Modified Activated Carbon Fiber Felt for the Electrosorption of Norfloxacin in Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Modification and Characterization of ACFF
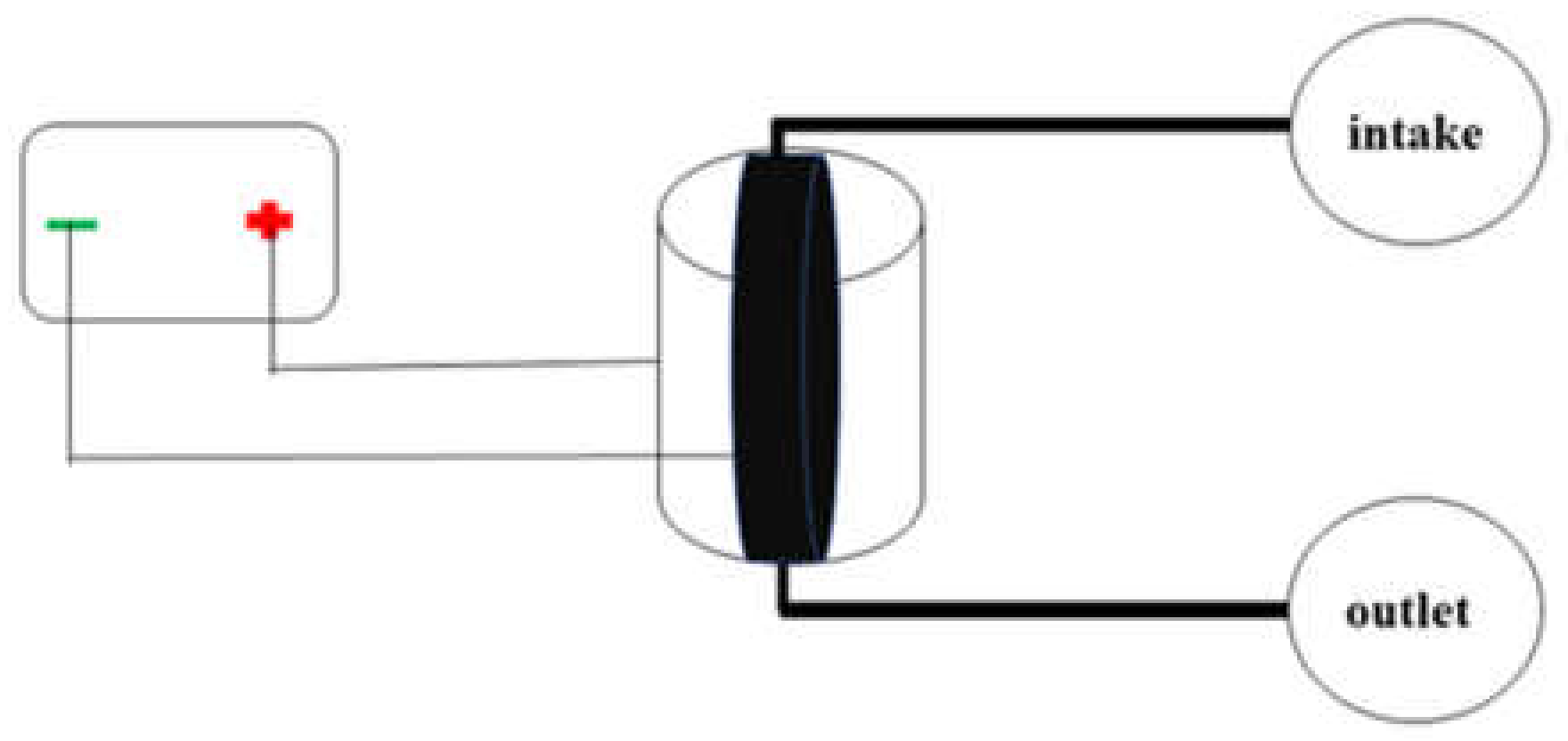
2.3. Preparation of Electrosorption Device
2.4. Electric Adsorption Test
2.5. Determination of NOR in Sewage
2.6. Adsorption Kinetics Test and Adsorption Isotherms
2.7. MACFF Regeneration Test
3. Results and Discussion
3.1. Structural Characterization of Activated Carbon Fiber Felt
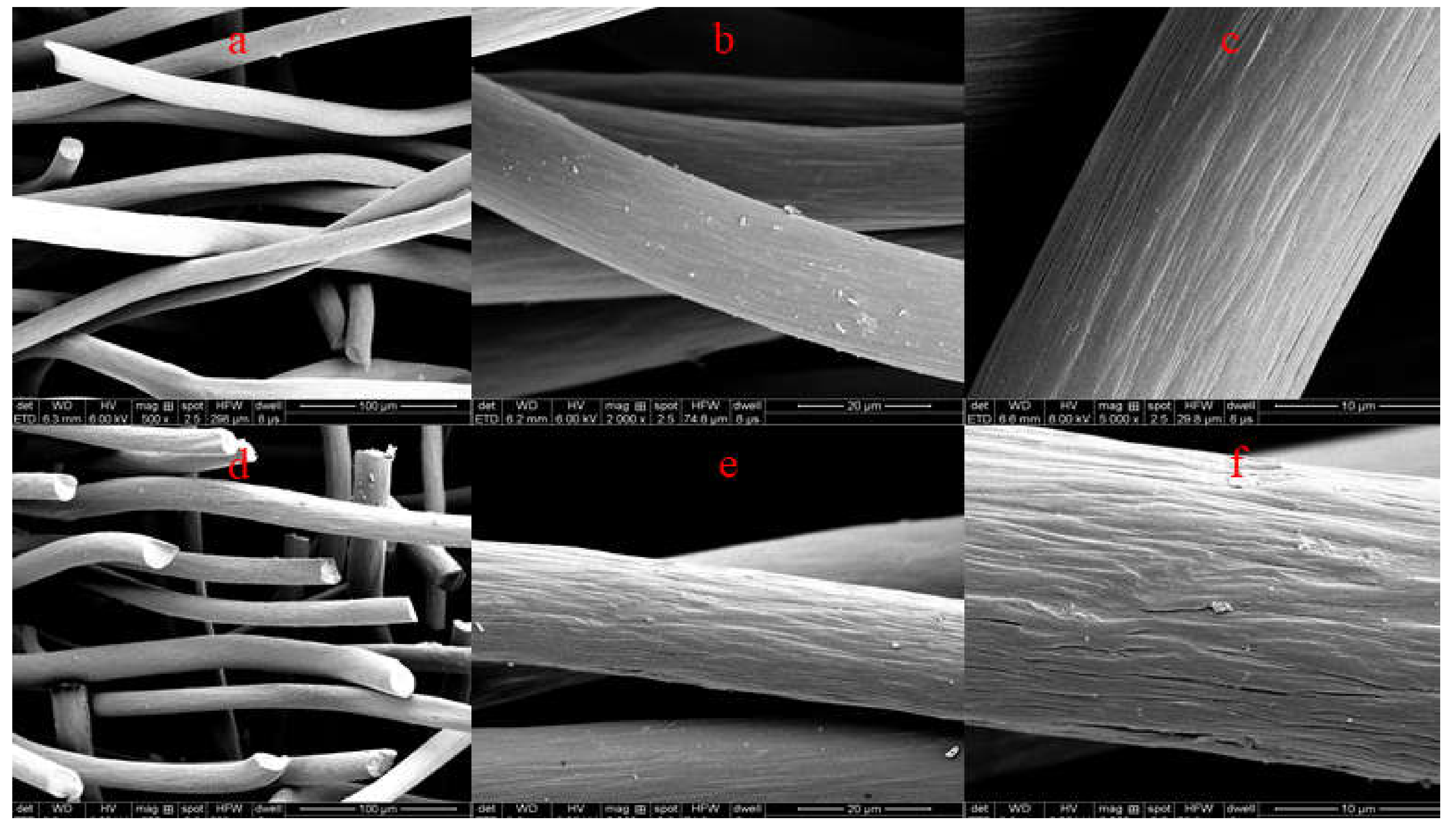
3.1.1. SEM Analysis
3.1.2. Analysis of Specific Surface Area and Pore Structure
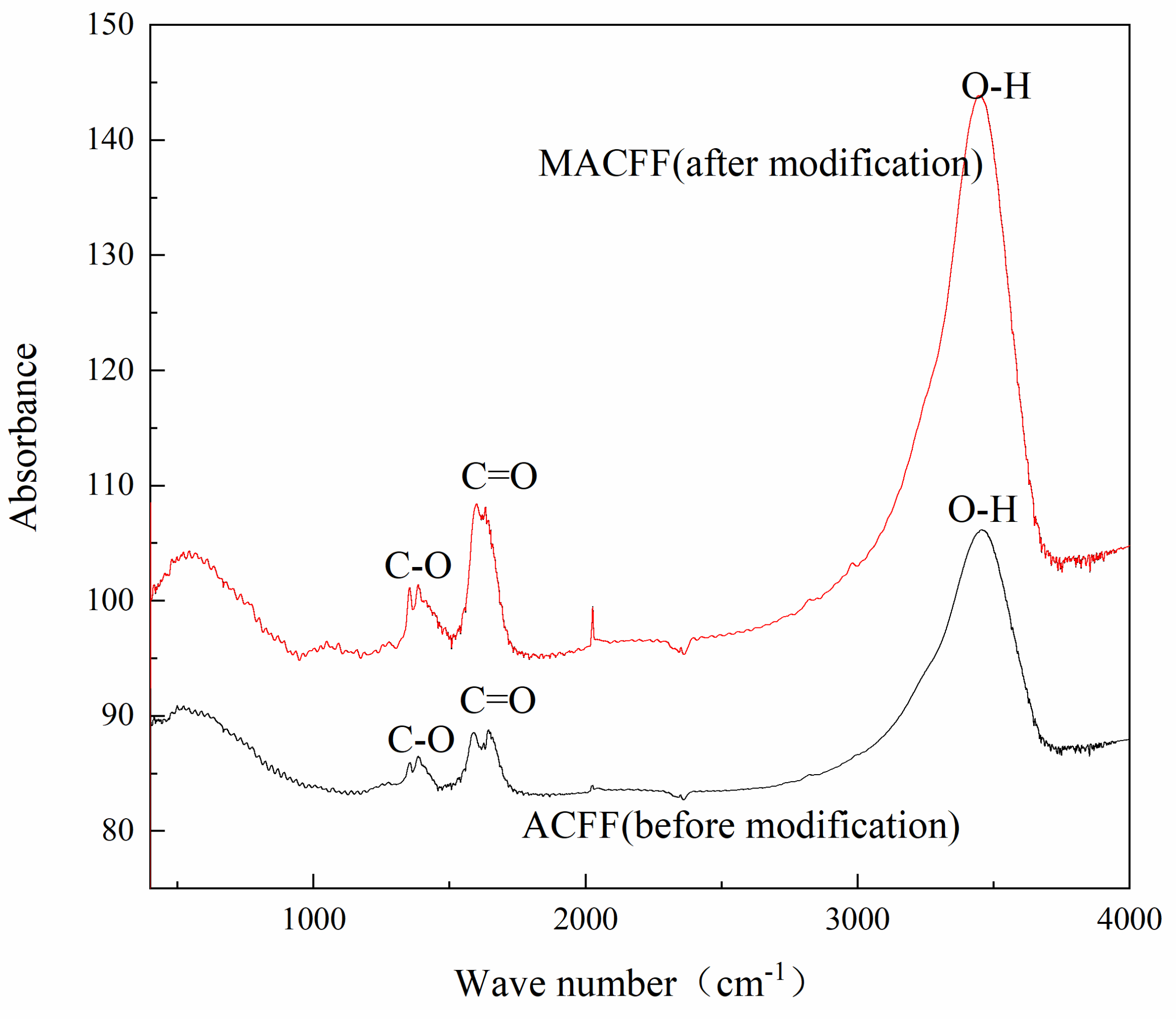
3.1.3. FTIR Analysis
3.2. Factors Affecting the Adsorption of NOR in Aqueous Solution by MACFF
3.2.1. Effect of pH
3.2.2. Effect of Voltage
3.2.3. Effect of Initial Concentration
3.2.4. Effect of Plate Spacing
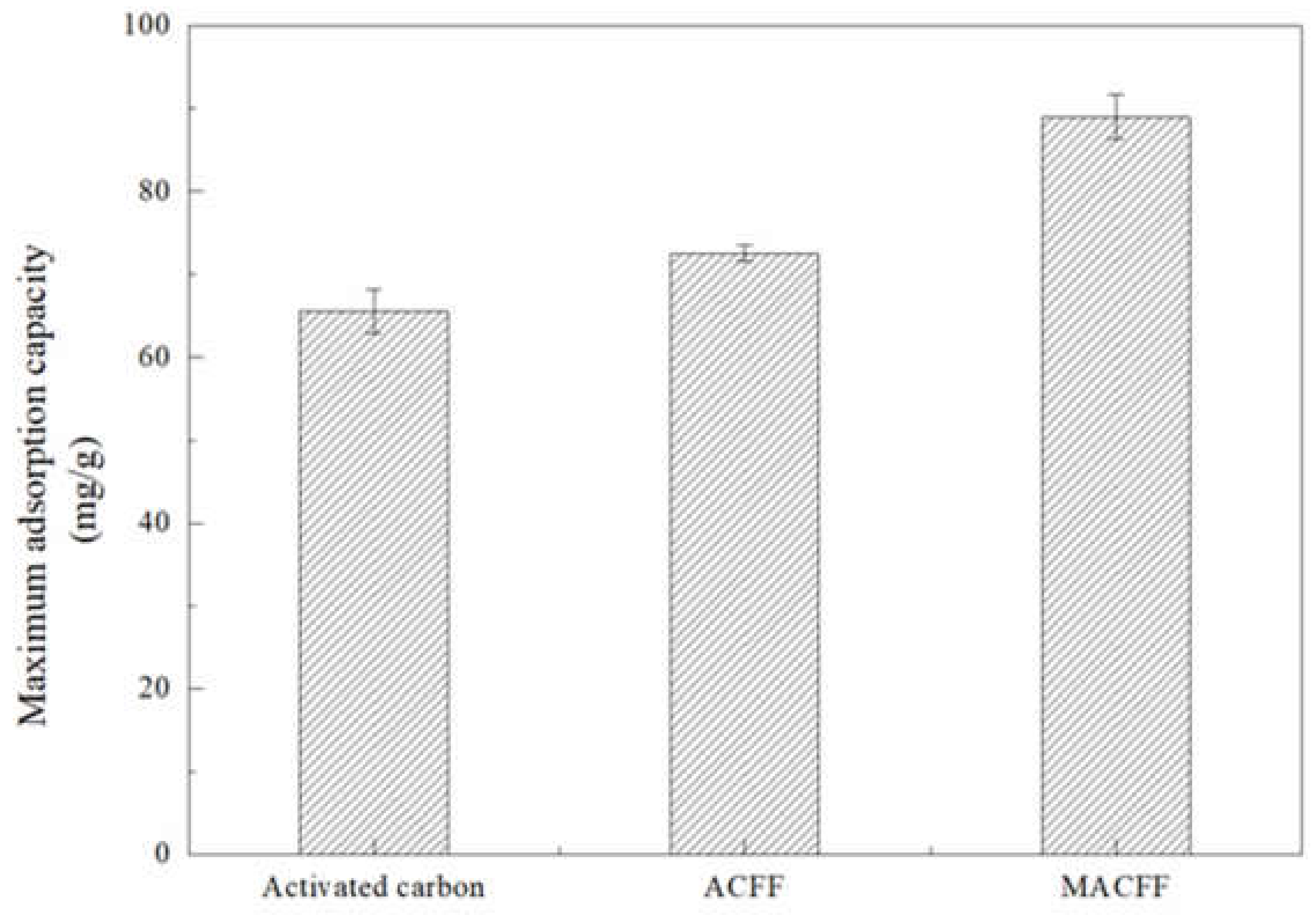
3.2.5. Comparative Study of Different Adsorption Electrodes
3.3. Adsorption Model and Mechanism Study
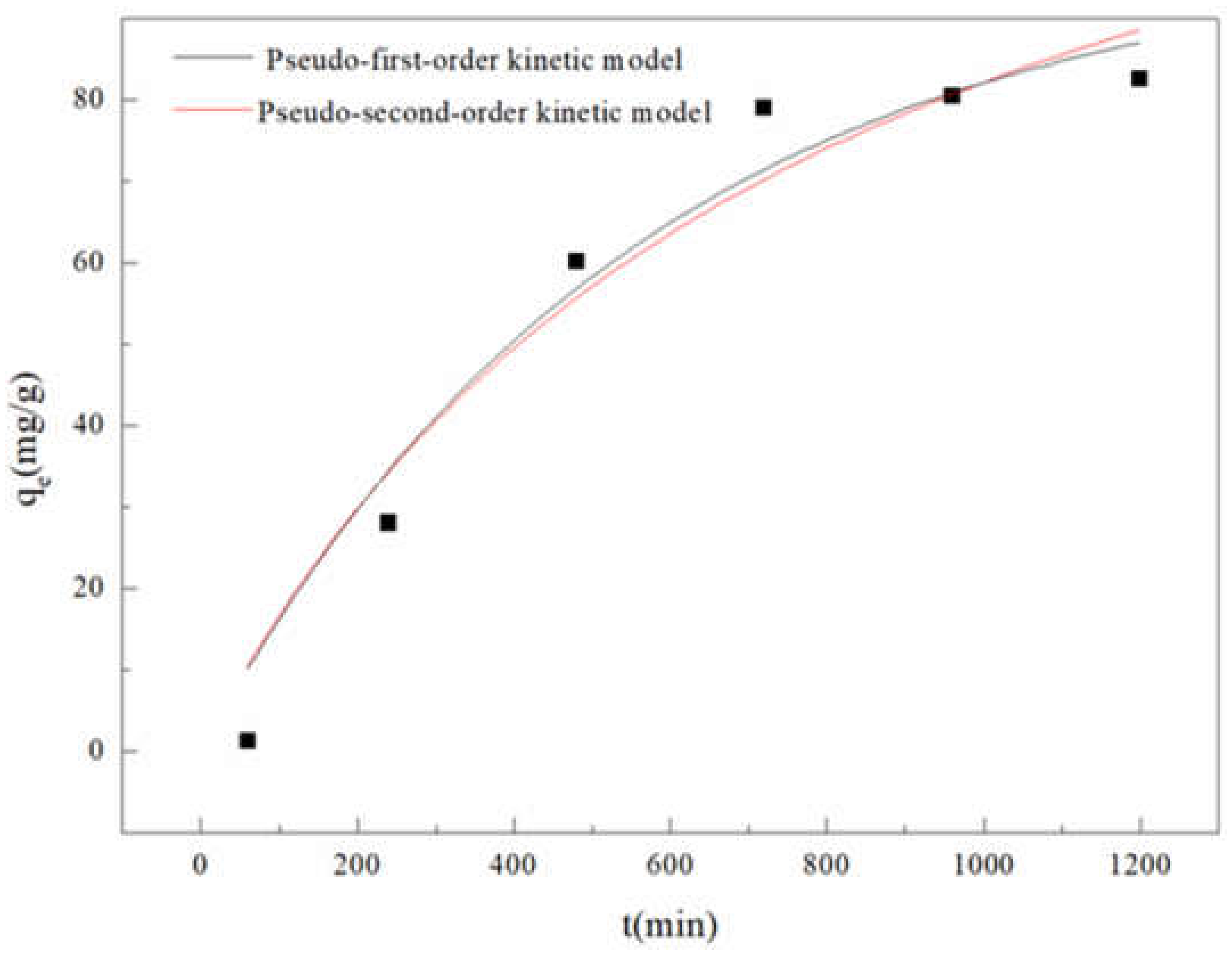
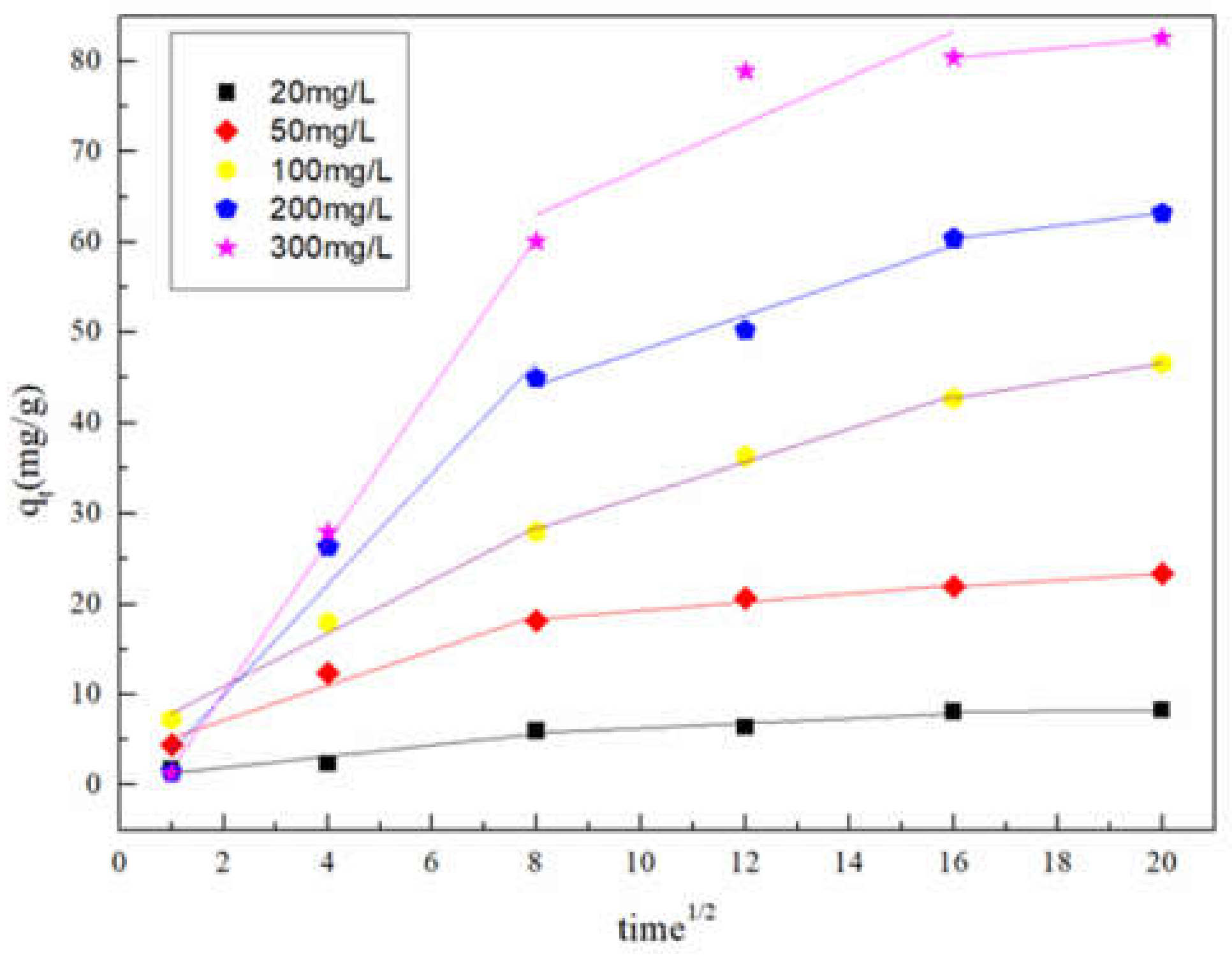
3.3.1. Adsorption Kinetics
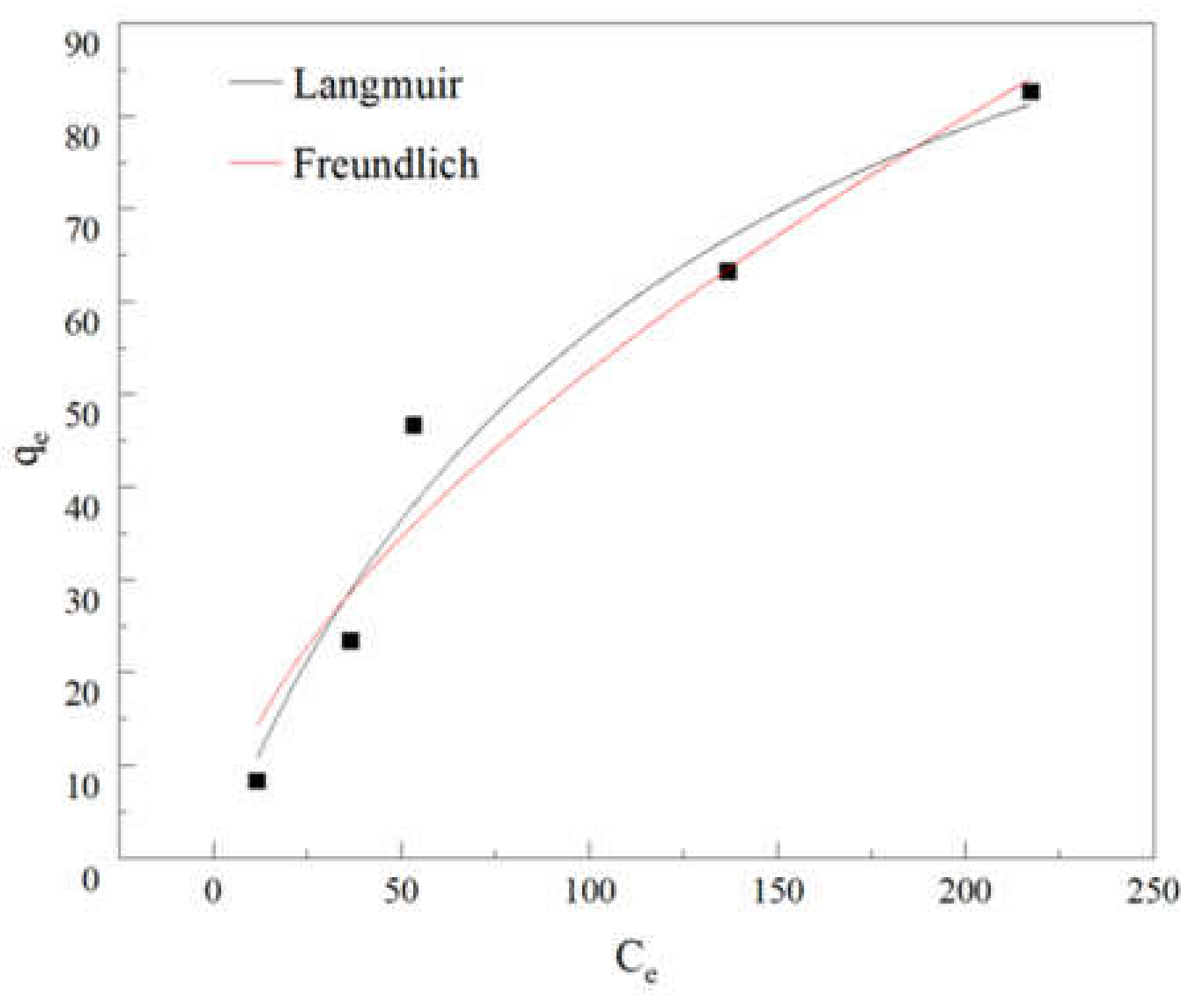
3.3.2. Adsorption Isotherms
3.3.3. Adsorption Mechanism
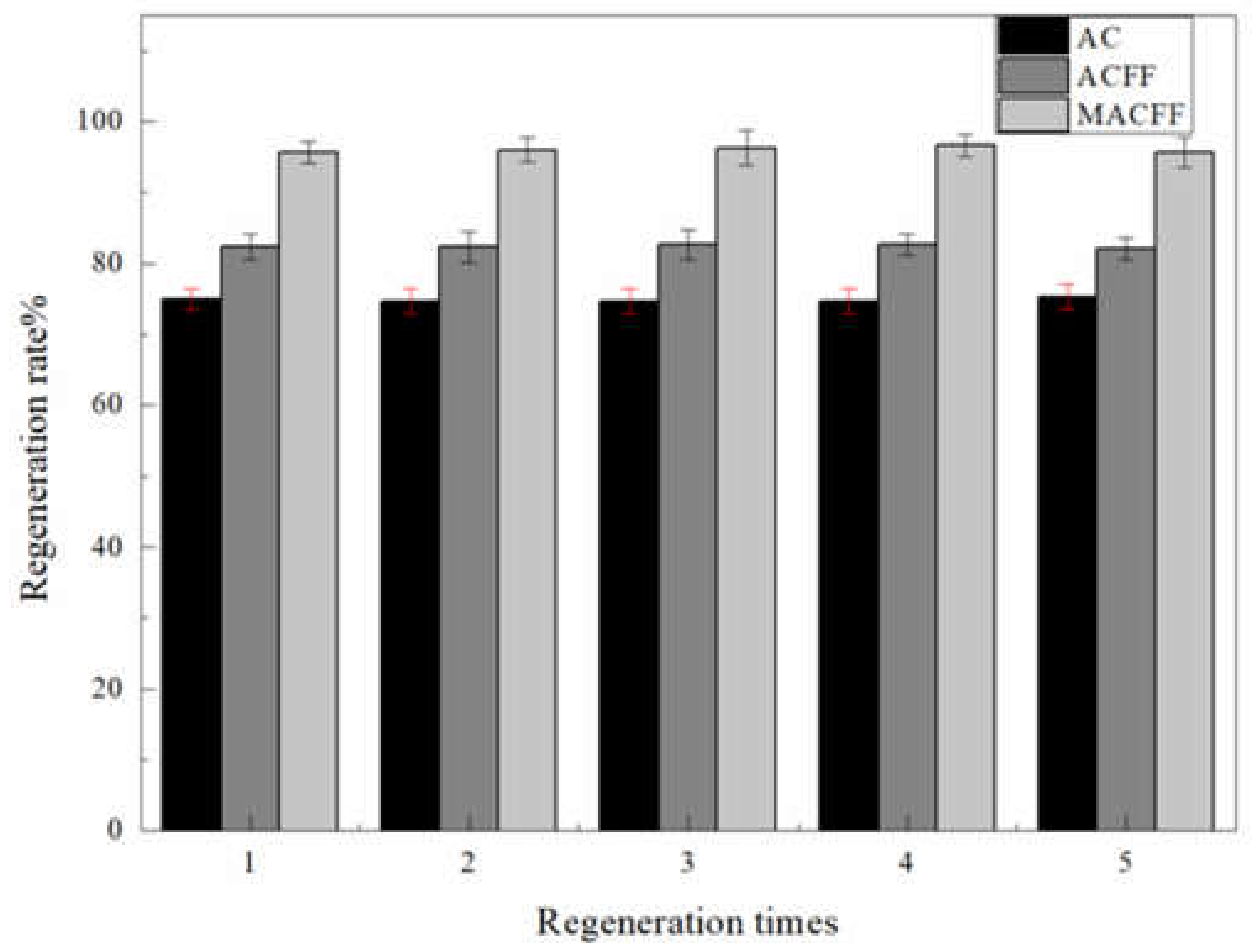
3.4. Backwash Regeneration of the Electrosorption Reactor
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2008, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Pouretedal, H.R.; Sadegh, N. Effective removal of Amoxicillin, Cephalexin, Tetracycline and Penicillin G from aqueous solutions using activated carbon nanoparticles prepared from vine wood. J. Water Process Eng. 2014, 1, 64–73. [Google Scholar] [CrossRef]
- Thai, P.K.; Ky, L.X.; Binh, V.N.; Nhung, P.H.; Nhan, P.T.; Hieu, N.Q. Occurrence of antibiotic residues and antibiotic-resistant bacteria in effluents of pharmaceutical manufacturers and other sources around Hanoi. Vietnam. Sci. Total Environ. 2018, 645, 393–400. [Google Scholar] [CrossRef]
- Hernández, F.; Calısto-Ulloa, N.; Gómez-Fuentes, C.; Gómez, M.; Ferrer, J.; González-Rocha, G.; Bello-Toledo, H.; Botero-Coy, A.M.; Boıx, C.; Ibáñez, M.; et al. Occurrence of antibiotics and bacterial resistance in wastewater and sea water from the Antarctic. J. Hazard. Mater. 2019, 363, 447–456. [Google Scholar] [CrossRef]
- Lei, K.; Zhu, Y.; Chen, W.; Pan, H.-Y.; Cao, Y.-X.; Zhang, X.; Guo, B.-B.; Sweetman, A.; Lin, C.-Y.; Ouyang, W.; et al. Spatial and seasonal variations of antibiotics in river waters in the Haihe River Catchment in China and ecotoxicological risk assessment. Environ. Int. 2019, 130, 104919. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Wang, Z.; Li, S.; Zhao, J.; Liang, G.; Xie, X. Mechanistic insights into removal of Norfloxacin from water using different natural iron ore—biochar composites: More rich free radicals derived from natural pyrite-biochar composites than hematite-biochar composites. Appl. Catal. B Environ. 2019, 255, 117752. [Google Scholar] [CrossRef]
- Homem, V.; Santos, L. Degradation and removal methods of anti-biotics from aqueous matrices—A review. J. Environ. Manag. 2011, 92, 2304–2347. [Google Scholar] [CrossRef]
- Li, C.; Gao, Y.; Li, A.; Zhang, L.; Ji, G.; Zhu, K.; Wang, X.; Zhang, Y. Synergistic effects of anionic surfactants on adsorption of norfloxacin by magnetic biochar derived from furfural residue. Environ. Pollut. 2019, 254, 113005. [Google Scholar] [CrossRef]
- Ferreira, V.R.A.; Amorim, C.L.; Cravo, S.M.; Tiritan, M.E.; Castro, P.M.L.; Afonso, C.M.M. Fluoroquinolones biosorption onto microbial biomass: Activated sludge and aerobic granular sludge. Int. Biodeter. Biodegr. 2016, 110, 53–60. [Google Scholar] [CrossRef]
- Xu, Z.; Xue, X.; Hu, S.; Li, Y.; Shen, J.; Lan, Y.; Zhou, R.; Yang, F.; Cheng, C. Degradation effect and mechanism of gas-liquid phase dielectric barrier discharge on norfloxacin combined with H2O2 or Fe2+. Sep. Purif. Technol. 2020, 230, 115862. [Google Scholar] [CrossRef]
- Jojoa-Sierra, S.D.; Silva-Agredo, J.; Herrera-Calderon, E.; Torres-palma, R.A. Torres-Palma of the antibiotic norfloxacin in municipal wastewater, urine and seawater by electrochemical oxidation on IrO2 anodes. Sci. Total Environ. 2017, 575, 1228–2123. [Google Scholar] [CrossRef]
- Da Silva, S.W.; Navarro, E.M.O.; Rodrigues, M.A.S.; Bernardes, A.M.; Pérez-Herranz, V. Using p-Si/BDD anode for the electrochemical oxidation of norfloxacin. J. Electroanal. Chem. 2019, 832, 112–120. [Google Scholar] [CrossRef]
- Xiang, Y.; Xu, Z.; Wei, Y.; Zhou, Y.; Yang, X. Carbon-based materials as adsorbent for antibiotics removal: Mechanisms and influencing factors. J. Environ. Manag. 2019, 237, 128–213. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K. Functionalization of tungsten oxide into MWCNT and its application for sunlight-induced degradation of rhodamine B. J. Colloid Interface Sci. 2011, 362, 337–344. [Google Scholar] [CrossRef]
- Pirkarami, A.; Olya, M.E.; Yousefi Limaee, N. Decolorization of azo dyes by photo electro adsorption process using polyaniline coated electrode. Prog. Org. Coat. 2013, 76, 682–688. [Google Scholar] [CrossRef]
- Li, N.; Yang, S.; Chen, J.; Gao, J.; He, H.; Sun, C. Electro-adsorption of tetracycline from aqueous solution bycarbonized pomelo peel and composite with aniline. Appl. Surf. Sci. 2016, 386, 460–466. [Google Scholar] [CrossRef]
- Macías-Garcíaa, A.; Carrasco-Amadorb, J.P.; Encinas-Sánchezc, V.; Díaz-Díeza, M.A.; Torrejón-Martína, D. Preparation of activated carbon from kenaf by activation with H3PO4. Kinetic study of the adsorption/electroadsorption using a system of supports designed in 3D, for environmental applications. J. Environ. Chem. Eng. 2019, 7, 103196. [Google Scholar] [CrossRef]
- Park, K.H.; Kwak, D.H. Electrosorption and electrochemical properties of activated-carbon sheet electrode for capacitive deionization. J. Electroanal. Chem. 2014, 732, 66–73. [Google Scholar] [CrossRef]
- Hou, C.H.; Huang, J.F.; Lin, H.R.; Wang, B.Y. Preparation of activated carbon sheet electrode assisted electrosorption process. J. Taiwan Inst. Chem. Eng. 2012, 43, 473–479. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. An overview of the modification methods of activated carbon for its water treatment applications. Chem. Eng. J. 2013, 219, 499–511. [Google Scholar] [CrossRef]
- Jung, C.H.; Lee, H.Y.; Moon, J.K.; Won, H.J.; Shul, Y.G. Electrosorption of uranium ions on activated carbon fibers. J. Radioanal. Nucl. Chem. 2011, 287, 833–839. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; Lealda Silva, E.; Oliveir, A.P.S.; Matsushima, J.T.; Cuña, A.; Marcuzzo, J.S. High-performance supercapacitor electrode based on activated carbon fiber felt/iron oxides. Mater. Today 2019, 16, 100553. [Google Scholar] [CrossRef]
- Huang, L.; Xue, J.; Jin, F.; Zhou, S.; Wang, M.; Liu, Q. Study on mechanism and influential factors of the adsorption properties and regeneration of activated carbon fiber felt (ACFF) for Cr (VI) under electrochemical environment. J. Taiwan Inst. Chem. Eng. 2014, 45, 2986–2994. [Google Scholar] [CrossRef]
- Baur, G.B.; Yuranov, I.; Kiwi-Minsker, L. Activated carbon fibers modified by metal oxide as effective structured adsorbents for acetaldehyde. Catal. Today 2015, 249, 252–258. [Google Scholar] [CrossRef]
- Gupta, V.K.; Saleh, T.A. Sorption of pollutants by porous carbon, carbon nanotubes and fullerene—An overview. Environ. Sci. Pollut. Res. 2013, 20, 2828–2843. [Google Scholar] [CrossRef]
- Zhu, N.W.; Chen, X.; Zhang, T.; Wu, P.X.; Li, P.; Wu, J.H. Improved performance of membrane free single-chamber air-cathode microbial fuel cells with nitric acid and ethylenediamine surface modified activated carbon fiber felt anodes. Bioresour. Technol. 2011, 102, 422–426. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, Y.S.; Li, F.Y.; Yang, D.T. Preparation of biochar by simultaneous carbonization, magnetization and activation for norfloxacin removal in water. Bioresour. Technol. 2017, 233, 159–165. [Google Scholar] [CrossRef]
- Matsuda, H.; Bernardo, E.C.; Fukuta, T.; Fujita, T.; Matsubara, T.; Kojima, Y. Effect of ultraviolet irradiation pretreatment on the removal of saccharin by activated carbon adsorption. In Asian Pacific Confederation of Chemical Engineering Congress Program and Abstracts; The Society of Chemical Engineers: Tokyo, Japan, 2004; pp. 100–1000. [Google Scholar]
- Nekouei, F.; Nekouei, S.; Tyagi, I.; Gupta, V.K. Kinetic, thermodynamic and isotherm studies for acid blue 129 removal from liquids using copper oxide nanoparticle-modified activated carbon as a novel adsorbent. J. Mol. Liq. 2015, 201, 124–133. [Google Scholar] [CrossRef]
- Priya, B.; Gupta, V.K.; Pathania, D.; Singha, A.S. Synthesis, characterization and antibacterial activity of biodegradable starch/PVA composite films reinforced with cellulosic fibre. Carbohydr. Polym. 2014, 109, 171–179. [Google Scholar] [CrossRef]
- Blok, W.J.G.D.; Mcgaugh, S.S. The dark and visible matter content of low surface brightness disc galaxies. Mon. Not. R. Astron. Soc. 1997, 290, 533–552. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, M.; Leifeld, J.; Fuhrer, J. Quantifying soil organic carbon fractions by infrared-spectroscopy. Soil Biol. Biochem. 2007, 39, 224–231. [Google Scholar] [CrossRef]
- Daifullah, A.A.M.; Yakout, S.M.; Elreefy, S.A. Adsorption of fluoride in aqueous solutions using KMnO4 modified activated carbon derived from steam pyrolysis of rice straw. J. Hazard. Mater. 2007, 147, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Xu, X.C. Nondestructive covalent functionalization of carbon nanotubes by selective oxidation of the original defects with K2FeO4. Appl. Surf. Sci. 2015, 346, 520–527. [Google Scholar] [CrossRef]
- Ayranci, E.; Duman, O. Adsorption behaviors of some phenolic compounds onto high specific area activated carbon cloth. J. Hazard. Mater. 2005, 124, 125–132. [Google Scholar] [CrossRef]
- Yang, W.; Lu, Y.; Zheng, F.; Xue, X.; Li, N.; Liu, D. Adsorption behavior and mechanisms of norfloxacin onto porous resins and carbon nanotube. Chem. Eng. J. 2012, 179, 112–118. [Google Scholar] [CrossRef]
- Saravanan, R.; Sacari, E.; Gracia, F.; Khan, M.M.; Mosquera, E.; Gupta, V.K. Conducting PANI stimulated ZnO system for visible light photocatalytic degradation of coloured dyes. J. Mol. Liq. 2016, 221, 1029–1033. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Siddiqui, M.N.; Agarwal, S. Chromium removal from water by activated carbon developed from waste rubber tires. Environ. Sci. Pollut. Res. 2012, 20, 1261–1268. [Google Scholar] [CrossRef]
- Li, Z.; Liu, G.; Su, Q.; Jin, X.; Wen, X.; Zhang, G. Kinetics and thermodynamics of NPX adsorption by γ-FeOOH in aqueous median. Arab. J. Chem. 2018, 11, 910–917. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Zou, L.; Morris, G.; Qi, D. Using activated carbon electrode in electrosorptive deionisation of brackish water. Desalination 2008, 225, 329–340. [Google Scholar] [CrossRef]
- Mohammadi, N.; Khani, H.; Gupta, V.K.; Amereh, E.; Agarwal, S. Adsorption process of methyl orange dye onto mesoporous carbon material-kinetic and thermodynamic studies. J. Colloid Interface Sci. 2011, 362, 457–462. [Google Scholar] [CrossRef]
- Chen, W.; Li, X.; Pan, Z.; Bao, Y.; Ma, S.; Li, L. Efficient adsorption of Norfloxacin by Fe-MCM-41 molecular sieves: Kinetic, isotherm and thermodynamic studies. Chem. Eng. J. 2015, 281, 397–403. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Gupta, V.K. Rice Husk and Its Ash as Low-Cost Adsorbents in Water and Wastewater Treatment. Ind. Eng. Chem. Res. 2011, 50, 13589–13613. [Google Scholar] [CrossRef]
- Khani, H.; Rofouei, M.K.; Arab, P.; Gupta, V.K.; Vafaei, Z. Multi-walled carbon nanotubes-ionic liquid-carbon paste electrode as a super selectivity sensor: Application to potentiometric monitoring of mercury ion (II). J. Hazard. Mater. 2010, 183, 402–409. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, X.; Pan, B.; Xing, B. Norfloxacin Sorption and Its Thermodynamics on Surface-Modified Carbon Nanotubes. Environ. Sci. Technol. 2010, 44, 978–984. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Q.; Zheng, C.; He, C. Sorption of Sulfadiazine, Norfloxacin, Metronidazole, and Tetracycline by Granular Activated Carbon: Kinetics, Mechanisms, and Isotherms. Water Air Soil Pollut. 2017, 228, 129. [Google Scholar] [CrossRef]
- Gupta, V.K.; Gupta, B.; Rastogi, A.; Agarwal, S.; Nayak, A. A comparative investigation on adsorption performances of mesoporous activated carbon prepared from waste rubber tire and activated carbon for a hazardous azo dye—Acid Blue 113. J. Hazard. Mater. 2011, 186, 891–990. [Google Scholar] [CrossRef]
- Gupta, V.K.; Nayak, A.; Agarwal, S.; Tyagi, I. Potential of activated carbon from waste rubber tire for the adsorption of phenolics: Effect of pre-treatment conditions. J. Colloid Interface Sci. 2014, 417, 420–430. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, X.; Liu, P.; Zhao, L.; Zou, W. Optimization adsorption of norfloxacin onto polydopamine microspheres from aqueous solution: Kinetic, equilibrium and adsorption mechanism studies. Sci. Total Environ. 2018, 639, 428–437. [Google Scholar] [CrossRef]
- Keiluweit, M.; Kleber, M. Molecular-level interactions in soils and sediments: The role of aromatic π-systems. Environ. Sci. Technol. 2009, 43, 3421–3429. [Google Scholar] [CrossRef]
- Yang, K.; Xing, B. Adsorption of organic compounds by carbon nanomaterials in aqueous phase: Polanyi theory and its application. Chem. Rev. 2010, 110, 5989–6008. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Pan, B.; Xing, B. Adsorption and desorption of oxytetracycline and carbamazepine by multiwalled carbon nanotubes. Environ. Sci. Technol. 2009, 43, 9167–9173. [Google Scholar] [CrossRef]
- Ji, L.; Chen, W.; Zheng, S.; Xu, Z.; Zhu, D. Adsorption of sulfonamide antibiotics to multiwalled carbon nanotubes. Langmuir 2009, 25, 11608–11613. [Google Scholar] [CrossRef]
- Gao, J.; Pedersen, J.A. Sorption of sulfonamide antimicrobial agents to humic acid–clay complexes. J. Environ. Qual. 2010, 39, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.K.; Nayak, A.; Agarwal, S. Bioadsorbents for remediation of heavy metals: Current status and their future prospects. Environ. Eng. Res. 2015, 20, 1–18. [Google Scholar] [CrossRef]
- Salvador, F.; Martin, N. Regeneration of activated carbons contaminated by phenol using supercritical water. J. Supercrit. Fluids 2013, 74, 1–7. [Google Scholar] [CrossRef]
- Savlak, O.; Karabacakoğlu, B. Electrochemical regeneration of Cr (VI) saturated granular and powder activated carbon: Comparison of regeneration efficiency. Ind. Eng. Chem. Res. 2004, 53, 13171–13179. [Google Scholar]


| Type | Specific Surface Area | Average Pore Size | Micropore Volume |
|---|---|---|---|
| (m2/g) | (nm) | (cm3/g) | |
| ACFF | 1035.21 | 2.04 | 0.22 |
| MACFF | 1355.09 | 2.11 | 0.3 |
| Model Name | Model Related Parameters |
|---|---|
| pseudo-first-order | k1 = 1.79 × 10−3(min−1), qe = 98.39(mg/g), R2 = 0.9425 |
| pseudo-second-order | k2 = 8.75 × 10−6(g/(mg· min)), qe = 146.24(mg/g), R2 = 0.9540 |
| Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|
| R2 | b | qm | R2 | n | kf |
| 0.9656 | 0.00791 | 128.5515 | 0.9495 | 0.6045 | 3.2484 |
| Absorbent | T(K) | Sorption | Reference |
|---|---|---|---|
| Capacity (mg/g) | |||
| Aminated Polystyrene Resin | 288 | 117.6 | [36] |
| Carboxylated Carbon Nanotube | 288 | 87 | [36] |
| Molecular Sieves | 298 | 102.9 | [44] |
| Activated Carbon | 298 | 112.48 | [46] |
| Graphitized Carbon Nanotubes | 298 | 57.55 | [46] |
| Carboxylated Carbon Nanotubes | 298 | 54.44 | [46] |
| Hydroxylated Carbon Nanotubes | 298 | 76.34 | [46] |
| Granular Activated Carbon | 298 | 112.86 | [47] |
| Modified Activated Carbon Fiber Felt | 298 | 128.55 | This work |
| Initial Concentration of NOR (mg/L) | Kid,1 | C1 | R2 | Kid,2 | C2 | R2 |
|---|---|---|---|---|---|---|
| (mg/g·min1/2) | (mg/g·min1/2) | |||||
| 20 | 0.6233 | 0.6538 | 0.8356 | 0.2671 | 3.6028 | 0.7687 |
| 50 | 1.9294 | 3.2786 | 0.9435 | 0.4702 | 14.5789 | 0.9368 |
| 100 | 2.9396 | 5.0107 | 0.9787 | 1.8369 | 13.618 | 0.9891 |
| 200 | 6.1399 | −2.3663 | 0.9449 | 1.9294 | 28.7292 | 0.9378 |
| 300 | 8.3863 | −6.5492 | 0.9983 | 2.5349 | 42.7352 | 0.6052 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Hu, Y.; She, D.; Shen, W.-B. Modified Activated Carbon Fiber Felt for the Electrosorption of Norfloxacin in Aqueous Solution. Sustainability 2020, 12, 3986. https://doi.org/10.3390/su12103986
Li X, Hu Y, She D, Shen W-B. Modified Activated Carbon Fiber Felt for the Electrosorption of Norfloxacin in Aqueous Solution. Sustainability. 2020; 12(10):3986. https://doi.org/10.3390/su12103986
Chicago/Turabian StyleLi, Xianzhen, Yue Hu, Diao She, and Wei-Bo Shen. 2020. "Modified Activated Carbon Fiber Felt for the Electrosorption of Norfloxacin in Aqueous Solution" Sustainability 12, no. 10: 3986. https://doi.org/10.3390/su12103986
APA StyleLi, X., Hu, Y., She, D., & Shen, W.-B. (2020). Modified Activated Carbon Fiber Felt for the Electrosorption of Norfloxacin in Aqueous Solution. Sustainability, 12(10), 3986. https://doi.org/10.3390/su12103986





