Abstract
The belowground bud bank plays an important role in plant communities succession and maintenance. In order to understand the response of the bud bank to the sod layer moisture, we investigated the bud bank distribution, size, and composition of six different water gradient alpine meadows through excavating in the Zoige Plateau. The results showed: (1) The alpine meadow plant belowground buds were mainly distributed in the 0–10 cm sod layer, accounting for 74.2%–100% of the total. The total bud density of the swamp wetland and degraded meadow was the highest (16567.9 bud/m3) and the lowest (4839.5 bud/m3). (2) A decrease of the moisture plant diversity showed a trend of increasing first and then decreasing. Among six alpine meadows the swamp meadow plant diversity was the highest, and species richness, Simpson, Shannon–Wiener, and Pielou were 10.333, 0.871, 0.944, and 0.931, respectively. (3) The moisture was significantly positively correlated with the total belowground buds and short rhizome bud density. There were significant positive correlations with sod layer moisture and tiller bulb bud density. This study indicates that the moisture affected bud bank distribution and composition in the plant community, and the results provide important information for predicting plant community succession in the alpine meadow with future changes in precipitation patterns.
1. Introduction
Plant community maintenance and regeneration is realized by the continuous renewal of plants [1]. In addition to seed propagation, plants can also conduct asexual propagation (population reproduction and renewal rely on belowground buds) [2,3]. Plant bud bank is defined as a collection of all buds that are potentially used for vegetative reproduction [4]. In natural conditions, belowground plant bud banks play an important role in population regeneration and maintenance [5]. Some ecological systems (especially overgrazing grassland ecosystem) cannot reach sexual maturity and engage in seed breeding due to many reasons such as animal eating and low temperature stress. [6,7]. Therefore, asexual reproduction has become the main reproduction method in many grassland ecosystems, especially in the perennial grassland where the role of a bud bank is even greater than that of the seed bank [8,9]. For example, 99% of the aboveground plant stems in North American grasslands were formed from the bud bank [10]; 84% of the alpine grassland area in the Alps is occupied by clonal plants [11].
As an important dynamic composition of grassland vegetation, belowground bud bank is the key to study plant adaptation to environmental stress, ecological restoration, and succession, and it directly affects the function of the terrestrial ecosystem [12,13]. In a grassland ecosystem, the size and composition of the plant bud bank can reflect the aboveground plant richness, species composition, and other characteristics, and the population renewal of plants in response to different disturbances and environmental stresses [2,3]. Plants respond to disturbance and stress such as grazing, burning, drought, and water flooding by regulating the input, output, and size of the bud bank, and this then affects the vegetation community structure [3,14]. In addition, asexual plants can enhance their competitiveness through clonal integration, which is important for plants to expand their growth space, tolerate stressful environments, and effectively utilize heterogeneous resources [15,16]. Therefore, the bud bank is of great significance and even plays a decisive role in ecosystem resilience, plant community structure, and productively copes with drought, grazing pressure, or invasion of alien plant species [9,17]. The ability of the grassland ecosystem to respond to disturbances is closely related to the rapid regeneration and growth of the belowground bud bank, which could be used to predict the plant community dynamics to a certain extent [18].
At present, the study about plant bud banks has been carried out worldwide. Studies have shown that water is one of the key factors affecting the density of belowground buds. The size and composition of plant buds is closely related to precipitation. The larger the precipitation, the more abundant the bud bank will be. For example, the density of buds in North American grasslands increases significantly with the increase of annual average precipitation [10]. Some plants have high photosynthesis efficiency under high soil moisture, and more organic matter is produced to translate rhizome buds, enabling plants to escape from an unfavorable habitat and seek a favorable habitat to survive and reproduce [5]. In habitats with relatively high-water stress, the number and types of belowground bud banks is poor, and plants tend to adopt a conservative strategy to produce a larger proportion of bulb buds and tiller buds to maintain their population survival. With the intensification of water stress, plants translate from expansive strategies to conservative strategies by producing more rhizome buds. Arid soil cannot provide sufficient water for plants, thus affecting photosynthesis, physiological functions, and the expansion of plant populations. This should be the result of intra-species competition within plant communities and the trade-off between sexual reproduction and asexual reproduction [5,9]. Therefore, the belowground bud bank is an important regulatory mechanism of the structure and composition of the grassland community in response to drought stress [19]. However, Zhang et al. [20] believed that drought conditions were not conducive to plant growth, so plants produce a large number of rhizome buds for plasticity. In this way plants escape from the adverse habitat and reach a resource-rich habitat [20]. If soil moisture is abundant, plants adopt a conservative mode to produce a large number of tiller buds for cloning and reproduction, so as to take full advantage of occupied resources. The differences in the above research conclusions should be attributed to the differences in plant physiology that lead to the adoption of different strategies in response to different environments. However, in the alpine meadow grassland, it is still unknown how the plants belowground bud bank changes with water distribution. Under the background of global climate change, how the bud bank and plant community composition of alpine meadow grassland change, especially in response to the change in water distribution patterns, has become an important issue in the study of alpine meadow grassland.
Alpine meadow is the main vegetation type of the Tibetan Plateau, accounting for about 50% of the available grassland area [21]. Alpine meadow is the material basis of animal husbandry production on the plateau, and also a huge ecological protective screen in the inland and surrounding areas of China. The alpine meadow ecosystem is fragile with poor anti-interference ability and is sensitive to climate change and human disturbance [1,22]. Alpine meadow soil differs greatly from other ecosystems; it has 0–30 cm soil which is called the sod layer and has a high content of organic matter, as well as an interwoven entanglement of living and dead roots that makes the sod layer soft and tough [23]. The sod layer is not only the hub of material circulation and energy flow in an alpine ecosystem, but also the distribution layer of belowground bud bank of alpine plants. Therefore, the change of sod layer’s physical and chemical properties has an important impact on the generation, germination, and distribution of plant buds. At present, the alpine meadow ecosystem has been degraded to varying degrees under the dual influences of natural and human activities. Herbage has been replaced by poisonous weed species, grassland has become bare, and the ecological role of the sod layer has been reduced to varying degrees. Alpine meadow restoration has become an important research topic in ecology and other fields. Relying on plant bud bank to restore local vegetation is the most effective method to restore plant diversity without damaging the genetic diversity [24]. Understanding the plant belowground buds’ contribution of different components is beneficial to (1) reveal the dynamic mechanisms of different plant communities along grassland succession sequences [5,25], (2) provide support for recovering degraded grassland, and 3) develop appropriate grassland management strategies and theoretical guidance for maintaining the community structure and productivity of existing plants [5,25].
This study’s primary purpose is to determine the response of the plant community belowground bud bank in the Zoige Plateau alpine meadow of China along different water gradients. The study attempts to answer the following scientific questions: (1) How does the size and composition of the bud bank in alpine sod layer respond to different water gradients? (2) Is there any difference in the response of different types of bud bank to alpine sod layer moisture? This study is helpful to understand and predict the dynamics of the grassland plant community in the alpine meadow, and reveal the general trend of the growth and change of the grassland plant community under climate change.
2. Materials and Methods
2.1. Site Description
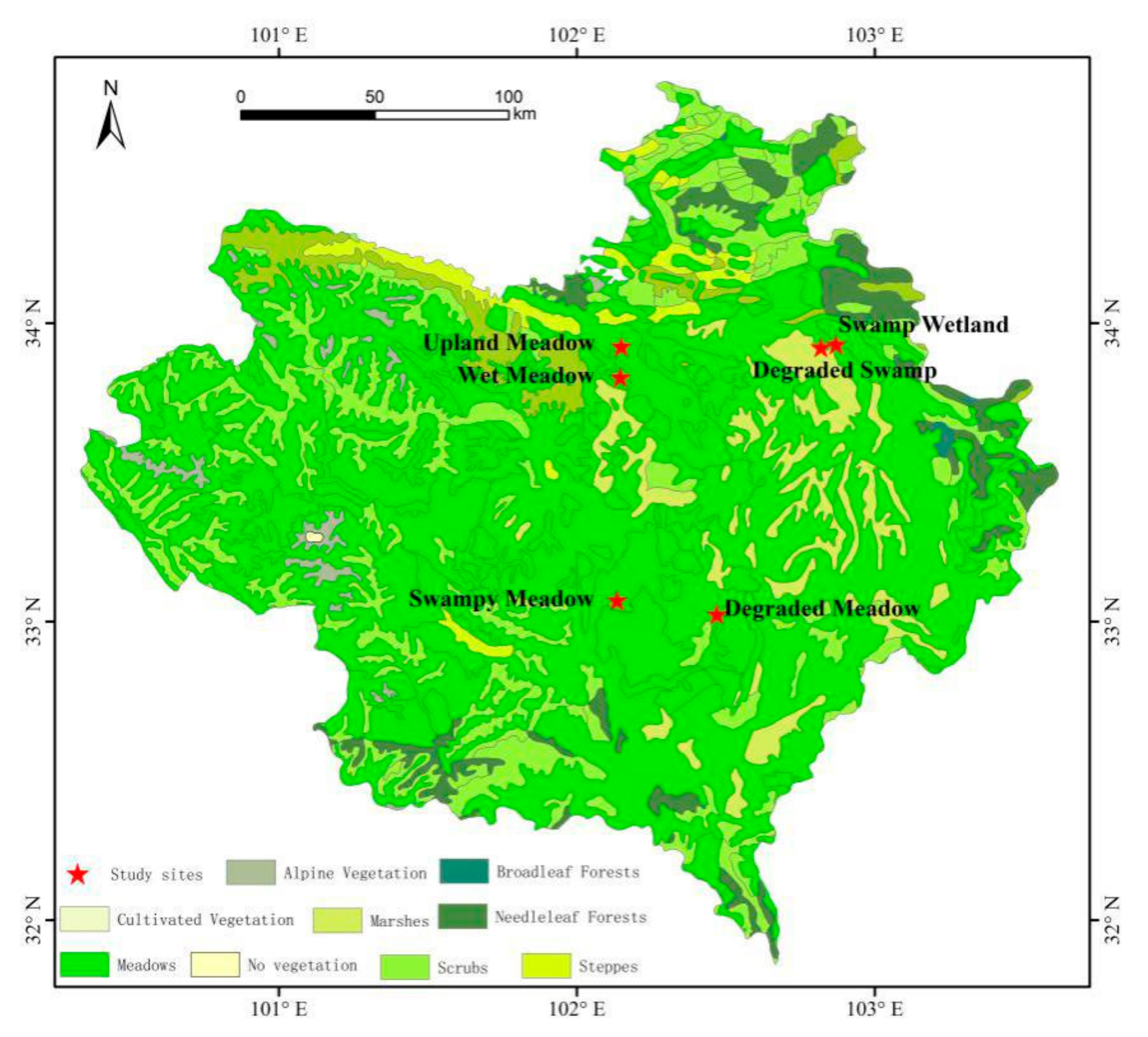
The study area was located in the eastern part of the Tibetan Plateau, named the Zoige Plateau (Figure 1). The area is a typical plateau wetland with an elevation of 3400–3800 m and an average elevation of 3500 m [23,26]. The climate of the continental plateau is a semi-humid monsoon climate, which is cold and humid, has a long frost period, and seasonal changes are not obvious. The annual average temperature is 0.7 °C, the annual average precipitation is 656.8 mm, and the rainy season is in June, July and August, when the precipitation accounts for 70% of the whole year. The annual average sunshine length is 2389 h, and the annual average evaporation is 1232 mm [27,28].
Figure 1.
Study sites distribution of alpine meadow in the Zoige Plateau.
In the Zoige Plateau, the alpine meadow was divided into swamp wetland, degraded swamp, swamp meadow, wet meadow, upland meadow (also known as dry meadow), and degraded meadow according to the surface water and vegetation conditions. Swamp wetland plots and degraded swamp plots are located in the Zoige Wetland Reserve, Ruoergai County, Sichuan Province (33°55′42″ N, 102°52′15″ E; 33°55′6″ N, 102°49′13″ E). Swamp meadow plots are located at the northern side of the Rierlang Mountain piedmont in Ruoergai County, Sichuan Province (33°4′15″ N, 102°8′11″ E). Wet meadow plots and upland meadow plots are located at the Hequ Horse Farm, Maqu County, Gansu Province (33°49′05″ N, 102°08′49″ E; 33°55′18″ N, 102°09′03″ E). Degraded meadow plots are located in Maixi Town, Ruoergai County, Sichuan Province (34°01′28″ N, 102°28′18″ E).
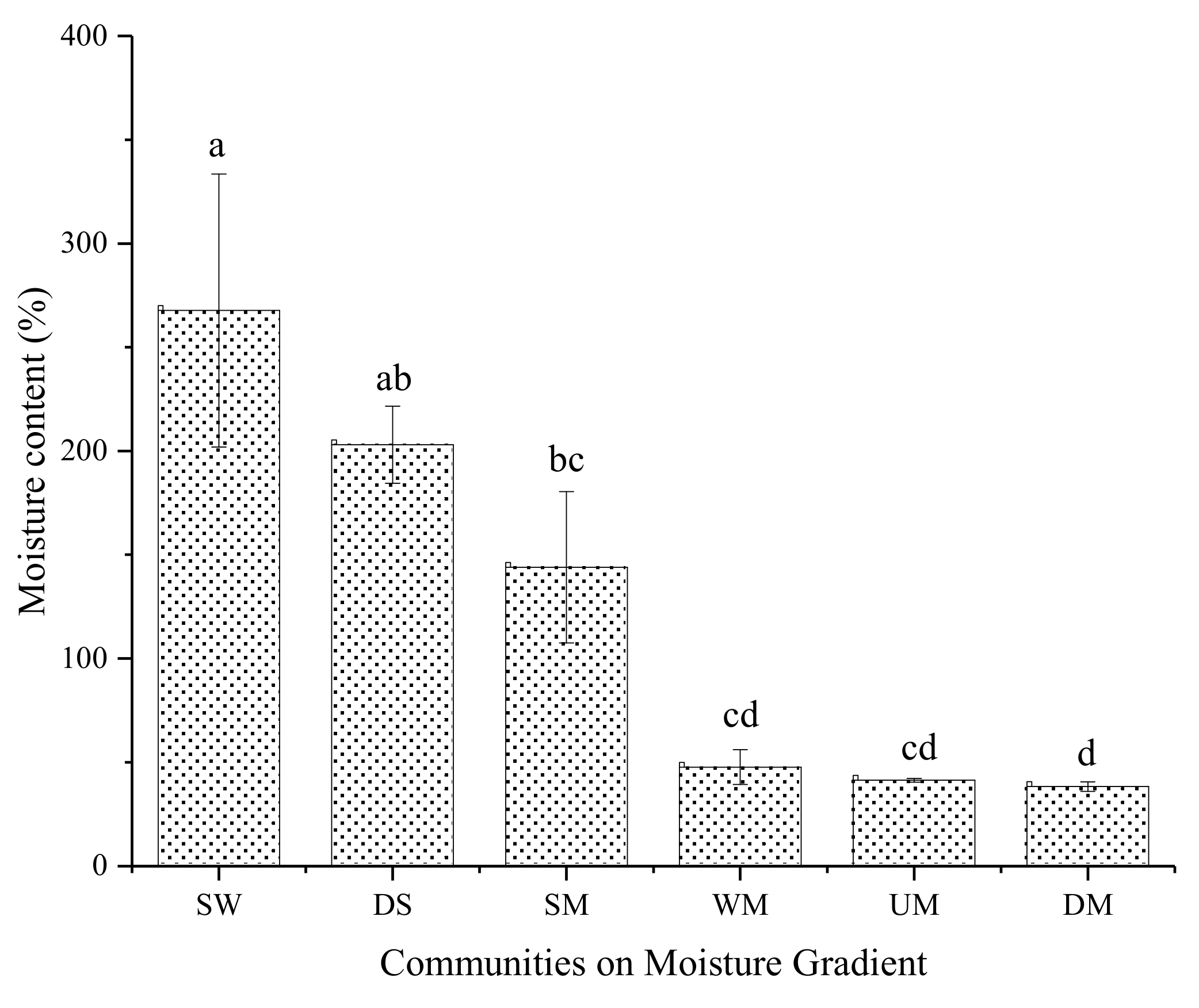
The alpine meadow sod layer moisture is shown in Figure 2.
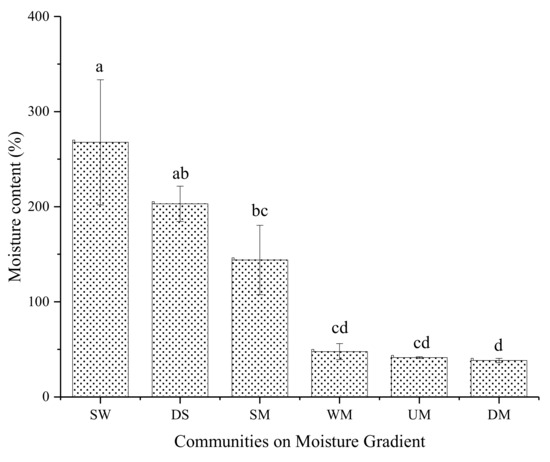
Figure 2.
Sod layer mass moisture of different alpine meadow. SW: swamp wetland; DS: degraded swamp; SM: swampy meadow; WM: wet meadow; UM: upland meadow or dry meadow; DM: degraded meadow. Data with different letters mean significant difference at 0.05 level in the same column.
2.2. Sampling Method
The size of the belowground bud bank was relatively stable from the end of the growing season to the second beginning [7,10]. Therefore, investigation and sampling studies on sample plots took place from 11–29 September, 2018. The composition and distribution of alpine meadow bud bank were studied along water gradient. There are different degrees of grazing in degraded swamp, swamp meadow, wet meadow, and degraded meadows. Swamp wetland plots were located in the Zoige Wetland Reserve, so swamp wetland was banned by the government in 2009. Upland meadow plots were banned to protect the test instrument in 2016. Therefore, the investigation of aboveground biomass of plants is only for reference, not for discussion, in this study.
The data of vegetation type, coverage, height, and number of individuals were randomly investigated with three 1 × 1 m samples. Clipped aboveground biomass within grazed plots was collected, dried at 60 °C for at least 48 h and weighed to the nearest 0.01 g.
A total of 3 bud samples, with a size of 15 cm × 15 cm, were randomly selected from each plot with a depth of 30 cm and divided into 3 layers: top (0–10 cm), middle (10–20 cm), and lower (20–30 cm). Soil was carefully removed from each soil core in the field. Then, samples were collected and placed in plastic bags and stored at 4 °C until they were brought back to the laboratory for processing [8,10]. The soil samples were crushed and washed with water within two weeks, and the buds were counted according to soil layer and bud type. In this study, only living buds were counted and dead buds were artificially removed. We assigned buds to species and types using bud morphology, phyllotaxy, morphology of the attached root system, and morphology of any remaining aboveground parts. Due to the differences in bud morphology, different types of buds were counted in different ways: rhizome buds could be counted directly, and tiller buds and bulb (bulbous) buds could be counted after anatomy [17,20].
Previous studies show that bud density is related to the soil moisture [8], so we measured sod layer moisture in our subplots to check the results.
2.3. Statistical Analyses
The following formulas are used to calculate the plant important values, Simpson index, Shannon–Wiener, and Pielou index [29].
- (1)
- Calculation method of important value (IV)where Rh is relative height, Rc is relative coverage, Rd is relative density, hi is the height of the species i, ci is the coverage of the species i, di is the abundance of the species i, i = 1,2,3,..., S.
- (2)
- Simpson index:where D is the Simpson index; Pi is the relative important value of species i, i = 1,2,3,..., S.
- (3)
- Shannon–Wiener:where H is Shannon–Wiener; Pi is the relative important value of species i, i = 1,2,3,..., S.
- (4)
- Pielou index:where J is the Pielou index and S is the total number of species.
Data including bud density, soil water content, and vegetation community characteristics for all quadrats were averaged to describe each vegetation community type. The number of buds per quadrat (for 10 cm depths) was converted to the number of buds per meter3 before analysis. Excel 2013 was used to calculate different bud types density and plant diversity. A one-way analysis of variance (ANOVA) was used to analyze the differences in four types of bud density and vegetation community characteristics for a different plant community. Pearson correlation coefficients of relationships between belowground bud bank and plant community in alpine meadow was calculated by SPSS 24.0. The significant difference level was 0.05, and the extremely significant difference level was 0.01. Drawing was done with Origin 9.0.
3. Results
3.1. Distribution of Belowground Buds
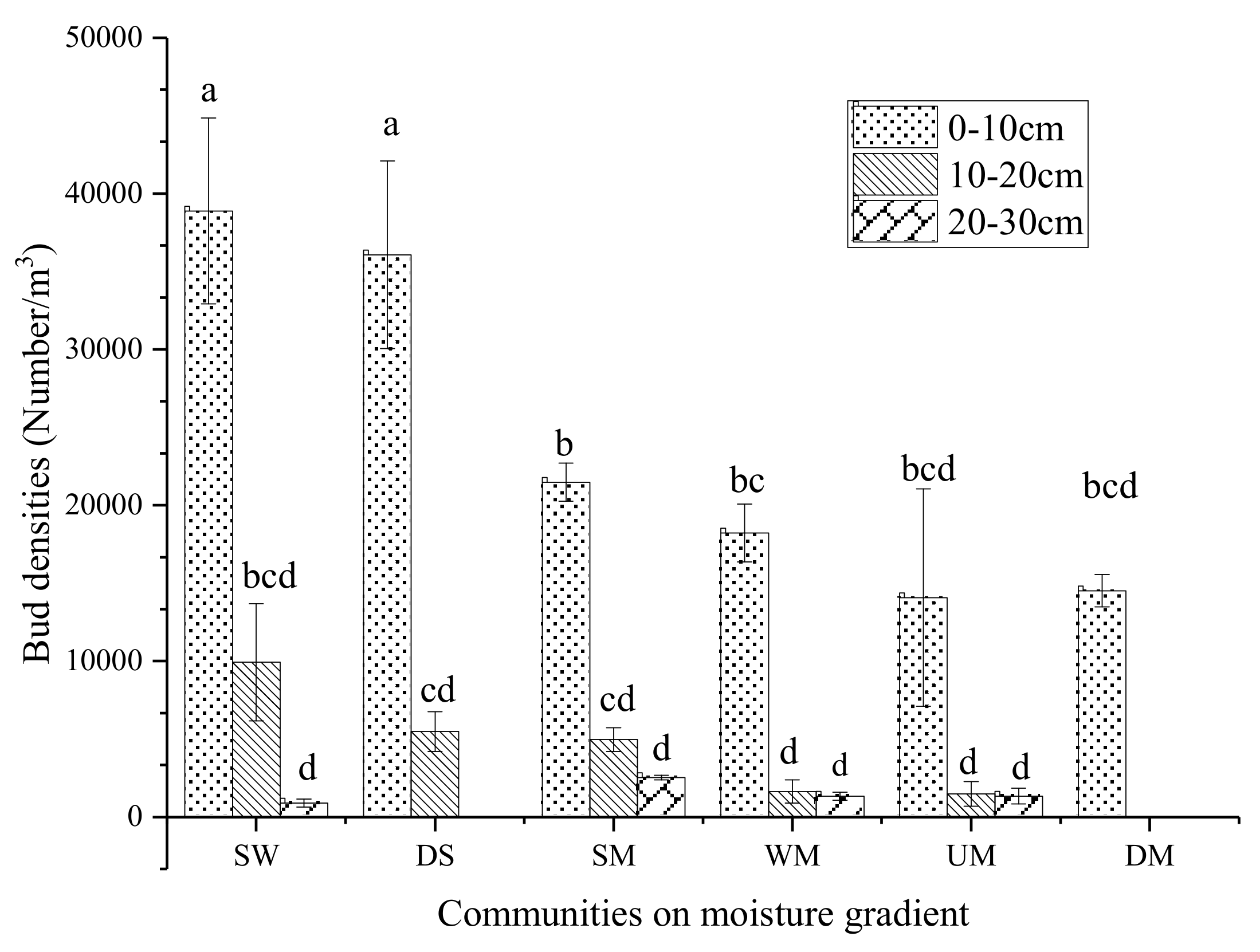
As a whole, in 0–30 cm alpine sod layers the total bud density decreases with the decrease of moisture (Figure 3). The total bud density of the swamp wetland was the highest (16567.9 bud/m3), followed by 13851.9 bud/m3 of degraded swamp with no significant difference. The belowground bud density of degraded meadow plants was the lowest at 4839.5 bud/m3.
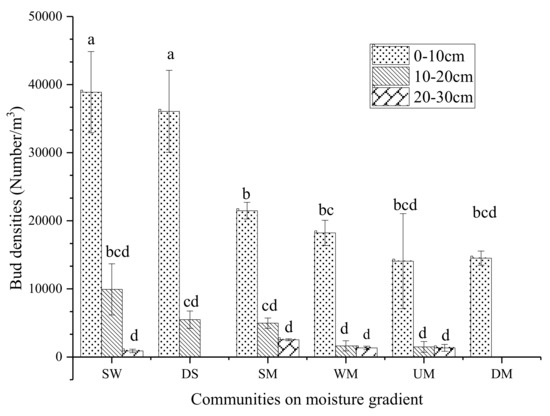
Figure 3.
Total bud density distribution in meadow sod layer. Data with different letters mean significant difference at 0.05 level in the same column.
The plant buds were mainly distributed in the 0–10 cm sod layer and account for 74.2%–100% of the total, which is rarely distributed in the 20–30 cm sod layer. Degraded meadow plant buds were only distributed in the 0–10 cm sod layer. On the whole, the higher the moisture of the sod layer, the higher the bud density of 0–10 cm sod layer. The Swamp wetland bud density of 0–10 cm sod layer is the largest (38888.9 bud/m3), followed by the degraded swamp (36074.1 bud /m3). The minimum bud density in 0–10 cm sod layer was found in the upland meadow (14074.1 bud/m3).
3.2. Composition of Plant Belowground Buds
On the whole, with the decrease of moisture in the sod layer, tiller buds, long rhizome buds, short rhizome buds, and bulb buds’ density decreased gradually, the percentage of tiller buds in total buds increased gradually, and the difference of tiller buds’ density among six alpine meadow community plants was not significant (Table 1). The swamp wetland plants density of tiller buds was the highest, and accounted for the lowest percentage of total buds (4444.4 bud/m3, 26.8%). The belowground tiller bud density of wet meadow plants was the lowest (1867.2 bud/m3). The highest percentage of tiller buds was 60.4% in the degraded meadow.

Table 1.
Different bud changes with the moisture gradients.
With the decrease of sod layer moisture, the long rhizome bud density decreased gradually (Table 1). The long rhizome bud density of degraded swamp plants was the highest at 2814.8 bud/m3, accounting for 20.5% of the total buds. The percentage of long rhizome bud in total buds of swamp meadow was the lowest (10.0%) and the percentage of wet meadow plants was the highest (27.1%).
The percentage of short rhizome buds in total buds decreased gradually with the decrease of moisture. The density of short rhizome buds in swamp wetland was the highest, accounting for the highest percentage of total buds, which were 9135.8 bud/m3 and 54.6%, respectively. The short rhizome bud density of upland meadow was the smallest and the percentage of total buds was the lowest, 1086.2 bud/m3 and 22.4%, respectively.
Bulb buds were distributed only in swamp wetland and degraded swamp, with densities of 1012.4 and 1209.9 bud/m3, accounting for 6.7% and 8.6% of total buds, respectively (Table 1).
3.3. Plant Community Characteristics
With the decrease of alpine sod layer moisture, the plant composition of different communities changed to some extent (Table 2). For example, the dominant plants in swamp wetland were Carex muliensis, Kobresia setchwanensis and Artemisia annua; the common plants were Deschampsia caespitosa and Koeleria cristata. The dominant plants in wet meadow were Blysmus sinocompressus and K. setchwanensis. The common plants were C. muliensis, C. moorcroftii and Deschampsia caespitosa. The dominant plants in degraded meadow were C. muliensis, K. setchwanensis, and Potentilla anserina, and the common plants were Ligularia virgaurea and Leontopodium haplophylloides.

Table 2.
Species composition in different plant communities.
With the decrease of sod layer moisture, the plant diversity increased first and then decreased (Table 3). Among the six alpine meadows with different water gradients, the plant diversity of swamp meadow was the highest. Compared with swamp meadow, plant diversity decreased in communities with high sod layer or low moisture. The plant diversity of upland meadow was the lowest, and was significantly lower than that of swamp meadow.

Table 3.
Diversity and aboveground biomass in different plant communities.
3.4. Relationship between Belowground Bud Bank and Aboveground Community
Among the six alpine meadow communities, the alpine sod layer moisture was extremely significantly positively correlated with total bud and short rhizome bud density. There was significantly positive correlation between moisture and tiller bud and bulb density. There was no significant correlation relationship between plant diversity and four types of buds’ density and moisture (Table 4).

Table 4.
Pearson correlation coefficients of relationships between belowground bud bank and plant community.
4. Discussion
In natural conditions, not every bud can initiate a new shoot for vegetation regeneration. Only when the belowground buds sprout to the plant aboveground part can the vegetation be renewed, and worthy of the energy input to the buds [20,30]. Most of belowground buds were distributed in the 0–10 cm sod layer, rarely buds were distributed in the 10–20 cm alpine sod layer, and very few were distributed in the 20–30 cm alpine sod layer. Zhang et al. [20] found that the belowground buds in sandy areas were mainly distributed in the 10–30 cm soil, and this should be the soil differences which affect the distribution of buds. In the 0–10 cm sandy soil, the plant buds are susceptible to drought, high temperature, etc., and the cost of plant bud preservation activity is relatively high [31,32]. In alpine meadow, compared with plant belowground buds distributed in the 10–30 cm sod layer, the belowground buds in the 0–10 cm sod layer were more likely to grow out, and rapid photosynthesis provided carbohydrates for plant growth. In alpine meadows and sandy environments, buds located in deep sod layer need more energy to maintain the consumption in a low oxygen environment [33,34], where buds’ germination ability is poor and more energy is needed to be put into the belowground buds to sprout out of the grass sod layer. In addition, according to the cost–benefit hypothesis, plants invest relatively little energy in deep soil layer buds because belowground buds in the 10–30 cm grass soil would germinate and grow only when they are brought to the shallow soil [31,35]. This part of the buds has a very low germination rate, so excessive investment will lead to a waste of plant energy, which is not conducive to plant growth and reproduction [36].
There are limited resources that plants could utilize throughout the growing season, therefore, there is a trade-off between reproduction and growth [2,5]. Plants constantly adapt to the environment changes depending on adjusting the resource allocation ratio between growth and reproduction. In a wetland ecosystem, plant growth and distribution largely depend on the water level gradient, which always changes with the elevation. Chen’s study showed that the bud density in the Dongting Lake wetland decreased with the increase of the distribution elevation [1,7]. In this study, the belowground bud density decreased gradually with the decrease of alpine sod layer moisture. Flood disturbance caused by rapid precipitation during the summer is the main factor affecting plant growth and distribution in swamp wetland, degraded swamp, and swamp meadow ecosystems [1]. In order to cope with flooding stress, plants always invest more resources towards producing a lot of buds [10]. After flooding, buds will supplement the restoration of aboveground vegetation, ensuring the normal growth of plant communities in the second year. Therefore, high bud density could rapidly increase the ability of plants to adapt to highly heterogeneous environments [18]. Flooding disturbance intensity and frequency generally decreases with the increase of elevation. Wet meadow, upland meadow, and degraded meadow were not affected by flooding stress, so there is no need for a large number of buds to replenish the plants aboveground, and the belowground bud density was lower than swamp wetland, degraded swamp, and swamp meadow.
Dalgleish and Hartnett (2006) [10] pointed out that with the increase of precipitation in the North American grassland, the plant bud density showed a significant increasing trend, which corresponded well with the vegetation pattern. In the alpine region, from the swamp wetland to the upland meadow, the plant belowground bud density decreased with the decrease of alpine sod layer moisture. Although the plant bud density increases with increasing water, the response mechanism should be different. In North American grassland, moisture is the main factor affecting plant growth. An increase in rainfall can alleviate plant drought stress, so plant bud density increases with the increase of precipitation [10]. However, the excessive water in alpine meadows brings flooding to some plant communities, so the plants produce more buds to restore the aboveground vegetation after flooding, ensuring the normal growth of plants in the second growing season.
As an important propagule of grassland plants, it has been confirmed that there are differences in the bud bank energy storage and expansion capacity due to the morphological differences of buds attached to clonal growth organs [31]. Therefore, different types of bud banks may endow plants with different adaptation strategies, and there may be differences in response to disturbances and utilization of resources [17]. Zhang et al. found that the proportion of Leymus secalinus belowground buds was different under different population densities in sandy land [20]. In alpine meadow, with the decrease of the alpine sod layer moisture, the percentage of tiller bud in the total buds increased, while that of short rhizome bud in the total buds decreased. Some studies have shown that when the environment conditions are favorable (wet meadow, dry meadow, and degraded meadow), the plant population adopts an intensive foraging strategy by increasing the proportion of tiller buds, which is conducive to plant population consolidation and fully occupying better habitats and reducing the probability of invasion by other species [37,38]. However, in poorer environments (swamp wetland, degraded swamp, swamp meadow), plant populations adopt a wide-ranging foraging strategy and seek favorable habitats by producing a high proportion of rhizome buds, which is conducive to clonal plants occupying more space for survival and reproduction, and also reduce competition among plant populations, promote plant ramet to escape from unfavorable habitats (escape from flooding), and improve the probability of ramet living in favorable habitats [39,40].
Plants buds are considered to be the key factors affecting aboveground biomass. For example, the study on the North American prairie verified the "meristem limitation hypothesis" [2,3,41,42,43,44]. However, the bud density in the 0–10 cm sod layer of alpine meadow plants is 14,074–38,889 bud/m3, which is relatively high compared with other ecosystems [3,5,10,45]. Therefore, the bud density in alpine meadow is not a key factor affecting the number of plant communities to a certain extent. Meristem limitation may only occur in specific grassland types or under specific environmental conditions [2,3,46,47,48]. It is necessary to conduct studies on belowground bud banks in different types of grasslands to verify the universality of the “meristem limitation hypothesis” in future studies. At the same time, more types and intensities of environmental factors should be integrated to explore the variation rules of the bud bank on a large geographical spatial scale.
5. Conclusions
The bud bank is the main propagule of alpine meadow plants. In this study, the belowground bud density was high and mainly distributed in the top layer (0–10 cm) sod layer, which indicates that the alpine meadow has a certain degree of self-recovery ability, and mildly degraded meadows can take self-recovery by enclosure. As the moisture content of the alpine sod layer changes, the size and composition of the belowground bud bank change, indicating that the plant adopts different strategies in response to environmental changes. The composition and diversity of alpine meadow plant community changed with moisture content, so the plant community dynamics caused by precipitation change could be predicted to a certain extent.
Author Contributions
Conceptualization, P.S.; formal analysis, X.D. and P.S.; funding acquisition, P.S.; investigation, X.D., Z.Z., and R.S.; methodology, X.D., Z.Z., and R.S.; resources, P.S. and Z.Z.; software, X.D. and R.S.; supervision, P.S.; writing—original draft, X.D., Z.Z., and R.S.; Writing—review and editing, X.D. and P.S.
Funding
This research was funded by the Strategic Priority Research Program of Chinese Academy of Sciences (XDA20050102), the National Natural Science Foundation of China (41701106, 41871043), and the People’s Livelihood Sciences and Technology Project of Gansu, China (17CX1FP076).
Acknowledgments
We would like to thank the Zoige Plateau Wetlands Ecosystem Research Station for help with the experimental conditions. We thank anonymous reviewers and the editor of the journal for their constructive comments and suggestions to the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, X.S.; Deng, Z.M.; Xie, Y.H.; Li, F.; Hou, Z.Y.; Li, X. Belowground bud banks of four dominant macrophytes along a small-scale elevational gradient in Dongting Lake wetlands, China. Aquat. Bot. 2015, 122, 9–14. [Google Scholar] [CrossRef]
- Benson, E.J.; Hartnett, D.C.; Mann, K.H. Belowground bud banks and meristem limitation in tallgrass prairieplant populations. Am. J. Bot. 2004, 91, 416–421. [Google Scholar] [CrossRef]
- Dalgleish, H.J.; Hartnett, D.C. The effects of fire frequency and grazing on tallgrass prairie productivity and plant composition are mediated through bud bank demography. Plant Ecol. 2009, 201, 411–420. [Google Scholar] [CrossRef]
- Harper, J.L. Population Biology of Plants; Academic Press: New York, NY, USA, 1977. [Google Scholar]
- Qian, J.Q.; Wang, Z.W.; Liu, Z.M.; Busso, C.A. Belowground bud bank responses to grazing intensity in the Inner-Mongolia Steppe, China. Land Degrad. Dev. 2014, 28, 822–832. [Google Scholar] [CrossRef]
- Klimešov, J.; Herben, T. Clonal and bud bank traits: Patterns across temperate plant communities. J. Veg. Sci. 2015, 26, 243–253. [Google Scholar] [CrossRef]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J.; Lamont, B.B.; Ojeda, F.; Burrows, G.E.; Enright, N.J.; Knox, K.J.E. Resprouting as a key functional trait: How buds, protection and resources drive persistence after fire. New Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, C.; Pang, X.P.; Jin, S.H.; Zhang, J.; Guo, Z.G. The disturbance and disturbance intensity of small and semi-fossorial herbivores alter the belowground bud density of graminoids in alpine meadows. Ecol. Eng. 2018, 113, 35–42. [Google Scholar] [CrossRef]
- Müllerová, V.; Hejcman, M.; Hejcmanová, P.; Pavlů, V. Effect of fertilizer application on Urtica dioica and its element concentrations in a cut grassland. Acta Oecol. 2014, 59, 1–6. [Google Scholar] [CrossRef]
- Dalgleish, H.J.; Hartnett, D.C. Below-ground bud banks increase along a precipitation gradient of the North American Great Plains:a test of the meristem limitation hypothesis. New Phytol. 2006, 171, 81–89. [Google Scholar] [CrossRef]
- Wellstein, C.; Kuss, P. Diversity and frequency of clonal traits along natural and land-use gradients in grasslands of the Swiss Alps. Folia Geobot. 2011, 46, 255–270. [Google Scholar] [CrossRef]
- Pausas, J.G.; Lamont, B.B.; Paula, S.; Appezzato-da-Glória, B.; Fidelis, A. Unearthing belowground bud banks in fire-prone ecosystems. New Phytol. 2018, 217, 1435–1448. [Google Scholar] [CrossRef]
- Ma, H.Y.; Liang, Z.W.; Lü, B.S.; Yang, H.Y.; Wang, S.H. Advances in research on the seed bank of a saline-alkali meadow in the Songnen Plain. Acta Ecol. Sin. 2012, 32, 4261–4269. (In Chinese) [Google Scholar]
- Yang, Y.F.; Li, J.D. Vegetative propagation of some rhizomatous on Song-Plain and there population stability. Acta Pratacult. Sin. 1996, 2, 43–48. (In Chinese) [Google Scholar]
- Van Groenendael, J.M.; Klimeš, L.; Klimešov, J.; Hendriks, R.J.J. Comparative ecology of clonal plants. Philos. Trans. R. Soc. Lond. B 1996, 351, 1331–1339. [Google Scholar]
- Moola, F.M.; Vasseur, L. The importance of clonal growth to the recovery of Gaultheria procumbens L. (Ericaceae) after forest disturbance. Plant Ecol. 2008, 201, 319–337. [Google Scholar] [CrossRef]
- Klimešová, J.; Klimeš, L. Bud banks and their role in vegetative regeneration—A literature review and proposal for simple classification and assessment. Perspect. Plant Ecol. 2007, 8, 115–129. [Google Scholar] [CrossRef]
- Yu, F.H.; Dong, M.; Krüsi, B. Clonal integration helps Psammochloa villosa survive sand burial in an inland dune. New Phytol. 2004, 162, 697–704. [Google Scholar] [CrossRef]
- Carter, D.L.; Vanderweide, B.L.; Blair, J.M. Drought-mediated stem and belowground bud dynamics in restored grasslands. Appl. Veg. Sci. 2012, 15, 470–478. [Google Scholar] [CrossRef]
- Zhang, D.M.; Zhao, W.Z.; Luo, W.C. Effect of the population density on belowground bud bank of a rhizomatous clonal plant Leymus secalinus in Mu Us sandy land. J. Plant Res. 2019, 132, 69–80. [Google Scholar] [CrossRef]
- Zhao, J.; Xiang, L.; Li, R.; Tian, L.; Tao, Z. Effect of grazing exclusion on ecosystem respiration among three different alpine grasslands on the central Tibetan Plateau. Ecol. Eng. 2016, 94, 599–607. [Google Scholar] [CrossRef]
- VanderWeide, B.L.; Hartnett, D.C. Belowground bud bank response to grazing under severe, short-term drought. Oecologia 2015, 178, 795–806. [Google Scholar] [CrossRef]
- Su, P.X.; Zhou, Z.J.; Shi, R.; Xie, T.T. Variation in basic properties and carbon sequestration capacity of an alpine sod layer along moisture and elevation gradients. Acta Ecol. Sin. 2018, 38, 1040–1052. (In Chinese) [Google Scholar]
- Zhang, W.J.; Zhou, Q.X.; Wei, W. Mechanisms of restoration of degraded ecosystem by clonal plants. Acta Agrestia Sin. 2016, 24, 485–490. [Google Scholar]
- Qian, J.Q.; Wang, Z.W.; Klimešov, J.; Lü, X.T.; Kuang, W.N.; Liu, Z.M.; Han, X.G. Differences in below-ground bud bank density and composition along a climatic gradient in the temperate steppe of northern China. Ann. Bot. 2017, 120, 755–764. [Google Scholar] [CrossRef]
- Su, P.; Shi, R.; Zhou, Z.; Xie, T.T. Characteristics and relationships of foliar element content and specific leaf volume of alpine plant functional groups. Int. J. Agric. Biol. 2018, 20, 1663–1671. [Google Scholar]
- Guo, X.; Du, W.; Wang, X.; Yang, Z. Degradation and structure change of humic acids corresponding to water decline in Zoige Peatland, Qinghai-Tibet Plateau. Sci. Total Environ. 2013, 445, 231–236. [Google Scholar] [CrossRef]
- Tang, J.; Ding, X.; Wang, L.; Xu, Q.; Yang, Z.; Zhao, J.; Sun, Q.; Feng, S.; Zhang, J. Effects of wetland degradation on bacterial community in the Zoige Wetland of Qinghai-Tibetan Plateau (China). World Microb. Biot. 2012, 28, 649–657. [Google Scholar] [CrossRef]
- Hector, A.; Schmid, B.; Beierkuhnlein, C.; Caldeira, M.C.; Diemer, M.; Dimitrakopoulos, P.G.; Finn, J.A.; Freitas, H.; Giller, P.S.; Good, J.; et al. Plant diversity and productivity experiments in european grasslands. Science 1999, 286, 1123–1127. [Google Scholar] [CrossRef]
- Signorelli, S.; Agudelo-Romero, P.; Foyer, C.H.; Considine, M.J.; Foyer, C.H.; Considine, M.J. Roles for light, energy and oxygen in the fate of quiescent axillary buds. Plant Physiol. 2017, 176, 1171–1181. [Google Scholar] [CrossRef]
- Vesk, P.A.; Westoby, M. Funding the bud bank: A review of the costs of buds. Oikos 2004, 106, 200–208. [Google Scholar] [CrossRef]
- Vesk, P.A.; Warton, D.I.; Westoby, M. Sprouting by semi-arid plants: Testing a dichotomy and predictive traits. Oikos 2010, 107, 72–89. [Google Scholar] [CrossRef]
- Klimeš, L.; Klimešov, J.; Osbornová, J. Regeneration capacity and carbohydrate reserves in a clonal plant Rumex alpinus: Effect of burial. Vegetation 1993, 109, 153–160. [Google Scholar] [CrossRef]
- Hendrickson, J.R.; Briske, D.D. Axillary bud banks of two semiarid perennial grasses: Occurrence, longevity, and contribution to population persistence. Oecologia 1997, 110, 584–591. [Google Scholar] [CrossRef]
- Raju, M.; Steeves, T.; Coupland, R. On the regeneration of root fragments of leafy spurge (Euphorbia esula L.). Weed Res. 1964, 4, 2–11. [Google Scholar] [CrossRef]
- Palacio, S.; Maestro, M.; Montserrat-Martí, G. Relationship between shoot-rooting and root-sprouting abilities and the carbohydrate and nitrogen reserves of Mediterranean dwarf shrubs. Ann. Bot. 2007, 100, 865–874. [Google Scholar] [CrossRef]
- Bai, W.M.; Sun, X.Q.; Wang, Z.W.; Li, L.H. Nitrogen addition and rhizome severing modify clonal growth and reproductive modes of Leymus chinensis population. Plant Ecol. 2009, 205, 13–21. [Google Scholar] [CrossRef]
- Wang, Z.W.; Xu, A.K.; Zhu, T.C. Plasticity in bud demography of a rhizomatous clonal plant Leymus chinensis L. in response to soil water status. J. Plant Biol. 2008, 51, 102–107. [Google Scholar] [CrossRef]
- Ott, J.P.; Hartnett, D.C. Bud bank dynamics and clonal growth strategy in the rhizomatous grass, Pascopyrum smithii. Plant Ecol. 2015, 216, 395–405. [Google Scholar] [CrossRef]
- Connolly, J.; Wayne, P. Assessing determinants of community biomass composition in two-species plant competition studies. Oecologia 2005, 142, 450–457. [Google Scholar] [CrossRef]
- Deng, J.M.; Wang, G.X.; Morris, E.C.; Wei, X.P.; Li, D.X.; Chen, B.M.; Zhang, C.M.; Liu, J.; Wang, Y. Plant mass–density relationship along a moisture gradient in north-west China. J. Ecol. 2006, 94, 953–958. [Google Scholar] [CrossRef]
- Enquist, B.J.; Brown, J.H.; West, G.B. Allometric scaling of plant energetics and population density. Nature 1998, 395, 163–165. [Google Scholar] [CrossRef]
- Obeso, J.R. The costs of reproduction in plants. New Phytol. 2002, 155, 321–348. [Google Scholar] [CrossRef]
- Russell, M.L.; Vermeire, L.T.; Ganguli, A.C.; Hendrickson, J.R. Season of fire manipulates bud bank dynamics in northern mixed-grass prairie. Plant Ecol. 2015, 216, 835–846. [Google Scholar] [CrossRef]
- Harris, D.; Davy, A.J. Regenerative potential of Elymus farctus from rhizome fragments and seed. J. Ecol. 1986, 74, 1057–1067. [Google Scholar] [CrossRef]
- Kleunen, M.V.; Schmid, F.B. Costs of plasticity in foraging characteristics of the clonal plant Ranunculus reptans. Evolution 2000, 54, 1947–1955. [Google Scholar] [CrossRef][Green Version]
- Weber, E. Strong regeneration ability from rhizome fragments in two invasive clonal plants (Solidago canadensis and S. gigantea). Biol. Invasions 2011, 13, 2947–2955. [Google Scholar] [CrossRef]
- Cordazzo, C.V.; Davy, A.J. Vegetative regeneration of Panicum racemosum from rhizome fragments on southern Brazilian coastal dunes. J. Coast. Res. 1999, 15, 520–525. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).