chem. 1995, 46, 105–139. [Google Scholar] [PubMed]
- Feller, G.; Gerday, C. Psychrophilic enzymes: Molecular basis of cold adaptation. Cell. Mol. Life Sci. 1997, 53, 830–841. [Google Scholar] [CrossRef]
- Gerday, C.; Aittaleb, M.; Arpigny, J.L.; Baise, E.; Chessa, J.-P.; Garsoux, G.; Petrescu, I.; Feller, G. Psychrophilic enzymes: A thermodynamic challenge. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1997, 1342, 119–131. [Google Scholar] [CrossRef]
- Carrasco, M.; Rozas, J.M.; Barahona, S.; Alcaíno, J.; Cifuentes, V.; Baeza, M. Diversity and extracellular enzymatic activities of yeasts isolated from King George Island, the sub-Antarctic region. BMC Microbiol. 2012, 12, 251. [Google Scholar] [CrossRef] [PubMed]
- Vaz, A.B.M.; Rosa, L.H.; Vieira, M.L.A.; de Garcia, V.; Brandaão, L.R.; Teixeira, L.C.R.S.; Molineé, M.; Libkind, D.; van Broock, M.; Rosa, C.A. The diversity, extracellular enzymatic activities and photoprotective compounds of yeasts isolated in Antarctica. Braz. J. Microbiol. 2011, 42, 937–947. [Google Scholar] [CrossRef]
- Tsuji, M.; Fujiu, S.; Xiao, N.; Hanada, Y.; Kudoh, S.; Kondo, H.; Tsuda, S.; Hoshino, T. Cold adaptation of fungi obtained from soil and lake sediment in the Skarvsnes ice-free area, Antarctic. FEMS Microbiol. Lett. 2013, 346, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Snider, C.S.; Hsiang, T.; Zhao, G.; Griffith, M. Role of ice nucleation and antifreeze activities in pathogenesis and growth of snow molds. Phytopathology 2000, 90, 354–361. [Google Scholar] [CrossRef]
- Hoshino, T.; Kiriaki, M.; Nakajima, T. Novel thermal hysteresis proteins from low temperature basidiomycete, Coprinus psychromorbidus. Cryo Lett. 2003, 24, 135–142. [Google Scholar]
- Hoshino, T.; Xiao, N.; Tkachenko, O.B. Cold adaptation in the phytopathogenic fungi causing snow molds. Mycoscience 2009, 50, 26–38. [Google Scholar] [CrossRef]
- Xiao, N.; Suzuki, K.; Nishiyama, Y.; Kondo, H.; Miura, A.; Tsuda, S.; Hoshino, T. Comparison of functional properties of two fungal antifreeze proteins from Antarctomyces pschrotrophicus and Typhula ishikariensis. FEBS J. 2010, 277, 394–403. [Google Scholar] [CrossRef]
- Xiao, N.; Inaba, S.; Tojo, M.; Degawa, Y.; Fujiu, S.; Hanada, Y.; Kudoh, S.; Hoshino, T. Antifreeze activities of various fungi and Stramenophilia isolated from Antarctica. N. Am. Fungi 2010, 5, 215–220. [Google Scholar]
- Pathan, A.A.K.; Bhadra, B.; Begum, Z.; Shivaji, S. Diversity of Yeasts from Puddles in the Vinicity of Midre Lovénbreen Glacier, Arctic and Bioprospecting for Enzymes and Fatty acids. Curr. Microbiol. 2010, 60, 307–314. [Google Scholar] [CrossRef]
- Turk, M.; Plemenitaš, A.; Gunde-Cimerman, N. Extremophilic yeasts: Plasma-membrane fluidity as determinant of stress tolerance. Fungal Biol. 2011, 115, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Tsuji, M.; Singh, S.M.; Roy, U.; Hoshino, T. Taxonomic characterization, adaptation strategies and biotechnological potential of cryophilic yeasts from ice cores of Midre Lovénbreen glacier, Svalbard, Arctic. Cryobiology 2013, 66, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M. Cold-stress responses in the Antarctic basidiomycetous yeast Mrakia blollopis. R. Soc. Open Sci. 2016, 3, 160106. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Yokota, Y.; Kudoh, S.; Hoshino, T. 2015 Comparative analysis of milk fat decomposition activity by Mrakia spp. isolated from Skarvsnes ice-free area, East Antarctica. Cryobiology 2015, 70, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Goshima, T.; Matsushika, A.; Kudoh, S.; Hoshino, T. Direct ethanol fermentation from lignocellulosic biomass by Antarctic Basidiomycetous yeast Mrakia blollopis under a low temperature condition. Cryobiology 2013, 67, 241–243. [Google Scholar] [CrossRef]
- Tsuji, M.; Kudoh, S.; Hoshino, T. Ethanol productivity of cryophilic basidiomycetous yeast Mrakia spp. correlates with ethanol tolerance. Mycoscience 2016, 57, 42–50. [Google Scholar] [CrossRef]
- Tsuji, M.; Kudoh, S.; Hoshino, T. Draft genome sequence of cryophilic basidiomycetous yeast Mrakia blollopis SK-4, isolated from an algal mat of Naga-ike Lake in the Skarvsnes ice-free area, East Antarctica. Genome Announc. 2015, 3, e01454-14. [Google Scholar] [CrossRef]
- Tsuji, M. Change in the secondary metabolite of Antarctic fungi by cold stress. In Proceedings of the Annual Meeting of the Society for Biotechnology, 11–14 September; The Society for Biotechnology: Tokyo, Japan, 2017. [Google Scholar]
| Species | Number of Strains | Psychrophile or Psychrotolerant |
|---|---|---|
| Cystobasidium lysinophilum | 2 | Psychrotolerant |
| Cystobasidium ongulense | 10 | Psychrotolerant |
| Cystobasidium tubakii | 2 | Psychrotolerant |
| Glaciozyma antarctica | 1 | Psychrophile |
| Glaciozyma martinii | 10 | Psychrophile |
| Goffeauzyma gilvescens | 2 | Psychrotolerant |
| Holtermanniella wattica | 3 | Psychrotolerant |
| Mrakia gelida | 5 | Psychrophile |
| Naganishia adeliensis | 1 | Psychrotolerant |
| Naganishia albidosimilis | 4 | Psychrotolerant |
| Naganishia friedmannii | 17 | Psychrotolerant |
| Phenoliferia glacialis | 8 | Psychrophile |
| Tausonia pullulans | 1 | Psychrotolerant |
| Udeniomyces puniceus | 1 | Psychrotolerant |
| Vishniacozyma carnescens | 6 | Psychrotolerant |
| Vishniacozyma victoriae | 22 | Psychrotolerant |
| Species | Strain | Accession Number |
|---|---|---|
| Cystobasidium laryngis | ABH-3 | AB774463 |
| Dioszegia fristingensis | ARJ-3 | AB774458 |
| Dioszegia fristingensis | HYT-1 | AB774459 |
| Glaciozyma watsonii | KGK-2 | AB774460 |
| Goffeauzyma gastrica | TKU1-1 | AB773891 |
| Goffeauzyma gastrica | BSS-1 | AB773892 |
| Goffeauzyma gastrica | MOA-2 | AB774233 |
| Mrakia blollopis | MOA-3 | AB775474 |
| Mrakia gelida | AGK-2 | AB774465 |
| Mrakia gelida | ABU1-1 | AB774468 |
| Mrakia gelida | EBH-3 | AB774470 |
| Mrakia gelida | EBH-4 | AB774471 |
| Mrakia gelida | NKU-1 | AB775661 |
| Mrakia gelida | NGU-1 | AB775662 |
| Mrakia gelida | NIN-6 | AB775663 |
| Mrakia gelida | BSU2-3 | AB775471 |
| Mrakia gelida | EBN-1 | AB775203 |
| Mrakia gelida | NRI-1 | AB775469 |
| Mrakia robertii | SMI-2 | AB775472 |
| Mrakia robertii | MOA-4 | AB775660 |
| Mrakia robertii | NRI-1 | AB775468 |
| Mrakia robertii | NRI-3 | AB775470 |
| Naganishia friedmannii | NHU-1 | AB773893 |
| Phenoliferia glacialis | NHT-2 | AB774464 |
| Vishniacozyma victoriae | OGA-2 | AB774232 |
| Vishniacozyma victoriae | ARI-3 | AB773887 |
| Vishniacozyma victoriae | NIK-1 | AB774234 |
| Vishniacozyma victoriae | NIK-2 | AB774235 |
| Vishniacozyma victoriae | NIK-3 | AB774236 |
| Vishniacozyma victoriae | NIN-5 | AB774237 |
| Vishniacozyma victoriae | ARJ-4 | AB773888 |
| Vishniacozyma victoriae | OGN2-4 | AB774230 |
| Vishniacozyma victoriae | ABH-4 | AB773886 |
| Vishniacozyma victoriae | JZN-4 | AB773890 |
| Vishniacozyma victoriae | OGA-1 | AB774231 |
| Species | Chemical Compound | Principal Applications of Chemical Compounds |
|---|---|---|
| Mrakia blollopis | Peltatol A | anti-HIV activity |
| Mrakia blollopis | Pinacidil | reduces blood pressure |
| Mrakia blollopis | Pirbuterol | bronchodilatation |
| Cystobasidium ongulense | Altretamine | anti-neoplastic agent |
| Cystobasidium ongulense | Lucyoside M | anti-inflammatory activity |
| Cystobasidium ongulense | Tegafur | anti-neoplastic agent |
| Tausonia pullulans | Acebutolol | the treatment of hypertension and arrhythmias |
| Tausonia pullulans | Epothilone D | anti-neoplastic agent |
| Tausonia pullulans | Isopentenyl adenosine | promotes cell division |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuji, M.; Kudoh, S. Soil Yeasts in the Vicinity of Syowa Station, East Antarctica: Their Diversity and Extracellular Enzymes, Cold Adaptation Strategies, and Secondary Metabolites. Sustainability 2020, 12, 4518. https://doi.org/10.3390/su12114518
Tsuji M, Kudoh S. Soil Yeasts in the Vicinity of Syowa Station, East Antarctica: Their Diversity and Extracellular Enzymes, Cold Adaptation Strategies, and Secondary Metabolites. Sustainability. 2020; 12(11):4518. https://doi.org/10.3390/su12114518
Chicago/Turabian StyleTsuji, Masaharu, and Sakae Kudoh. 2020. "Soil Yeasts in the Vicinity of Syowa Station, East Antarctica: Their Diversity and Extracellular Enzymes, Cold Adaptation Strategies, and Secondary Metabolites" Sustainability 12, no. 11: 4518. https://doi.org/10.3390/su12114518
APA StyleTsuji, M., & Kudoh, S. (2020). Soil Yeasts in the Vicinity of Syowa Station, East Antarctica: Their Diversity and Extracellular Enzymes, Cold Adaptation Strategies, and Secondary Metabolites. Sustainability, 12(11), 4518. https://doi.org/10.3390/su12114518





