The Key Role of Variety and Method of Sowing Selection in Pea Roots’ Parameters Development under Sustainable Practice
Abstract
1. Introduction
2. Materials and Methods Materials and Methods
2.1. Site Description, Experimental Design, and Sampling
2.2. Ordinal Regression Model
2.3. Statistical Analysis
3. Results
3.1. Model Interpretation
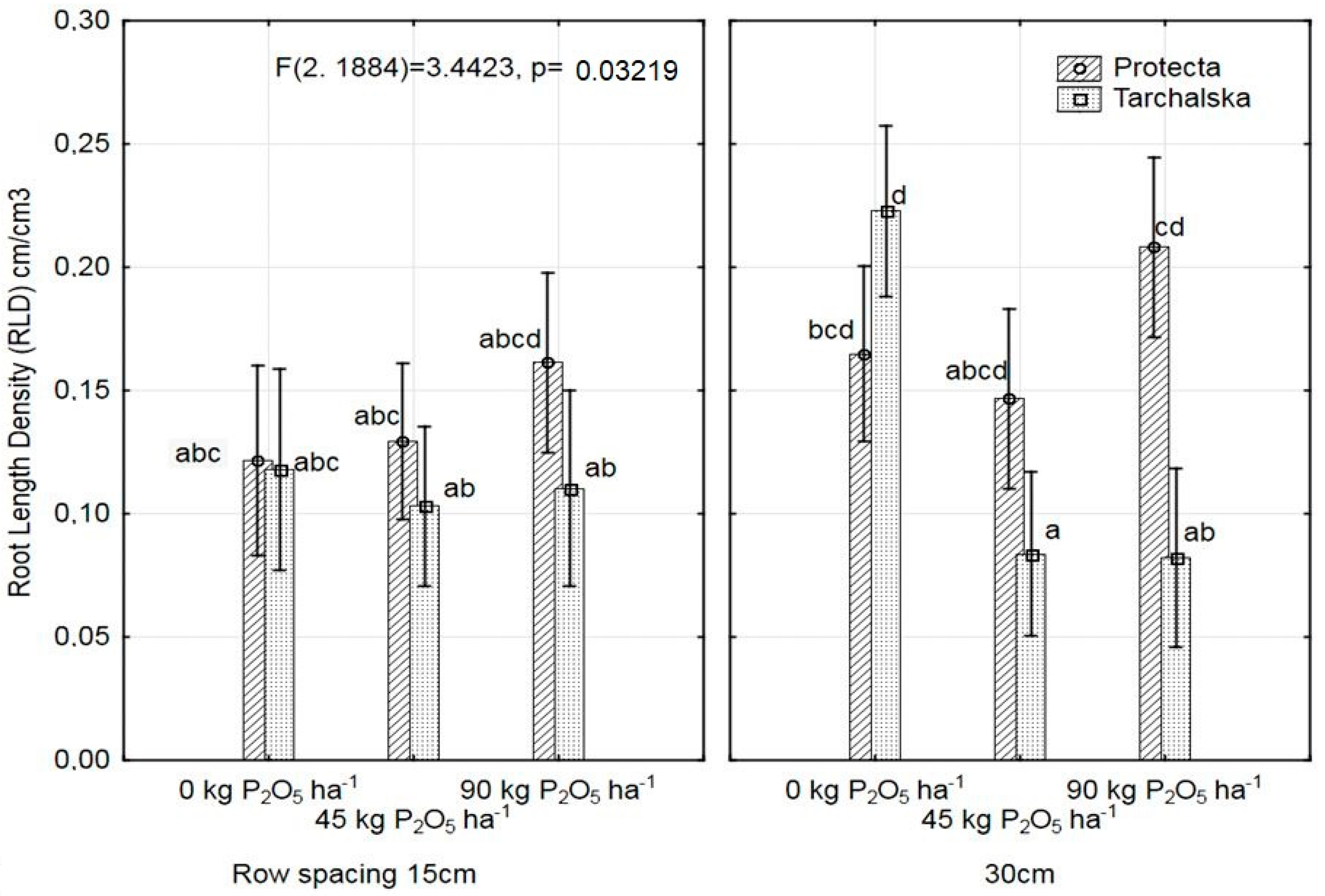
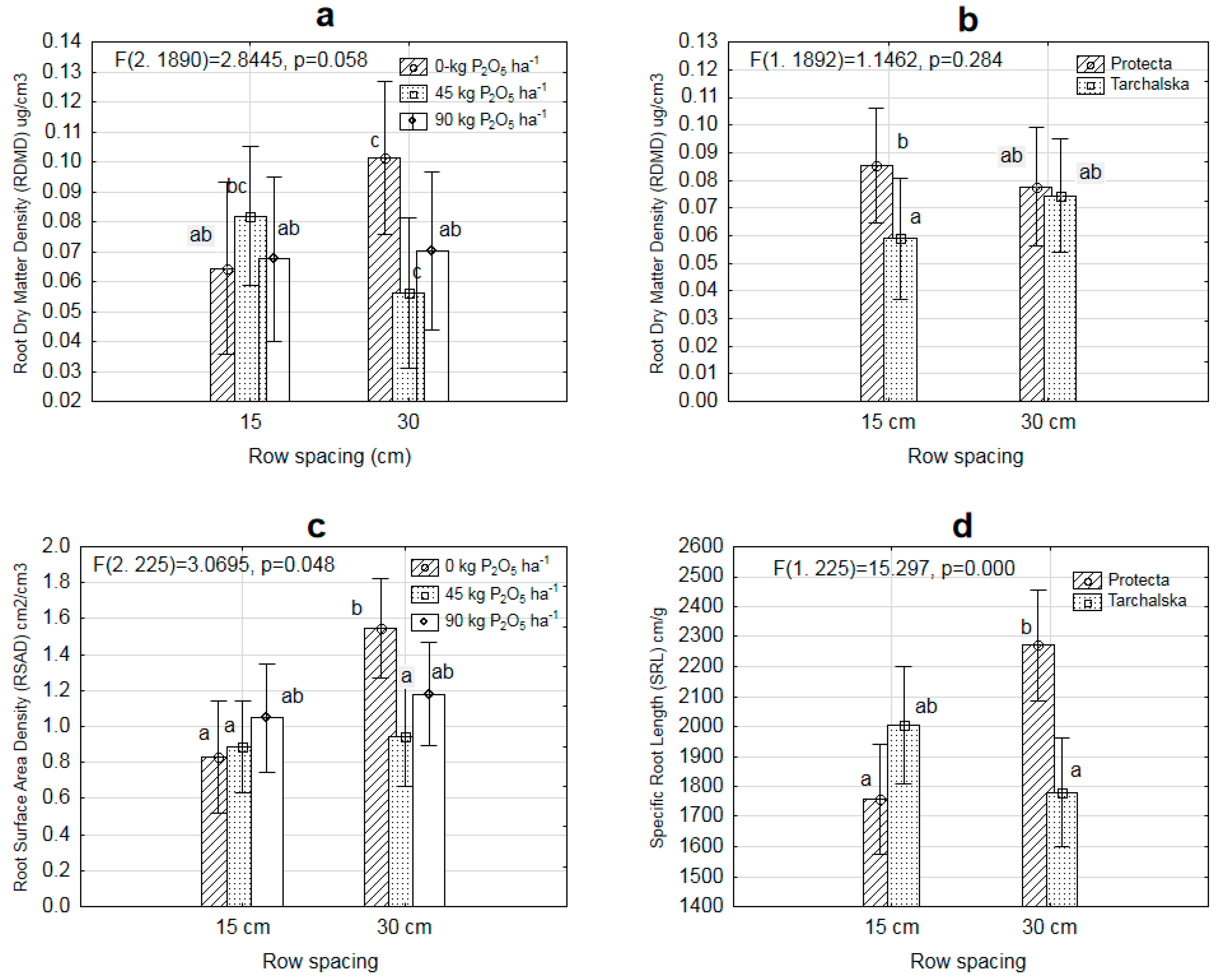
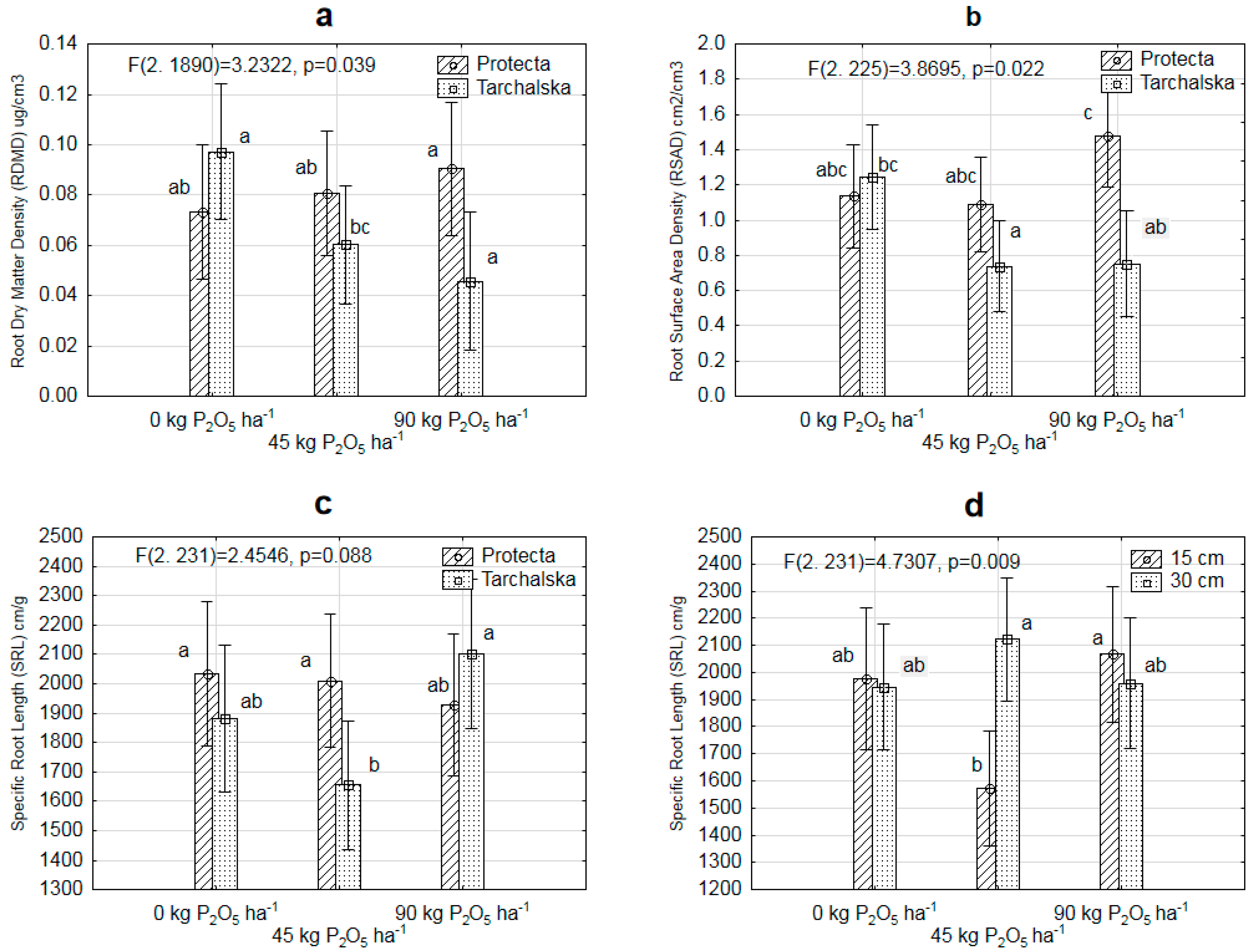
3.2. Diversity in Root Architecture of Two Pea Cultivars Grown under Different Levels of P Supply and Different Row Spacing
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, X.; Wang, Y.; Tian, J.; Lim, B.; Yan, X.; Liao, H. Overexpressing AtPAP15 Enhances phosphorus efficiency in soybean. Plant Physiol. 2009, 151, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.S.; Oki, Y.; Adachi, T. Intraspecific variations of phosphorus absorption and remobilization, P forms, and their internal buffering in Brassica cultivars exposed to a P-stressed environment. J. Integr. Plant Biol. 2008, 50, 703–716. [Google Scholar] [CrossRef]
- Wang, X.; Yan, X.; Liao, H. Genetic improvement for phosphorus efficiency in soybean: A radical approach. Ann. Bot. 2010, 106, 215–222. [Google Scholar] [CrossRef]
- Paez-Garcia, A.; Motes, C.; Scheible, W.R.; Chen, R.; Blancaflor, E.; Monteros, M. Root traits and phenotyping strategies for plant improvement. Plants J. 2015, 4, 334–355. [Google Scholar] [CrossRef]
- Katra, I.; Gross, A.; Swet, N.; Tanner, S.; Krasnov, H.; Angert, A. Substantial dust loss of bioavailable phosphorus from agricultural soils. Sci. Rep. 2016, 6, 24736. [Google Scholar] [CrossRef]
- Manschadi, A.M.; Manske, G.G.B.; Vlek, P.L.G. Root architecture and resource acquisition—Wheat as a Model Plant. In Plant Roots: The Hidden Half, 4th ed.; Eschel, A., Beeckman, T., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2013; pp. 22.1–22.18. [Google Scholar]
- Lynch, J. Root architecture and plant productivity. Plant Physiol. 1995, 109, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Gahoonia, T.S.; Care, D.; Nielsen, N.E. Root hairs and phosphorus acquisition of wheat and barley cultivars. Plant Soil 1997, 191, 181–188. [Google Scholar] [CrossRef]
- Gahoonia, T.S.; Nielsen, N.E. Barley genotypes with long root hairs sustain high grain yields in low-P field. Plant Soil 2004, 262, 55–62. [Google Scholar] [CrossRef]
- Wissuwa, M. How do plants achieve tolerance to phosphorus deficiency: Small causes with big effects. Plant Physiol. 2003, 133, 1947–1958. [Google Scholar] [CrossRef]
- Gibson, D. Methods in Comparative Plant Population Ecology, 2nd ed.; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Jiang, C.F.; Gao, X.H.; Liao, L.; Harberd, N.P.; Fu, X.D. Phosphate starvation root architecture and anthocyanin accumulation responses are modulated by the gibberellin-DELLA signaling pathway in Arabidopsis. Plant Physiol. 2007, 145, 1460–1470. [Google Scholar] [CrossRef]
- Lynch, J.P.; Brown, K.M. Topsoil foraging: An architectural adaptation of plants to low phosphorus availability. Plant Soil 2001, 237, 225–237. [Google Scholar] [CrossRef]
- Farley, R.A.; Fitter, A.H. The responses of seven co-occuring woodland herbaceous perennials to localized nutrient-rich patches. J. Ecol. 1999, 87, 849–859. [Google Scholar] [CrossRef]
- Liao, H.; Rubio, G.; Yan, X.; Cao, A.; Brown, K.M.; Lynch, J.P. Effect of phosphorus availability on basal root shallowness in common bean. Plant Soil 2001, 232, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.; Rosas, J.; Brown, K.; Lynch, J. Root architectural tradeoffs for water and phosporus acquisition. Funct. Plant Biol. 2005, 32, 737–748. [Google Scholar] [CrossRef]
- Walk, T.C.; Jaramillo, R.; Lynch, J.P. Architectural tradeoffs between adventitious and basal roots for phosphorus acquisition. Plant Soil 2006, 279, 347–366. [Google Scholar] [CrossRef]
- Ma, Z.; Baskin, T.I.; Brown, K.M.; Lynch, J.P. Regulation of root elongation under phosphorus stress involves changes in ethylene responsiveness. Plant Physiol. 2003, 131, 1381–1390. [Google Scholar] [CrossRef]
- Borch, K.; Bouma, T.; Lynch, J.P.; Brown, K.M. Ethylene: A regulator of root architectural responses to soil phosphorus availability. Plant Cell Environ. 1999, 22, 425–431. [Google Scholar] [CrossRef]
- Callaway, M.M.; Dawson, T.E.; Richards, J.H. Hydraulic lift: Consequences of water efflux from the roots of plants. Oecologia 1998, 113, 151–161. [Google Scholar]
- Lynch, J.P.; Ho, M.D. Rhizoeconomics: Carbon costs of phosphorus acquisition. Plant Soil 2005, 269, 45–56. [Google Scholar] [CrossRef]
- Nord, E.A.; Zhang, C.; Lynch, J.P. Root responses to neighbouring plants in common bean are mediated by nutrient concentration rather than self/non-self recognition. Funct. Plant Biol. 2011, 38, 941–952. [Google Scholar] [CrossRef]
- Yang, X.; Post, W.M.; Thornton, P.E.; Jain, A. The distribution of soil phosphorus for global biogeochemical modeling. Biogeosciences 2013, 10, 2525–2537. [Google Scholar] [CrossRef]
- Miguel, M.A.; Widrig, A.; Vieira, R.F.; Brown, K.M.; Lynch, J.P. Basal root whorl number: A modulator of phosphorus acquisition in common bean (Phaseolus vulgaris). Ann. Bot. 2013, 112, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Klimek-Kopyra, A.; Rębilas, K. Dependence of pea root mass distribution on weather conditions under varying levels of phosphorus application. Int. Agrophys. 2018, 32, 365–372. [Google Scholar] [CrossRef]
- Klimek Kopyra, A.; Strojny, J.; Zając, T.; Ślizowska, A.; Klimesova, J.; Neugschwandtner, R.W. Ordinal regression model for pea seed mass. Ein ordinales Regressionsmodell für das Samengewicht von Erbse. Die Bodenkul. J. Land Manag. Food Environ. 2017, 68, 81–87. [Google Scholar] [CrossRef]
- McCullagh, P.; Nelder, J.A. Generalized Linear Models, 2nd ed.; Chapman and Hall: London, UK, 1989. [Google Scholar]
- Schneider, K.; Turrion, M.B.; Gallardo, J.F. A modified method to measure acid phosphatase activities in forest soils with high organic matter content. Commun. Soil Sci. Plant Anal. 2000, 31, 1–17. [Google Scholar] [CrossRef]
- Grossman, J.D.; Rice, K.J. Evolution of root plasticity responses to variation in soil nutrient distribution and concenstration. Evol. Appl. 2012, 5, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Thalji, T. Impacts of row spacing on faba bean L. growth under Mediterranean rainfed conditions. J. Agron. 2006, 5, 527–532. [Google Scholar]
- Ndor, E.; Dauda, N.S.; Abimuku, E.O.; Azagaku, D.E.; Anzaku, H. Effect of phosphorus fertilizer and spacing on growth, nodulation count and yield of cowpea (Vigna unguiculata (L) Walp) in Southern Guinea Savanna Agroecological Zone, Nigeria. Asian J. Agric. Sci. 2012, 4, 254–257. [Google Scholar]
- Zhu, J.M.; Lynch, J.P. The contribution of lateral rooting to phosphorus acquisition efficiency in maize (Zea mays) seedlings. Funct. Plant Biol. 2004, 31, 949–958. [Google Scholar] [CrossRef]
- White, P.J.; Hammond, J.P. The Ecophysiology of Plant-Phosphorus Interactions; Springer: Berlin, Germany, 2008. [Google Scholar]
| Model | −2 Log Likelihood | Chi-Square | df | Sig. |
|---|---|---|---|---|
| Intercept Only | 655.636 | |||
| Final Model | 0.000 | 655.636 | 8 | 0.000 |
| Model Component | Variable Level | Estimate | Std. Error | Wald | df | Sig. | 95% Confidence Interval | |
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| Threshold | RDMD = 1 | −2.690 | 1.157 | 5.410 | 1 | 0.020 | −4.957 | −0.423 |
| RDMD = 2 | 6.001 | 1.264 | 22.539 | 1 | 0.000 | 3.524 | 8.478 | |
| RDMD = 3 | 11.738 | 1.669 | 49.461 | 1 | 0.000 | 8.467 | 15.009 | |
| Location | Fertilization-0 | 1.001 | 0.588 | 2.893 | 1 | 0.089 | −0.152 | 2.154 |
| Fertilization-45 | −0.006 | 0.564 | 0.011 | 1 | 0.916 | −1.166 | 1.047 | |
| Fertilization-90 | 0(a) | . | . | 0 | . | . | . | |
| row spacing = 15 | 2.042 | 0.506 | 16.262 | 1 | 0.000 | 1.049 | 3.034 | |
| row spacing = 30 | 0(a) | . | . | 0 | . | . | . | |
| RSAD | 8.055 | 2.108 | 14.604 | 1 | 0.000 | 3.924 | 12.187 | |
| RLD | 3.202 | 2.215 | 2.089 | 1 | 0.148 | −1.140 | 7.543 | |
| MRD | 17.171 | 3.470 | 24.492 | 0.000 | 10.370 | 23.971 | ||
| SRL | −0.007 | 0.001 | 66.230 | 1 | 0.000 | −0.009 | −0.005 | |
| Cv. Tarchalska | 1.199 | 0.571 | 4.407 | 1 | 0.036 | 0.008 | 2.318 | |
| Cv. Proctecta | 0(a) | . | . | 0 | . | . | . | |
| Treatments | RLD cm/cm3 | RDMD ěg/cm3 | RSAD cm2/cm3 | MRD cm | SRL cm/g | |
|---|---|---|---|---|---|---|
| Cultivars | Protecta | 0.154b | 0. 6b | 1.228b | 0.266 | 1991 |
| Tarchalska | 0.120a | 0. 5a | 0.918a | 0.262 | 1856 | |
| Phosphoruslevel (kg P2O5 ha−1) | 0 | 0.161b | 0. 74 | 0.237 | 0.276 | 1958 |
| 45 | 0.114a | 0. 63 | 0.901 | 0.271 | 1826 | |
| 90 | 0.142ab | 0. 53 | 1.113 | 0.244 | 2010 | |
| Row spacing | 15 | 0.124a | 0. 53 | 0.927a | 0.273 | 1833 |
| 30 | 0.150b | 0. 64 | 1.215b | 0.257 | 2011 | |
| Treatments | RLD cm/cm3 | RDMD ěg/cm3 | RSAD cm2/cm3 | MRD cm | SRL cm/g |
|---|---|---|---|---|---|
| Cultivar-C | 0.000 * | 0.007 * | 0.007 * | 0.989 | 0.200 |
| Fertilization-F | 0.004 * | 0.055 | 0.128 | 0.449 | 0.390 |
| Row-R | 0.009 * | 0.317 | 0.011 * | 0.499 | 0.125 |
| C × F | 0.000 * | 0.000 * | 0.022 * | 0.432 | 0.023 * |
| C × R | 0.434 | 0.039 * | 0.475 | 0.378 | 0.000 * |
| F × R | 0.008 * | 0.000 * | 0.048 * | 0.857 | 0.002 * |
| C × F × R | 0.032 * | 0.099 | 0.252 | 0.543 | 0.325 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimek-Kopyra, A.; Głąb, T.; Strojny, J. The Key Role of Variety and Method of Sowing Selection in Pea Roots’ Parameters Development under Sustainable Practice. Sustainability 2019, 11, 1824. https://doi.org/10.3390/su11071824
Klimek-Kopyra A, Głąb T, Strojny J. The Key Role of Variety and Method of Sowing Selection in Pea Roots’ Parameters Development under Sustainable Practice. Sustainability. 2019; 11(7):1824. https://doi.org/10.3390/su11071824
Chicago/Turabian StyleKlimek-Kopyra, Agnieszka, Tomasz Głąb, and Jacek Strojny. 2019. "The Key Role of Variety and Method of Sowing Selection in Pea Roots’ Parameters Development under Sustainable Practice" Sustainability 11, no. 7: 1824. https://doi.org/10.3390/su11071824
APA StyleKlimek-Kopyra, A., Głąb, T., & Strojny, J. (2019). The Key Role of Variety and Method of Sowing Selection in Pea Roots’ Parameters Development under Sustainable Practice. Sustainability, 11(7), 1824. https://doi.org/10.3390/su11071824







