Simple Soil Tests for On-Site Evaluation of Soil Health in Orchards
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Simple Soil Testing Strategies
2.1.1. Experimental Field Sites
2.1.2. Simple Physical Tests
2.1.3. Laboratory Physical Tests
2.1.4. Simple Biological Tests
2.1.5. Laboratory Biological Tests
2.1.6. Simple Chemical Tests
2.1.7. Laboratory Chemical Tests
2.2. Statistical Analysis
2.3. Training Sessions with Growers, and Collection of Feedback
3. Results and Discussion
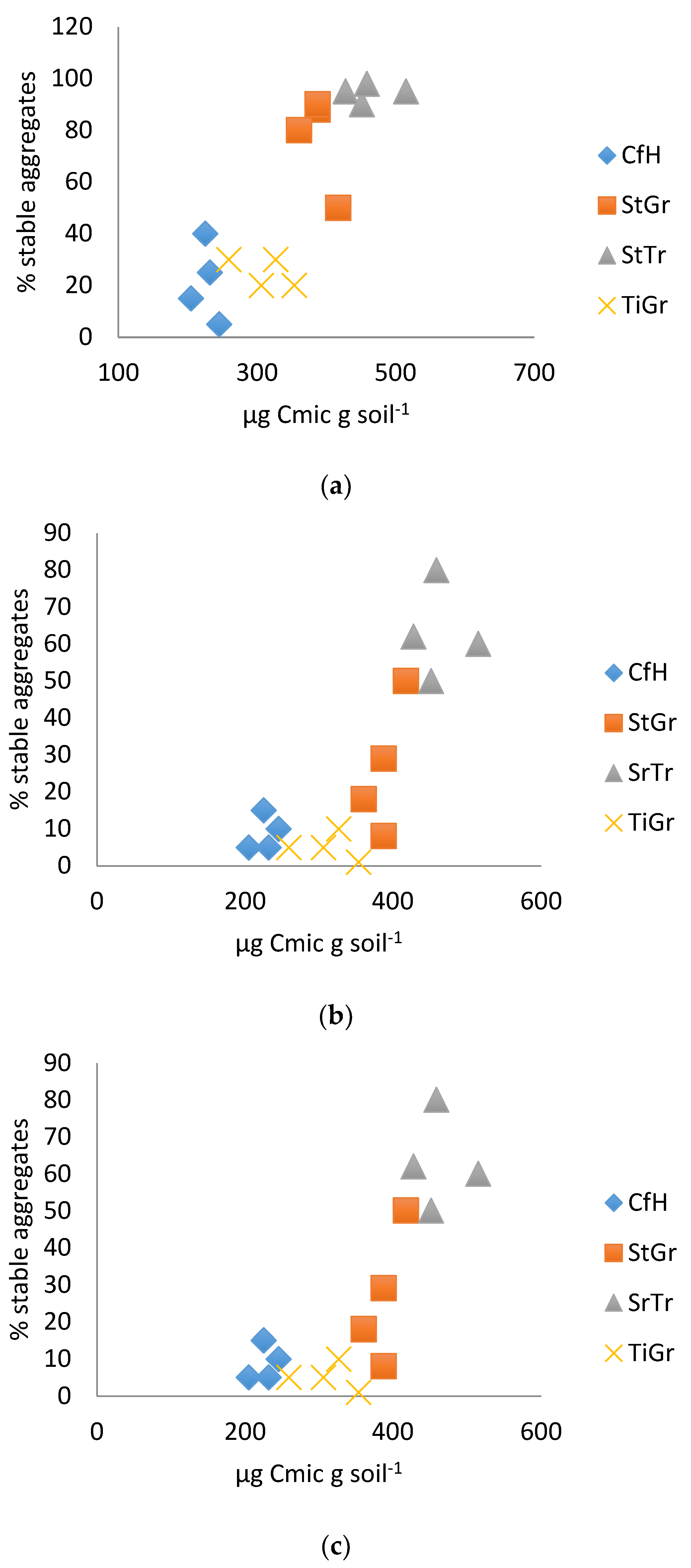
3.1. Physical Tests
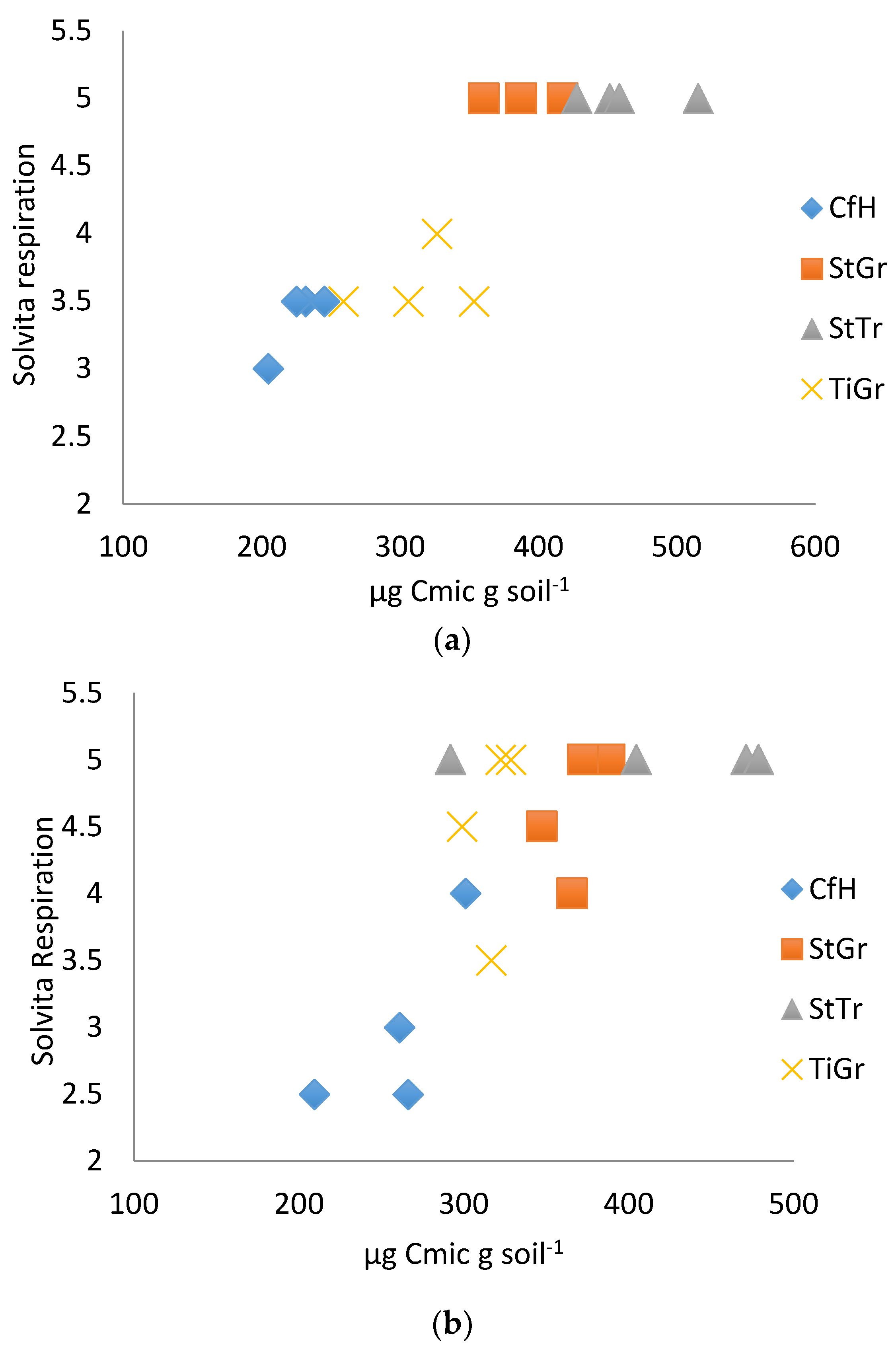
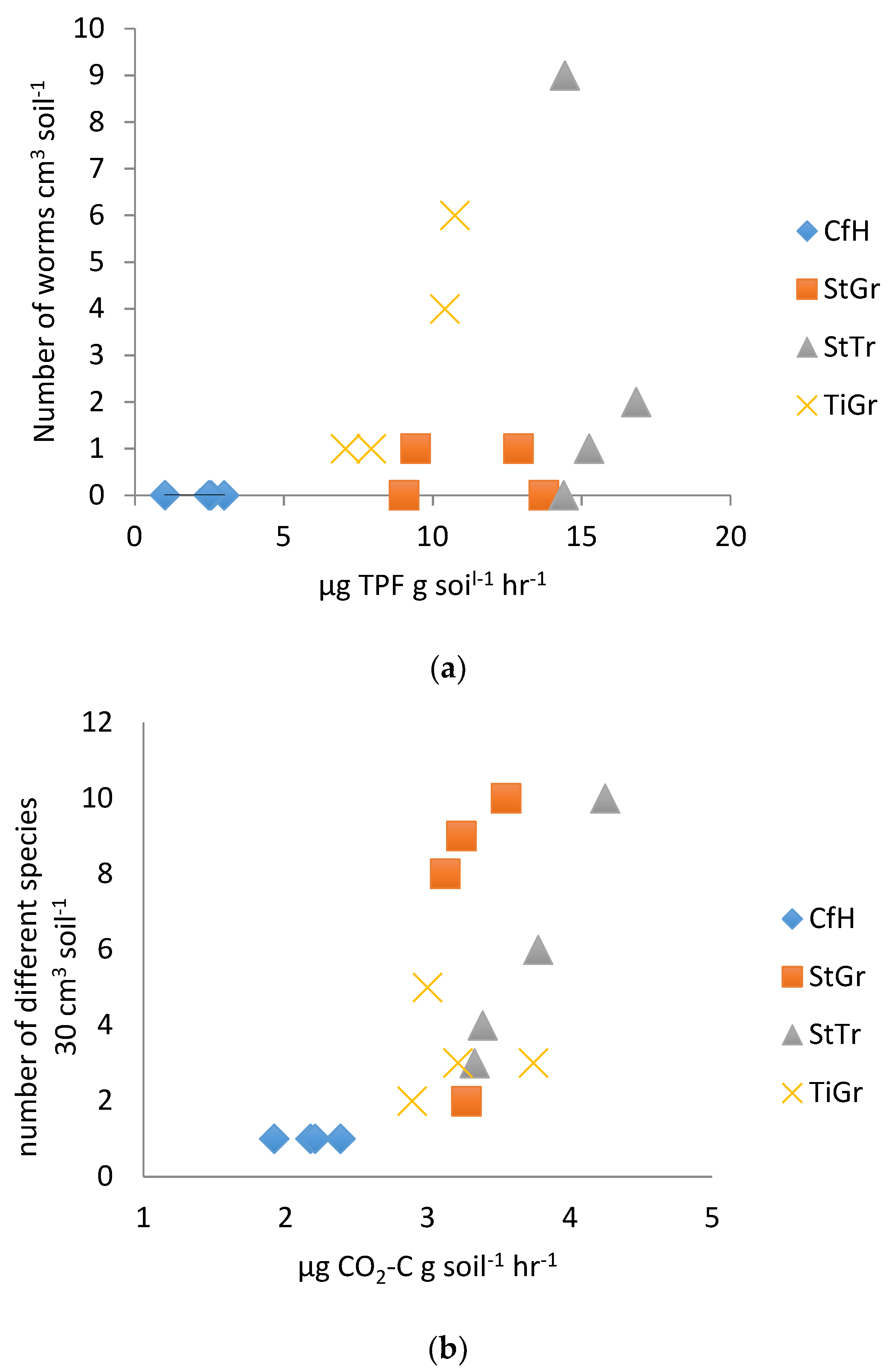
3.2. Biological Test Results
3.3. Chemical Tests
3.4. Grower Feedback
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Doran, J.W.; Parkin, T.B. Defining and assessing soil quality. In Defining Soil Quality for a Sustainable Environment; Doran, J.W., Coleman, D.C., Bezdicek, D.F., Stewart, B.A., Eds.; Soil Society Science of America: Madison, WI, USA, 1994; pp. 3–21. [Google Scholar]
- Harris, R.F.; Bezdicek, D.F. Descriptive aspects of soil quality/health. In Defining Soil Quality for a Sustainable Environment; Doran, J.W., Coleman, D.C., Bezdicek, D.F., Stewart, B.A., Eds.; Soil Society Science of America: Madison, WI, USA, 1994; pp. 23–35. [Google Scholar]
- Wienhold, B.J.; Andrews, S.S.; Karlen, D.L. Soil quality: A review of the science and experiences in the USA. Environ. Geochem. Health 2004, 26, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D.; Harvey, C.; Resosudarmo, P.; Sinclair, K.; Kurz, D.; McNair, M.; Crist, S.; Shpritz, L.; Fitton, L.; Safouri, R.; et al. Environmental and economic costs of soil erosion and conservation benefits. Science 1995, 267, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Liebman, M.; Helmers, M.J.; Schulte, L.A.; Chase, C.A. Using biodiversity to link agricultural productivity with environmental quality: Results from three field experiments in Iowa. Renew. Agric. Food Syst. 2013, 28, 115–128. [Google Scholar] [CrossRef]
- Herrick, J.E. Soil quality: An indicator of sustainable land management? Appl. Soil Ecol. 2000, 15, 75–83. [Google Scholar] [CrossRef]
- de Bruyn, L.L.; Andrews, S. Are Australian and United States farmers using soil information for soil health management? Sustainability 2016, 8, 304. [Google Scholar] [CrossRef]
- Friedman, D.; Hubbs, M.; Tugel, A.; Seybold, C.; Sucik, M. Guidelines for Soil Quality Assessment in Conservation Planning; USDA NRCS: Washington, DC, USA, 2001. Available online: https://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs142p2_051259.pdf (accessed on 6 September 2019).
- Solvita®. Soil Health Reference Program–Is your Lab Participating? Available online: https://solvita.com/soil/map/ (accessed on 6 September 2019).
- Cornell Soil Health Testing Laboratory. Comprehensive Assessment of Soil Health. Available online: https://soilhealth.cals.cornell.edu/ (accessed on 6 September 2019).
- Stott, D.E. Recommended Soil Health Indicators and Associated Laboratory Procedures. NRCS Soil Health Technical Note No.450-03. 2019. Available online: https://directives.sc.egov.usda.gov/OpenNonWebContent.aspx?content=43754.wba (accessed on 6 September 2019).
- Kemper, W.D.; Koch., E.J. Aggregate Stability of Soils from the Western Portions of the United States and Canada; USDA Tech. Bull. 1355; U.S. Gov. Print. Office: Washington, DC, USA, 1966.
- Kemper, W.D.; Rosenau, R.C. Aggregate stability and size distribution. In Methods of Soil Analysis, Part 1, 2nd ed.; Physical and Mineralogical Methods-Agronomy Monograph no. 9; Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1986; pp. 425–442. [Google Scholar]
- Diaz-Zorita, M.; Perfect, E.; Grove, J.H. Disruptive methods for assessing soil structure. Soil Till. Res. 2002, 64, 3–22. [Google Scholar] [CrossRef]
- Moncada, P.M.; Gabriels, D.; Cornelis, W.; Lobo, D. Comparing aggregate Stability tests for soil physical quality indicators. Land Degrad. Dev. 2015, 26, 843–852. [Google Scholar] [CrossRef]
- Lal, R.; Shukla, M.K. Principles of Soil Physics; Marcel Dekker: New York, NY, USA, 2004; ISBN 0-8247-5324-0. [Google Scholar]
- Emerson, W.W. A classification of soil aggregates based on their coherence in water. Aust. J. Soil Res. 1967, 5, 47–57. [Google Scholar] [CrossRef]
- Bruce-Okine, E.; Lal, R. Soil erodibility as determined by the raindrop technique. Soil Sci. 1975, 119, 149–157. [Google Scholar] [CrossRef]
- Yoder, R.E. A direct method of aggregate analysis and a study of the physical nature of erosion losses. J. Am. Soc. Agron. 1936, 28, 337–351. [Google Scholar] [CrossRef]
- Herrick, J.E.; Whitford, W.G.; de Soyza, A.G.; Van Zee, J.W.; Havstad, K.M.; Seybold, C.A.; Walton, M. Field soil aggregate stability kit for soil quality and rangeland health evaluations. Catena 2001, 44, 27–35. [Google Scholar] [CrossRef]
- Pankhurst, C.E.; Doube, B.M.; Gupta, V.V.S.R. Biological indicators of soil health: Synthesis. In Biological Indicators of Soil Health; Pankhurst, C.E., Doube, B.M., Gupta, V.V.S.R., Eds.; CAB International: New York, NY, USA, 1997; pp. 419–435. [Google Scholar]
- Guillard, K.; McIntosh, P.; Brinton, W. Solvita® Soil Test Kits to Categorize Turfgrass Site Responsiveness to Nitrogen Fertilization; 2014 Annual Turfgrass Research Report; University of Connecticut: Storrs, CT, USA, 2015. [Google Scholar]
- Keuskamp, J.A.; Dingemans, B.J.J.; Lehtinen, T.; Sarneel, J.M.; Hefting, M.M. Tea Bag Index: A novel approach to collect uniform decomposition data across ecosystems. Method Ecol. Evol. 2013, 4, 1070–1075. [Google Scholar] [CrossRef]
- Fauvel, G. Diversity of Heteroptera in agroecosystems: Role of sustainability and bioindication. Agric. Ecosyst. Environ. 1999, 74, 275–303. [Google Scholar] [CrossRef]
- Larink, O. Springtails and mites: Important knots in the food web of soils. In Fauna in Soil Ecosystems: Recycling Processes, Nutrient Fluxes, and Agricultural Production; Benckiser, G., Dekker, M., Eds.; CRC Press: New York, NY, USA, 1997; pp. 225–264. [Google Scholar]
- Hopkin, S.P. Biology of the Springtails (Insecta: Collembola); Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Macfadyen, A. Notes on methods for the extraction of small soil arthropods. J. Anim. Ecol. 1953, 22, 65–77. [Google Scholar] [CrossRef]
- Macfadyen, A. Improved funnel-type extractors for soil arthropods. J. Anim. Ecol. 1961, 30, 171–184. [Google Scholar] [CrossRef]
- Sabu, T.K.; Shiju, R.T. Efficacy of pitfall trapping, Winkler and Berlese extraction methods for measuring ground-dwelling arthropods in moist-deciduous forests in the Western Ghats. J. Insect Sci. 2010, 10, 98. [Google Scholar] [CrossRef]
- Saunders, L.G. Methods for studying forcipomyia midges, with special reference to cacao-pollinating species (diptera, ceratopogonidae). Can. J. Zool. 1959, 27, 33–51. [Google Scholar] [CrossRef]
- Norton, R.A.; Kethley, J.B. A collapsible, full-sized Berlese-funnel system. Entomol. News 1988, 991, 41–47. [Google Scholar]
- Culumber, C.M.; Reeve, J.R.; Black, B.L.; Ransom, C.V.; Alston, D.G. Organic orchard floor management impact on soil quality indicators: Nutrient fluxes, microbial biomass and activity. Nutr. Cycl. Agroecosyst. 2019, 115, 101–115. [Google Scholar] [CrossRef]
- Reeve, J.R.; Culumber, C.M.; Black, B.L.; Tebeau, A.S.; Ransom, C.V.; Rowley, M.A.; Alston, D.G.; Lindstrom, T. Establishing peach trees for organic production in Utah and the Intermountain West. Sci. Hort. 2017, 214, 242–251. [Google Scholar] [CrossRef]
- NRCS. Soil Quality Test Kit Guide; United States Department of Agriculture: Washington, DC, USA, 2001. Available online: https://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs142p2_050956.pdf (accessed on 14 September 2019).
- Anderson, J.P.E.; Domsch, K.H. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Davidson, E.A.; Galloway, L.F.; Strand, M.K. Assessing available carbon: Comparison of techniques across selected forest soils 1. Commun. Soil Sci. Plan. 1987, 18, 45–64. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis, Part 2. Microbiological and Biochemical Properties; SSSA Book Series, no. 5, 775-833; Bigham, J.M., Ed.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 778–826. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; U. S. Department of Agriculture Circular No. 939; Banderis, A.D., Barter, D.H., Anderson, K., Eds.; Agricultural and Advisor: Washington, DC, USA, 1954.
- Paul, B.K.; Vanlauwe, B.; Ayuke, F.; Gassner, A.; Hoogmoed, M.; Hurisso, T.T.; Pulleman, M.M. Medium-term impact of tillage and residue management on soil aggregate stability, soil carbon and crop productivity. Agric. Ecosyst. Environ. 2013, 164, 14–22. [Google Scholar] [CrossRef]
- Beare, M.H.; Bruce, R.R. A comparison of methods for measuring water-stable aggregates: Implications for determining environmental effects on soil structure. Geoderma 1993, 56, 87–104. [Google Scholar] [CrossRef]
- Kemper, W.D.; Rosenau, R.C. Soil cohesion as affected by time and water content. Soil Sci. Soc. Am. J. 1984, 48, 1001–1006. [Google Scholar] [CrossRef]
- Fernández-Ugalde, O.; Virto, I.; Barré, P.; Gartzia-Bengoetxea, N.; Enrique, A.; Imaz, M.J.; Bescansa, P. Effect of carbonates on the hierarchical model of aggregation in calcareous semi-arid Mediterranean soils. Gerderma 2011, 164, 203–214. [Google Scholar] [CrossRef]
- Kheyrodin, H. Importance of soil quality and soil agricultural indicators. Acad. J. Agric. Res. 2014, 2, 231–238. [Google Scholar] [CrossRef]
- Haney, R.; Brinton, W.; Evans, E. Estimating Soil Carbon, Nitrogen and Phosphorous Mineralization from Short-Term Carbon Dioxide Respiration. Commun. Soil Sci. Plant Anal. 2008, 39, 17–18. [Google Scholar] [CrossRef]
- Pelosi, C.; Bertrand, M.; Thénard, J.; Mougin, C. Earthworms in a 15 years agricultural trial. Appl. Soil Ecol. 2015, 88, 1–8. [Google Scholar] [CrossRef]
- McDaniel, M. What is soil health, how do we measure it, and why the emphasis on soil biology? Proc. Int. Crop Manag. 2017, 32, 193–198. [Google Scholar]
- Griess, P. Bemerkungen zu der Abhandlung der HH. Weselsky und Benedikt ‘Uber einige Azoverbindungen’. Chem. Berichte 1879, 12, 426–428. [Google Scholar] [CrossRef]
- Mulvaney, R.L. Nitrogen–Inorganic forms. In Methods of Soil Analysis: Part 3—Chemical Analysis; Sparks, D., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1123–1184. [Google Scholar]
- Helmke, P.A.; Sparks, D.L. Lithium, Sodium, Potassium, Rubidium, and Cesium. In Methods of Soil Analysis: Part 3—Chemical Analysis; Sparks, D., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 551–574. [Google Scholar]
- Kuo, S. Phosphorus. In Methods of Soil Analysis: Part 3—Chemical Analysis; Sparks, D., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 869–920. [Google Scholar]
| Integrated Orchard | Organic Orchard | ||||
|---|---|---|---|---|---|
| Treatment Code | Fertilizer Treatment | Weed Control Treatment | Treatment Code | Tree-Row | Alleyway |
| CfH | Conventional fertilizer | Herbicide | StGr | Straw | Grass |
| CfHO | Conventional fertilizer | Herbicide | StTr | Straw | Birdsfoot trefoil |
| OfH | Compost + feathermeal | Herbicide | LmGr | Living mulch | Grass |
| CfM | Conventional fertilizer | Paper mulch | LmTr | Living mulch | Birdsfoot trefoil |
| OfM | Compost + feathermeal | Paper mulch | WfGr | Weed fabric | Grass |
| TiGr | Tillage | Grass | |||
| Lab Tests | ||||||
|---|---|---|---|---|---|---|
| Field Tests | DHA 2014 | DHA 2015 | MicC 2014 | MicC 2015 | Machine Slake Test 2014 | Machine Slake Test 2015 |
| Surface test | 0.76 | 0.38 | 0.83 | 0.64 | −0.05 | −0.25 |
| Hose test | 0.73 | 0.26 | 0.80 | 0.42 | 0.04 | −0.15 |
| NRCS test | 0.68 | 0.13 | 0.58 | 0.38 | 0.31 | 0.03 |
| Lab Tests | ||||||
|---|---|---|---|---|---|---|
| Field Tests | DHA 2014 | DHA 2015 | BR 2014 | BR 2015 | Cmic 2014 | Cmic 2015 |
| Solvita® Respiration | 0.83 | 0.74 | 0.64 | 0.81 | 0.88 | 0.70 |
| Earthworm Abundance test | 0.38 | −0.02 | 0.31 | 0.22 | 0.32 | 0.06 |
| Organism Diversity count | 0.31 | 0.35 | 0.35 | 0.68 | 0.33 | 0.55 |
| Total Organism count | 0.48 | 0.30 | 0.65 | 0.56 | 0.49 | 0.40 |
| Berlese Funnel test | 0.43 | 0.22 | 0.29 | 0.68 | 0.48 | 0.55 |
| Simple Tests | Lab Tests | |||||||
|---|---|---|---|---|---|---|---|---|
| Integrated/Conventional Orchard | Organic Orchard | |||||||
| N | P | K | pH | N | P | K | pH | |
| Rapid test 2014 | 0.74 | 0.02 | −0.14 | 0.00 | 0.69 | 0.28 | 0.29 | 0.00 |
| LaMotte 2014 | 0.78 | 0.13 | 0.45 | 0.03 | −0.21 | 0.32 | 0.72 | 0.37 |
| Hanna pH meter 2014 | -- | -- | -- | 0.20 | -- | -- | -- | 0.24 |
| Mosser 2015 | -- | -- | -- | -- | 0.80 | −0.09 | 0.60 | 0.09 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomsen, E.O.; Reeve, J.R.; Culumber, C.M.; Alston, D.G.; Newhall, R.; Cardon, G. Simple Soil Tests for On-Site Evaluation of Soil Health in Orchards. Sustainability 2019, 11, 6009. https://doi.org/10.3390/su11216009
Thomsen EO, Reeve JR, Culumber CM, Alston DG, Newhall R, Cardon G. Simple Soil Tests for On-Site Evaluation of Soil Health in Orchards. Sustainability. 2019; 11(21):6009. https://doi.org/10.3390/su11216009
Chicago/Turabian StyleThomsen, Esther O., Jennifer R. Reeve, Catherine M. Culumber, Diane G. Alston, Robert Newhall, and Grant Cardon. 2019. "Simple Soil Tests for On-Site Evaluation of Soil Health in Orchards" Sustainability 11, no. 21: 6009. https://doi.org/10.3390/su11216009
APA StyleThomsen, E. O., Reeve, J. R., Culumber, C. M., Alston, D. G., Newhall, R., & Cardon, G. (2019). Simple Soil Tests for On-Site Evaluation of Soil Health in Orchards. Sustainability, 11(21), 6009. https://doi.org/10.3390/su11216009




