Effect of Stand Density and Soil Layer on Soil Nutrients of a 37-year-old Cunninghamia lanceolata Plantation in Naxi, Sichuan Province, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Soil and Biomass Sampling
2.3. Statistical Analysis
3. Results
3.1. The Density and Soil Layer Effect of Soil Nutrients
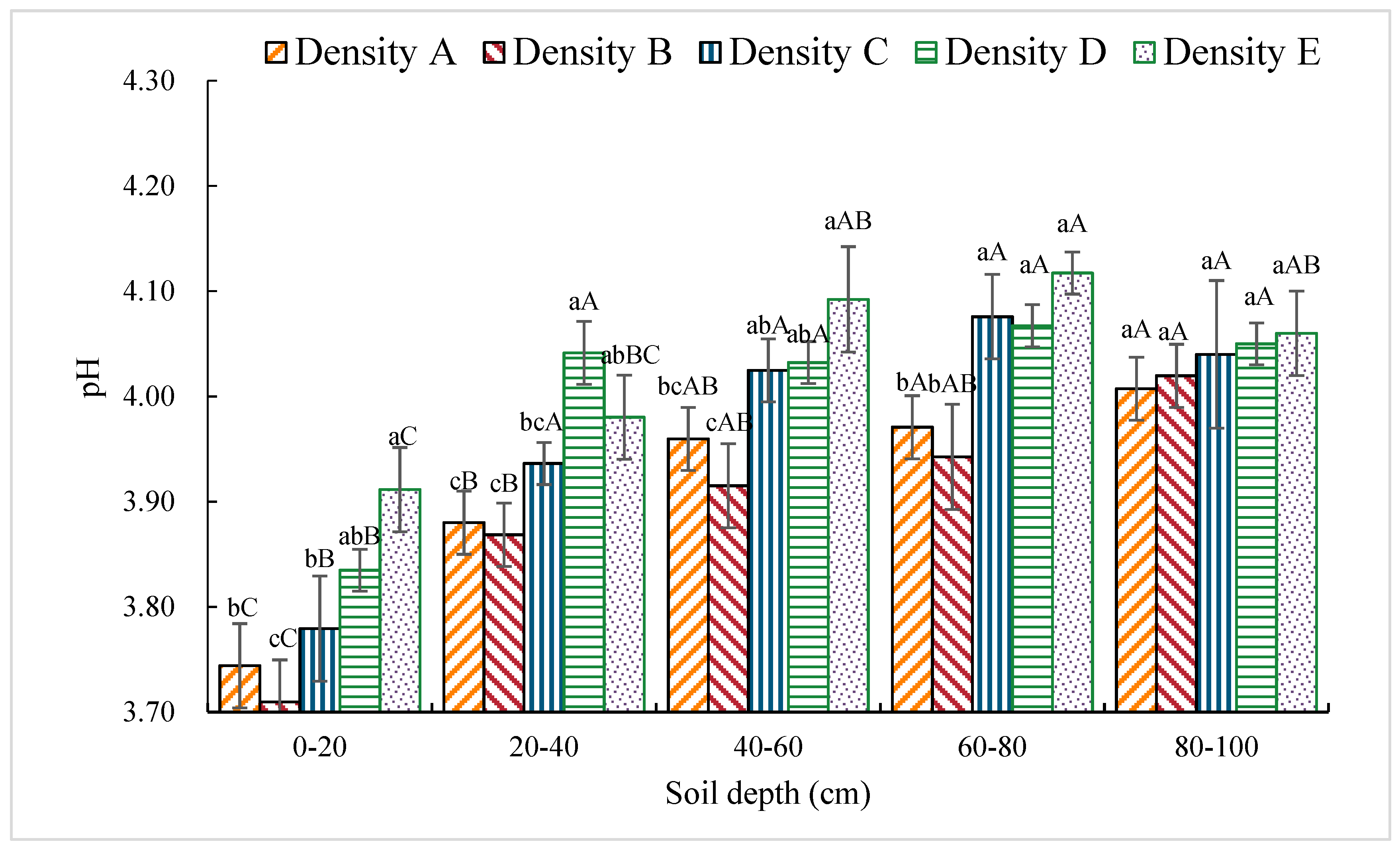
3.1.1. pH
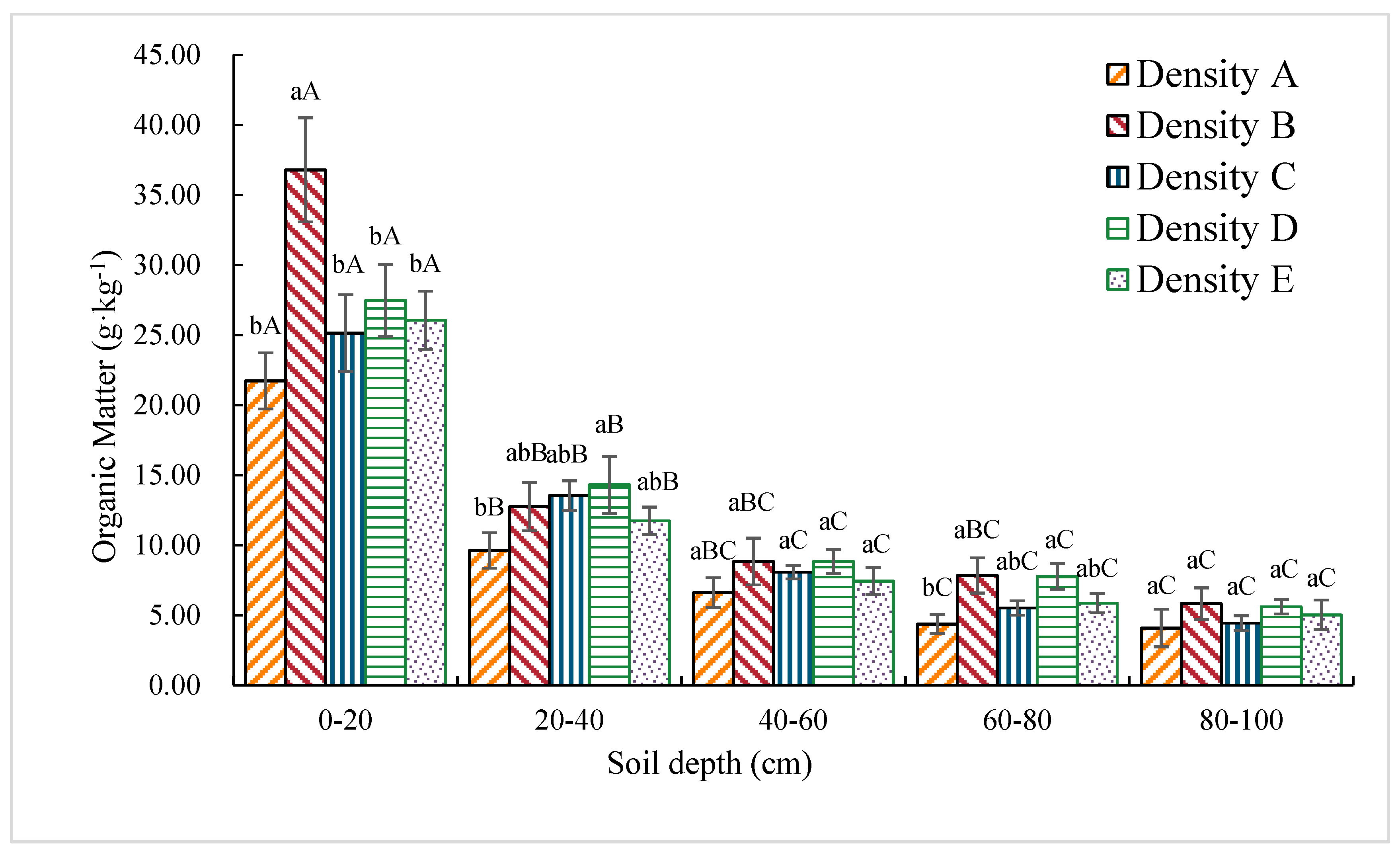
3.1.2. Organic Matter
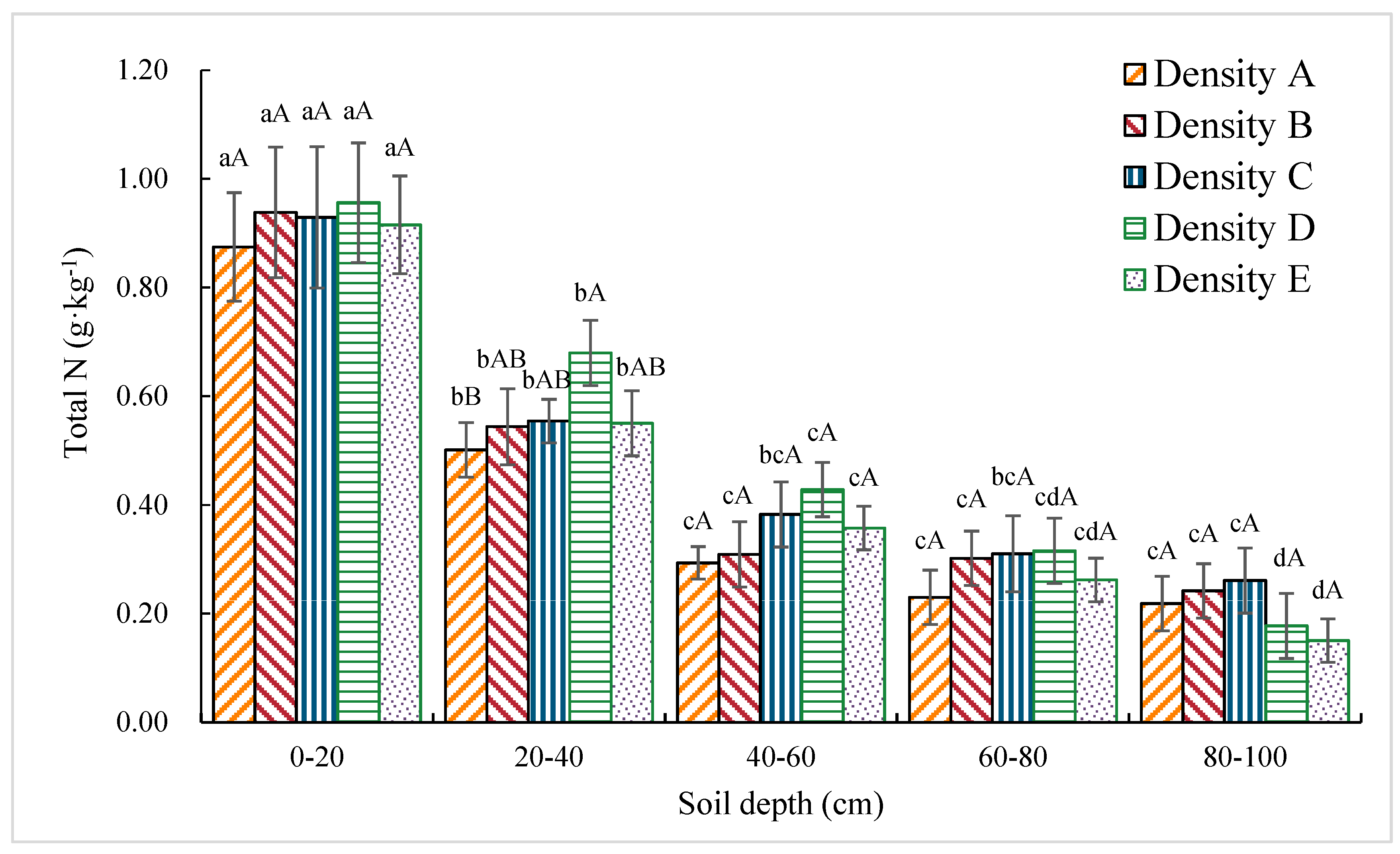
3.1.3. Soil Total N Content
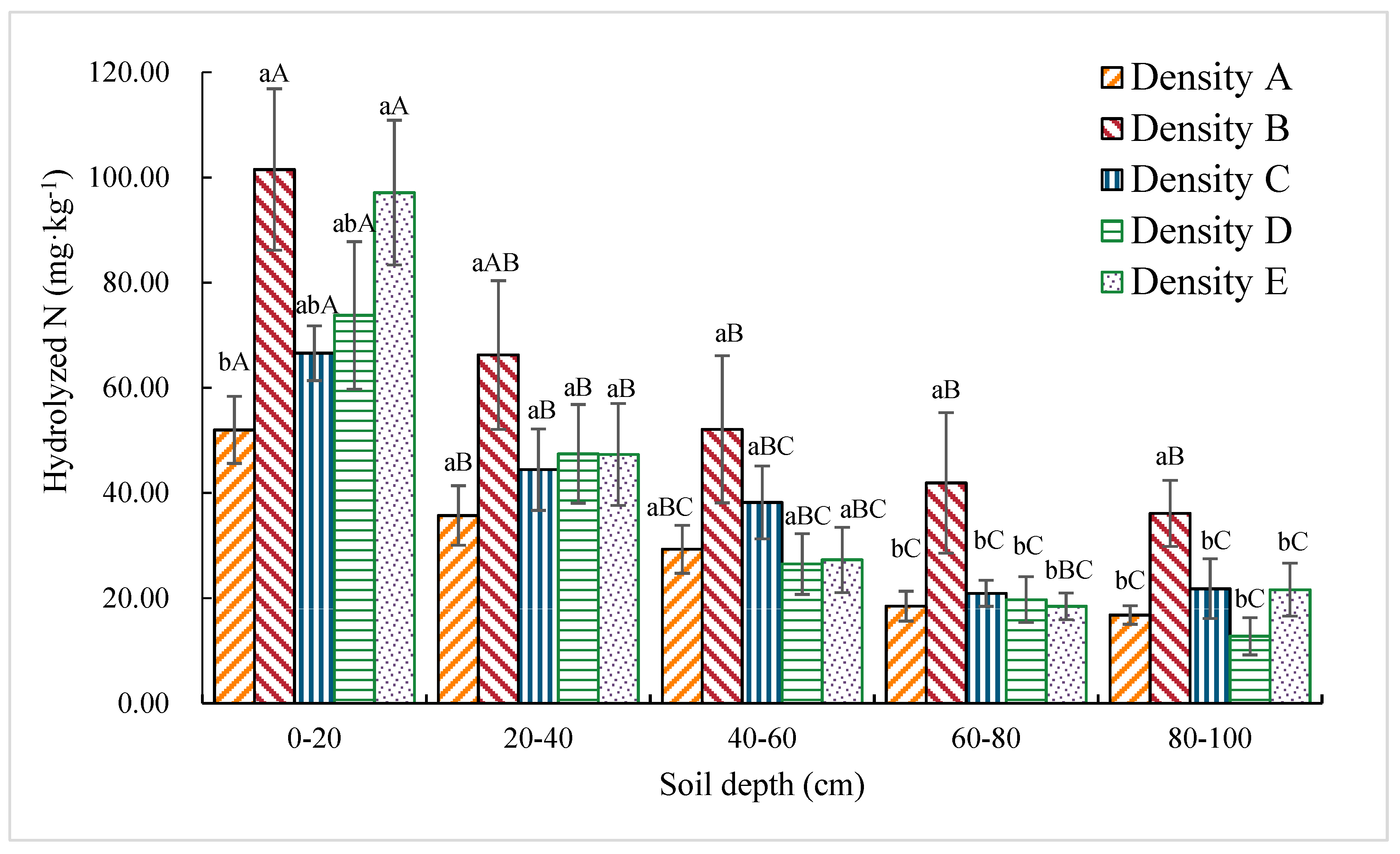
3.1.4. Hydrolytic N Content
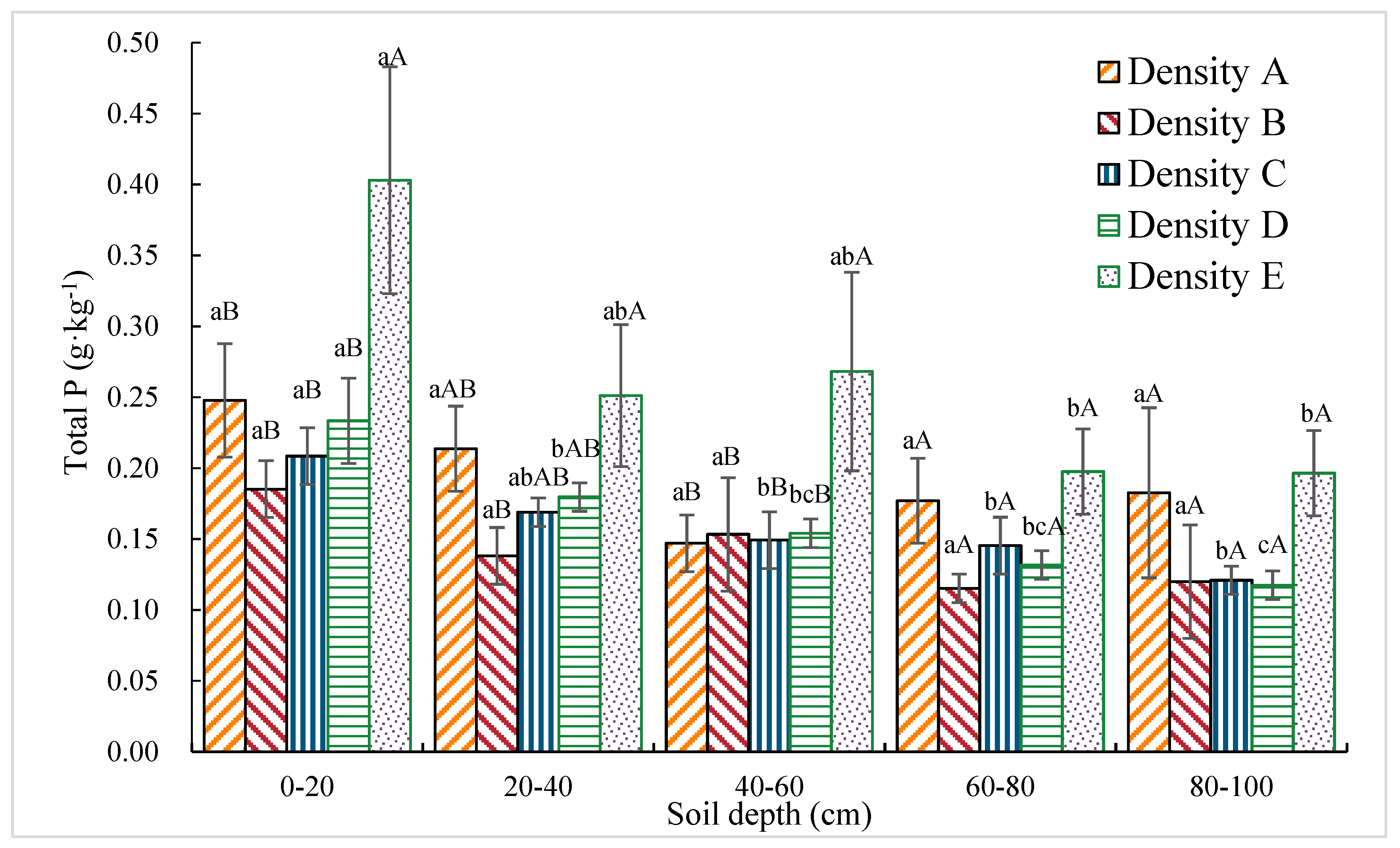
3.1.5. Total P Content
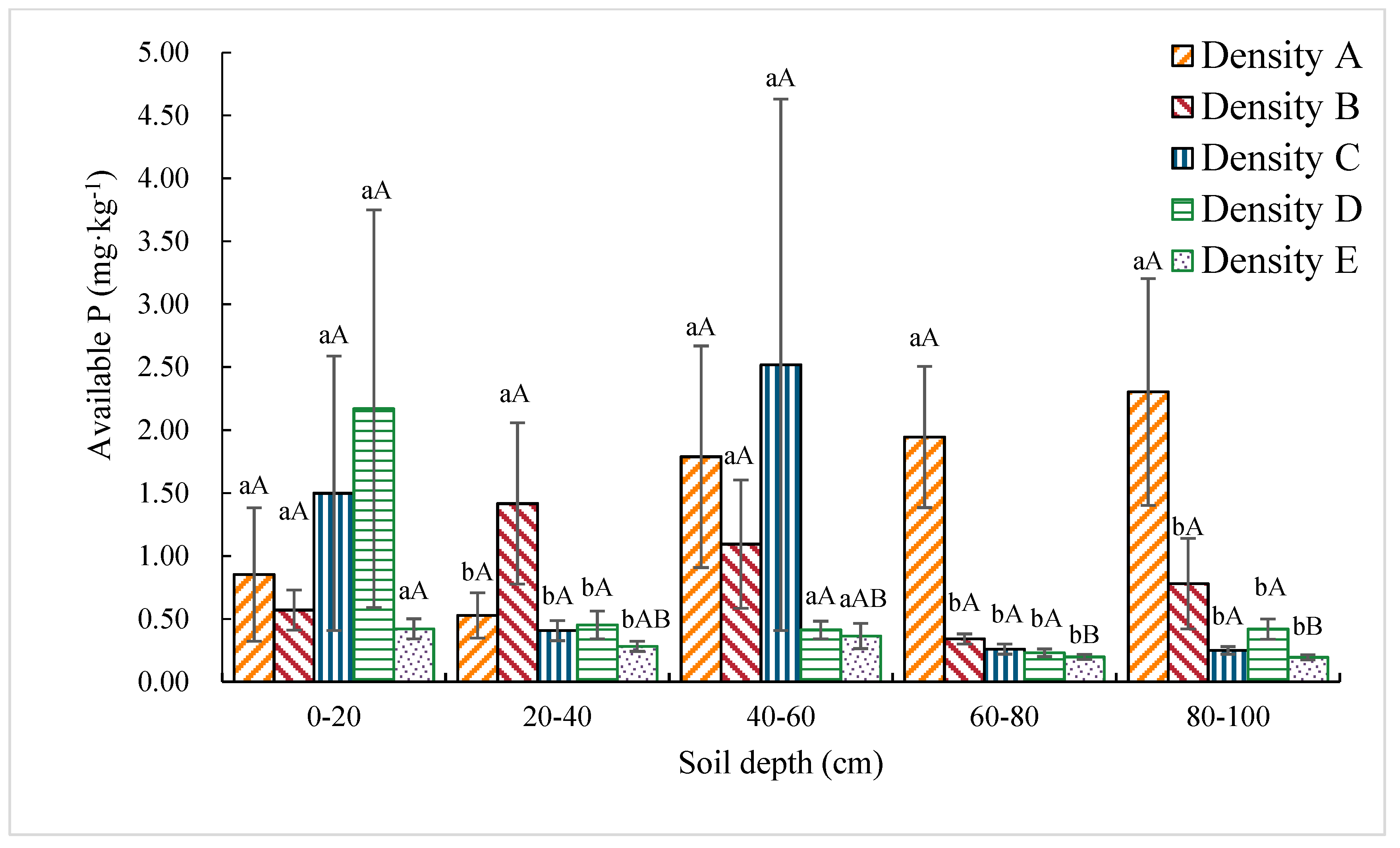
3.1.6. Available P Content
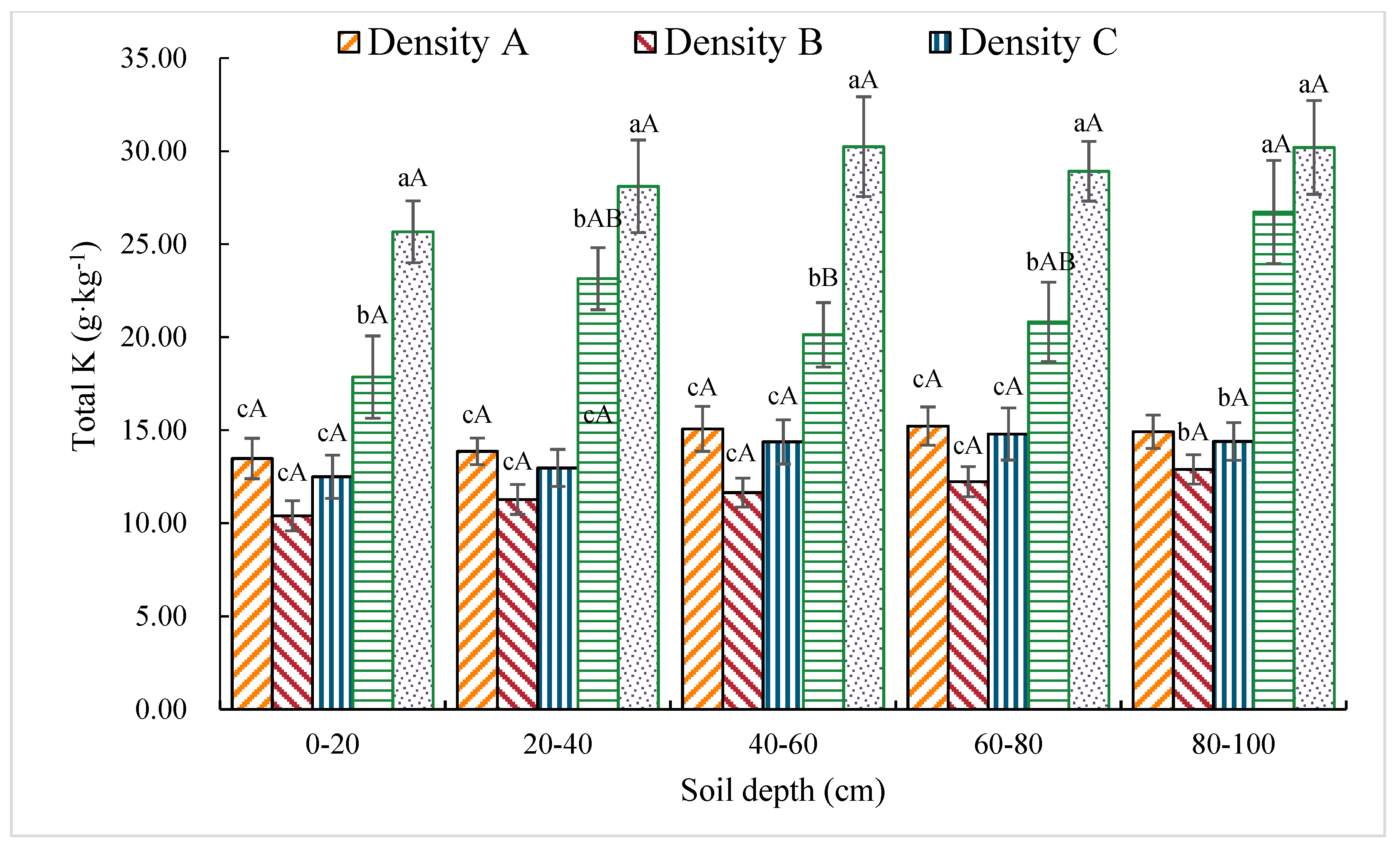
3.1.7. Total K Content
3.1.8. Available K Content
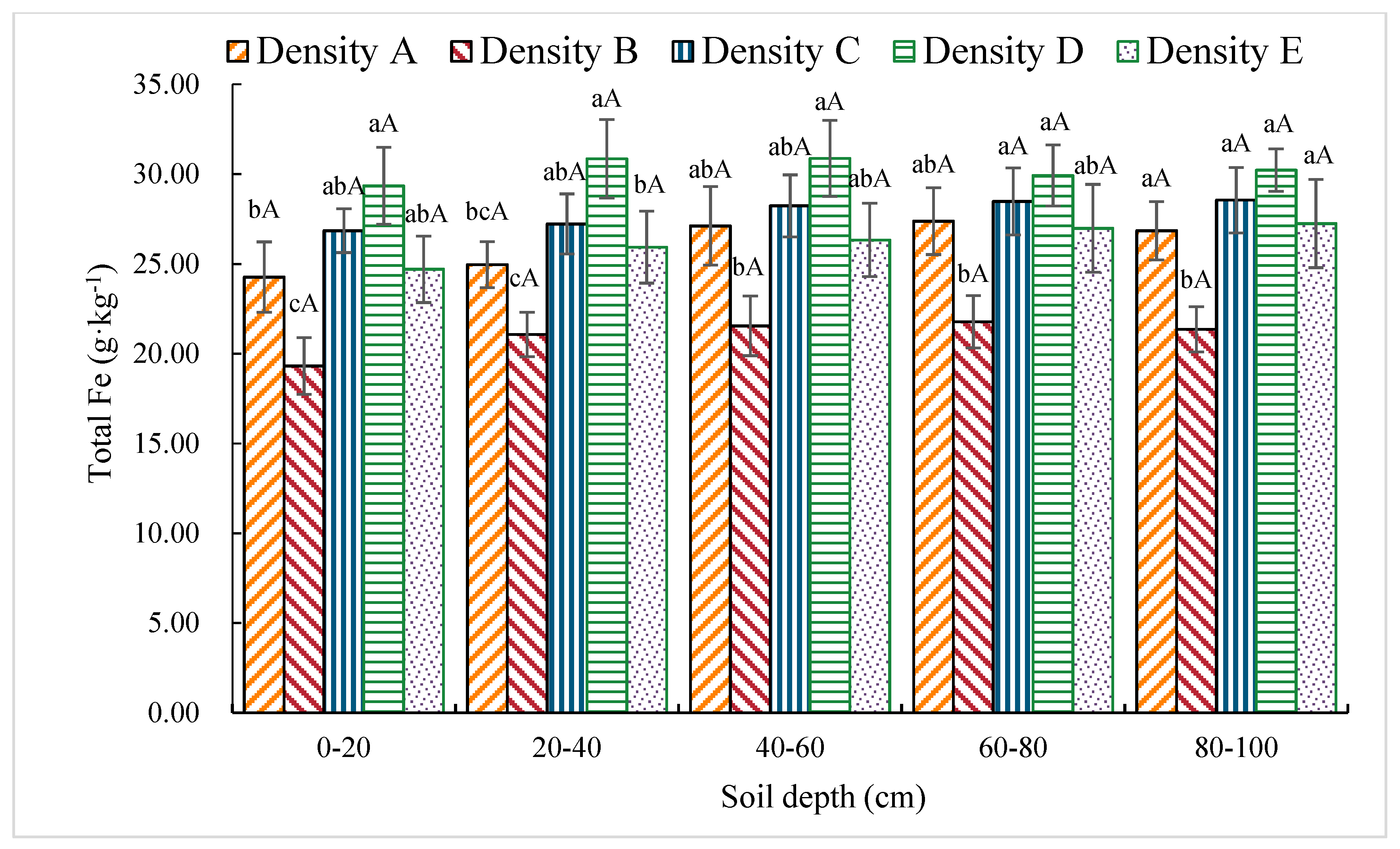
3.1.9. Total Fe Content
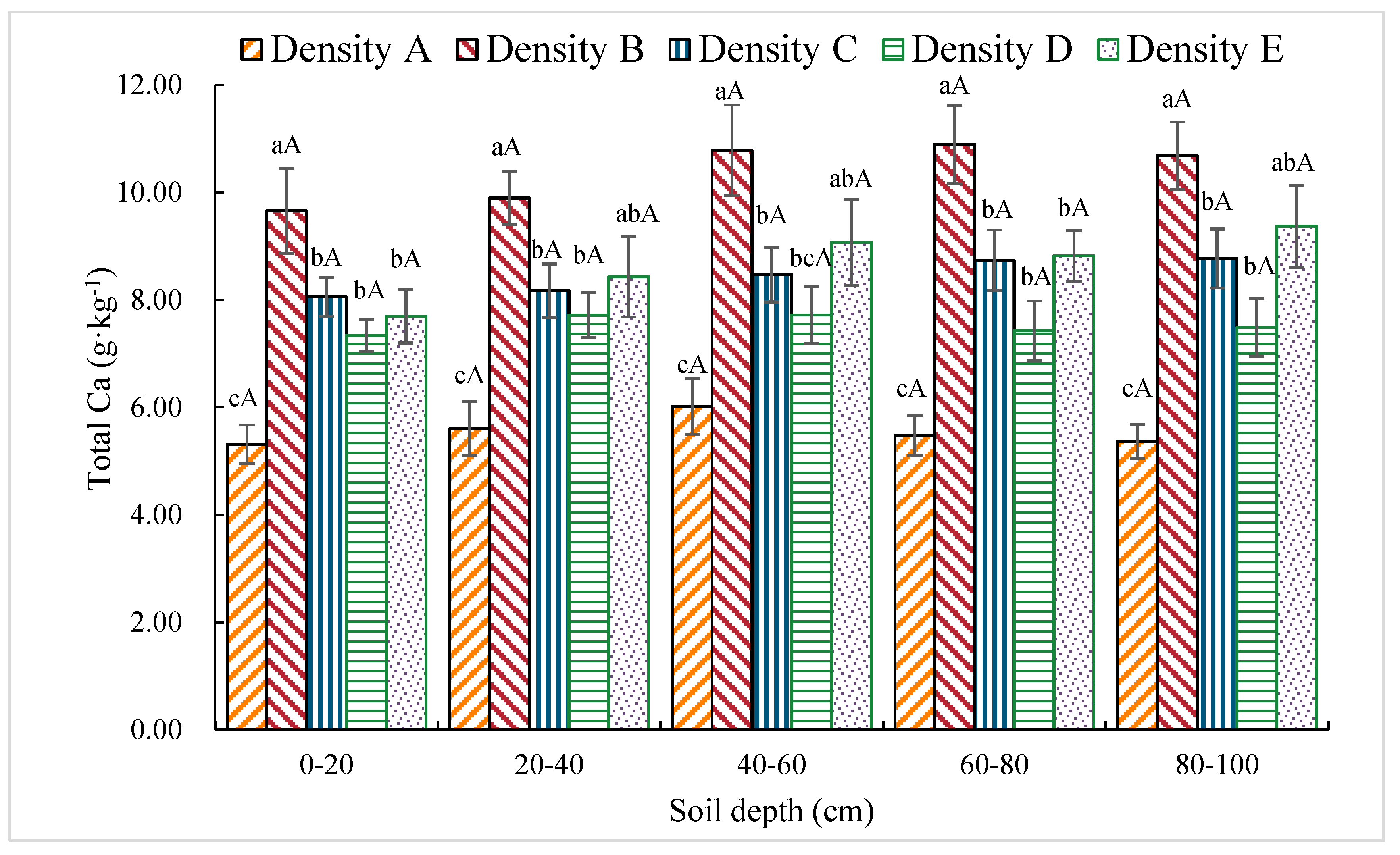
3.1.10. Total Ca Content
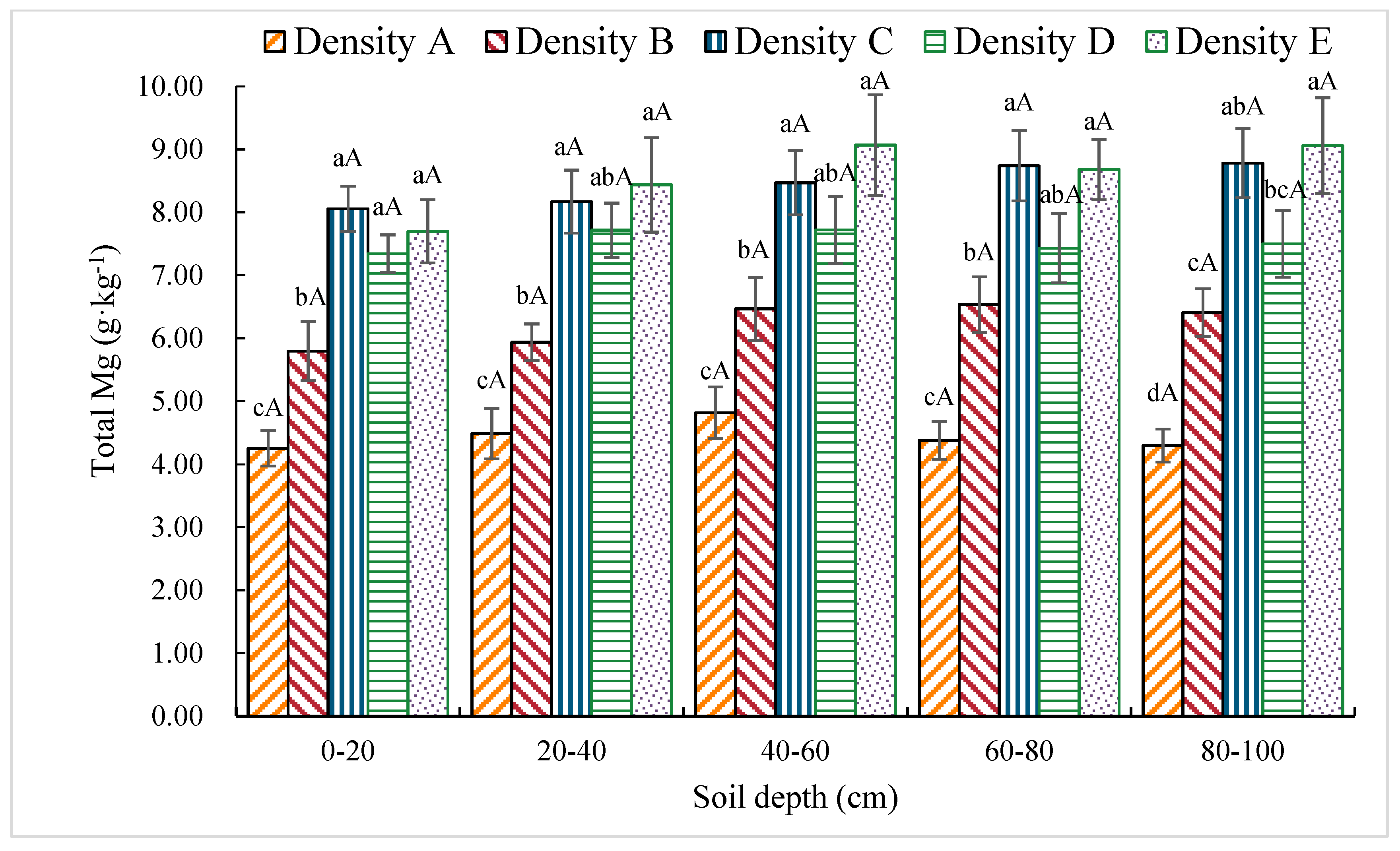
3.1.11. Total Mg Content
3.2. Correlation of Soil Nutrient Elements in the Studied Site
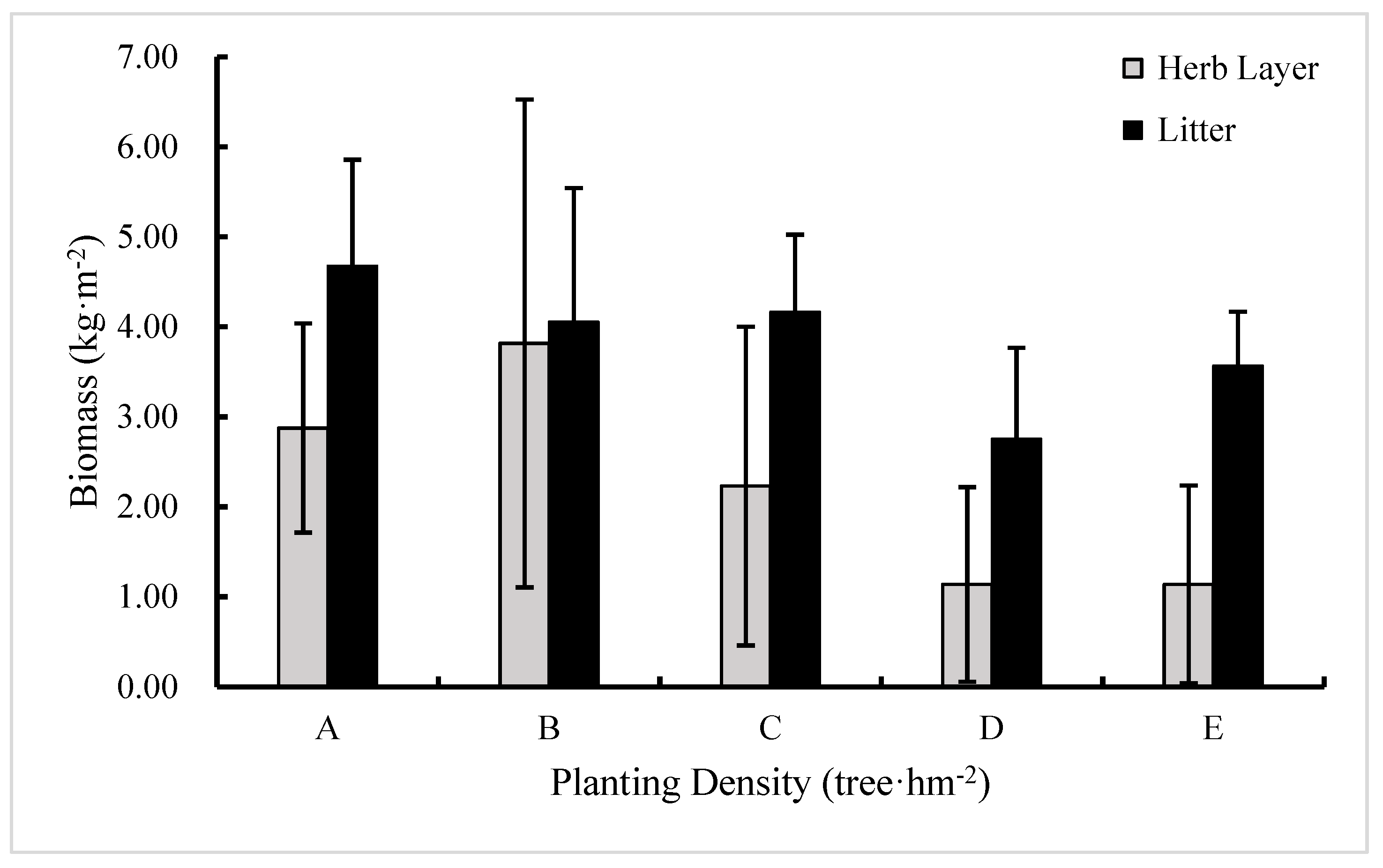
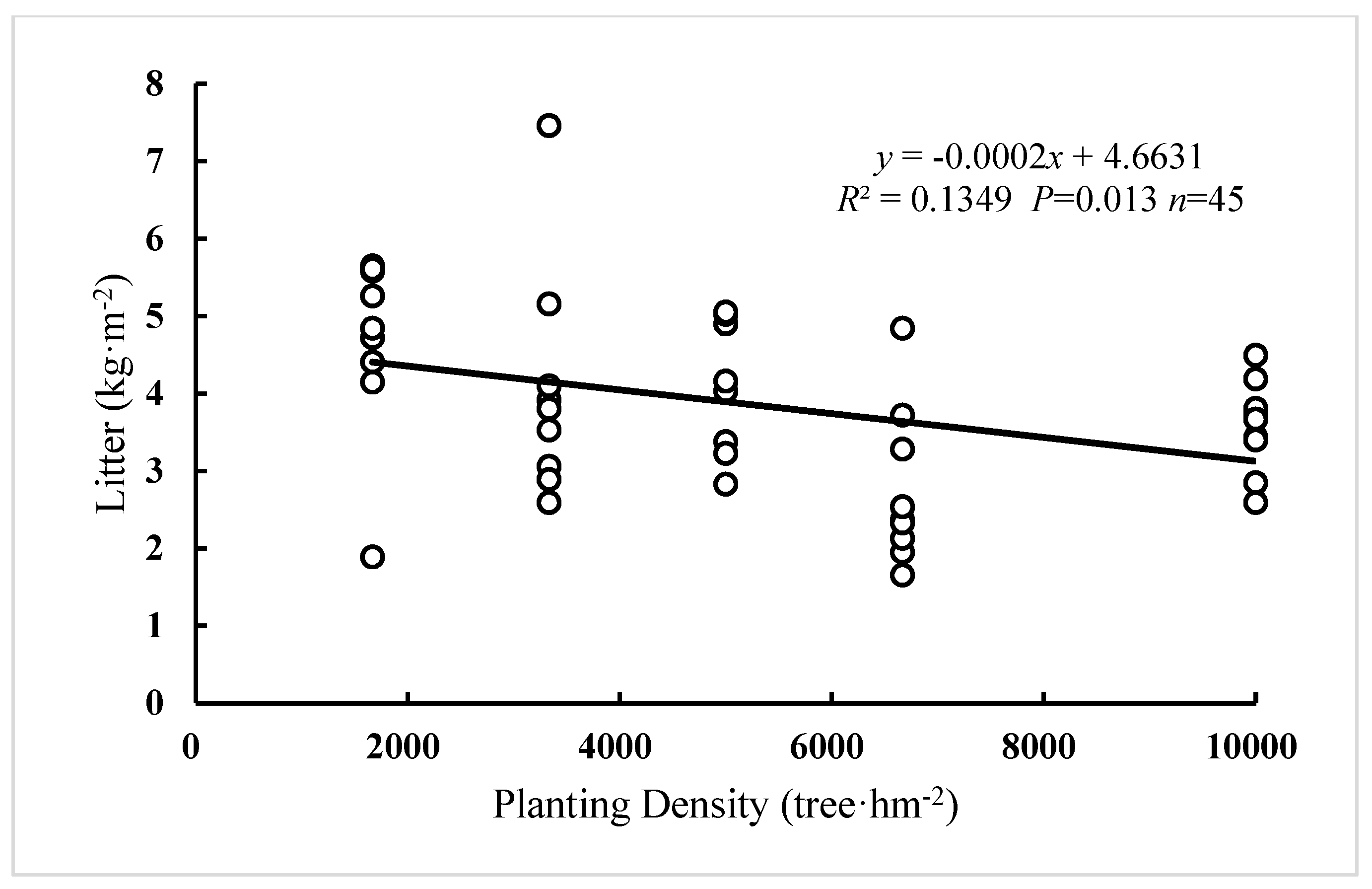
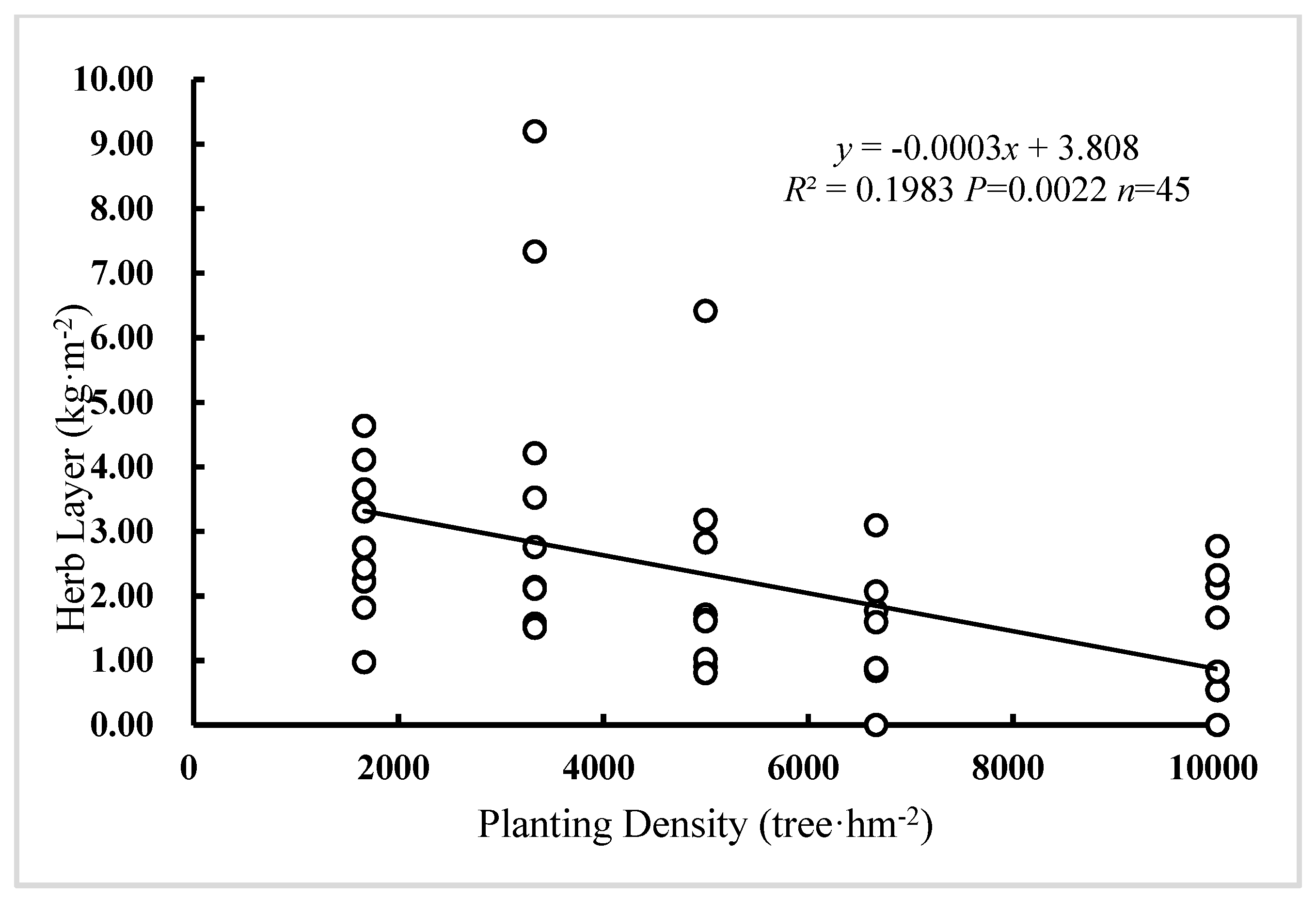
3.3. The Effect of Planting Density on Litter and Herb Layer Biomass
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xie, J.T. Mangshan Evergreen Broad-Leaved Forest Soil Nutrient Distribution Research; Central South University ofForestry and Technology: Changsha, China, 2014. [Google Scholar]
- Zhao, D.; Kane, M.; Teskey, R.; Markewitz, D.; Greene, D.; Borders, B. Impact of management on nutrients, carbon, and energy in aboveground biomass components of mid-rotation loblolly pine (Pinus taeda L.) plantations. Ann. For. Sci. 2014, 71, 843–851. [Google Scholar] [CrossRef][Green Version]
- Hartemink, A.E.; Hartemink, A.E. Soil fertility decline in the tropics: With case studies on plantations. Soil Sci. 2003, 170, 149–151. [Google Scholar]
- Jurgensen, M.; Tarpey, R.; Pickens, J.; Kolka, R.; Palik, B. Long-term effect of silvicultural thinnings on soil carbon and nitrogen pools. Soil Sci. Soc. Am. J. 2012, 76, 1418–1425. [Google Scholar] [CrossRef]
- Chen, Y.L.; Han, S.J.; Zhou, Y.M. Characteristics of available P in the rhizosphere soil pure Juglans mandshurica and Larix gmelinii and their mixed plantation. Chin. J. Appl. Ecol. 2002, 13, 790–794. [Google Scholar]
- Peng, X.H.; Zhang, B.; Zhao, Q.G. A review on relationship between soil organic carbon pools and soil structure stability. Acta. Pedol. Sin. 2004, 41, 618–623. [Google Scholar]
- Li, S.L.; Fang, X.; Xiang, W.H. Soil microbial biomass carbon and nitrogen concentrations in four subtropical forests in hilly region of central Hunan province, China. Sci. Silvae Sin. 2014, 50, 8–16. [Google Scholar]
- Zhao, W.W.; Liang, W.J.; Wei, X. Soil nutrient characteristics of Larix Principis Rupprechtii plantation with different stand densities. J. Southwest China Norm. Univ. (Nat. Sci. Ed.) 2019, 44, 84–92. [Google Scholar]
- Huang, C.Y. Soil; China Agriculture Press: Beijing, China, 2002. [Google Scholar]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zhang, G. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Hobbie, S.E.; Tali, L.; Ellsworth, D.S.; West, J.B.; David, T.; Knops, J.M.H.; Shahid, N.; Jared, T. Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 2006, 440, 922–925. [Google Scholar] [CrossRef]
- Zhao, Q.; Zeng, D.H. Phosphorus cycling in terrstrial ecosystems and its controlling factors. Acta Physiol. Sin. 2005, 29, 153–163. [Google Scholar]
- Chen, F.S.; Niklas, K.J.; Yu, L.; Fang, X.M.; Wan, S.Z.; Wang, H. Nitrogen and phosphorus additions alter nutrient dynamics but not resorption efficiencies of Chinese fir leaves and twigs differing in age. Tree Physiol. 2015, 35, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.X.; Chen, F.S.; Hu, X.F.; Gan, L.; Zhu, Y.L. Soil nitrogen and phosphorus availability in forest ecosystems at different stages of succession in the central subtropical region. Acta Ecol. Sin. 2009, 29, 4673–4680. [Google Scholar]
- Kennedy, P.C.; Wilson, L.F. Understory vegetation associated with saratoga spittlebug damage in Michigan Red Pine plantations. Can. Entomol. 1971, 103, 1421–1426. [Google Scholar] [CrossRef]
- Whittaker, R.H. Classification of Plant Communities; Classif. Plant Communities Series; Springer: Dordrecht, The Netherlands; Berlin, Germany, 1978; Volume 5-1. [Google Scholar]
- Nilsson, M.-C.; Wardle, D.A. Understory vegetation as a forest ecosystem driver: evidence from the northern Swedish boreal forest. Front. Ecol. Environ. 2005, 3, 421–428. [Google Scholar] [CrossRef]
- Kimmins, J.P. Forest Ecology: Afoundation for Sustainable Management; Prentice Hall PTR: Upper Saddle River, NJ, USA, 1997. [Google Scholar]
- Moeur, M. Characterising spatial patterns of trees using stem-mapped data. For. Sci. 1993, 39, 756–775. [Google Scholar]
- Bolat, İ. The effect of thinning on microbial biomass C, N and basal respiration in black pine forest soils in Mudurnu, Turkey. Eur. J. For. Res. 2014, 133, 131–139. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.Y.; Yang, X.J.; Xu, L.; Ren, L.N. Soil Organic Carbon and Nutrients in Natural Larix olgensis at Different Stand Densities. J. Northeast For. Univ. 2013, 41, 51–55. [Google Scholar]
- Zhao, R.D.; Fan, J.B.; He, Y.Q.; Song, C.L.; Tu, R.F.; Tan, B.C. Effects of stand density on soil nutrients and enzyme activities in Pinus massoniana plantation. Soils 2012, 44, 297–301. [Google Scholar]
- Barron-Gafford, G.; Will, R.; Burkes, E.C.; Shiver, B.; Teskey, R. Nutrient concentrations and contents, and their relation to stem growth, of intensively managed Pinus taeda and Pinus elliottii stands of different planting densities. For. Sci. 2003, 49, 291–300. [Google Scholar]
- Hu, Y.L.; Wang, S.L.; Zeng, D.H. Effects of Single Chinese Fir and Mixed Leaf Litters on Soil Chemical, Microbial Properties and Soil Enzyme Activities. Plant Soil 2006, 282, 379–386. [Google Scholar] [CrossRef]
- SFA. National Forest Resources Inventory. Available online: http://124.205.185.89:8085/8/tongji/queryForPage?lm=zyyy&Model.GUID=B9E05DF2F0CD4B00938FEFC9F1C0AB20 (accessed on 9 August 2019).
- Fang, X.; Tian, D.L. Dynamic of carbon stock and carbon sequestration in Chinese fir plantation. Guihaia 2006, 26, 516–522. [Google Scholar]
- Ding, Y.X.; Chen, J.L. Effect of continuous plantation of Chinese Fir on soil fertility. Pedosphere 1995, 5, 57–66. [Google Scholar]
- Evans, J. Sustainability of forest plantations: a review of evidence and future prospects. Int. For. Rev. 1999, 1, 153–162. [Google Scholar]
- Li, Y.M.; Hu, J.C.; Zhang, J.; Wang, S.L.; Wang, S.J. Microbial diversity in continuously planted Chinese fir soil. Chin. J. Appl. Ecol. 2005, 16, 1275–1278. [Google Scholar]
- Chen, L.C.; Wang, S.L.; Chen, C.Y. Degradation mechanismof Chinese fir plantation. Chin. J. Appl. Ecol. 2004, 15, 1953–1957. [Google Scholar]
- Sheng, W.T.; Fan, S.H. Long Term Productivity of Chinese Fir Plantation; Science Press: Beijing, China, 2005. [Google Scholar]
- Wang, J.H.; Guo, F.T.; Wu, P.F.; Zhou, L.L.; Su, Z.W.; Ma, X.Q. The estimation model for the biomass of Chinese fir plantation in the central zone of distribution area at different development stages. J. Anhui Agric. Sci. 2014, 42, 5104–5108. [Google Scholar]
- Chen, C.S.; Si, F.F. Diameter distribution models of Chinese Fir in Dagangshan, Jiangxi Provicne. For. Sci. Technol. 2018, 12, 17–19. [Google Scholar]
- He, G.P.; Xu, J.L.; Xu, Y.Q.; Chen, Y.H.; Shen, F.Q.; XU, L.Y. Growth differences and selection of Chinese fir families in young plantations with different site conditions. J. Zhejiang A&F Univ. 2018, 35, 453–458. [Google Scholar]
- Wang, Z.; Chen, A.L.; Cao, G.Q.; Zhang, Y.Q.; Zhang, H.Y.; Wang, F.L.; Wang, F. Effects of different litter compositions on soil microbial biomass carbon and nitrogen contents in Chinese fir plantation. J. South. Agric. 2017, 48, 1849–1857. [Google Scholar]
- Hu, X.Y.; Duan, A.G.; Zhang, J.G.; Du, H.L.; Zhang, X.Q.; Guo, W.F.; Guo, G.Z. Effect of stand density on soil nutrient of Chinese Fir mature plantations in south Asia subtropical zone. For. Res. 2018, 31, 15–23. [Google Scholar]
- Nelson, D.W.; Sommers, L.E.; Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, C.T.; Sumner, M.E. Total carbon, organic carbon, and organic matter. Methods Soil Anal. 1982, 9, 961–1010. [Google Scholar]
- Venanzi, R.; Picchio, R.; Piovesan, G. Silvicultural and logging impact on soil characteristics in Chestnut (Castanea sativa Mill.) Mediterranean coppice. Ecol. Eng. 2016, 92, 82–89. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; China Agriculture Press: Beijng, China, 2000. [Google Scholar]
- Wu, Z.L. Chinese Fir; China Forestry Publishing House: Beijing, China, 1984. [Google Scholar]
- Wang, Y.; Wang, H.Y.; Xu, L.I.; Yang, X.J.; Liu, L.; Wei-Song, L.I.; University, B.F. Soil physical and chemical characteristics of different depths in semi-natural mixed larch-spruce-fir at different stand densities. Pratacult. Sci. 2014, 37, 68–70. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Wang, G.D.; Zhang, L. Time-space distributive feature of soil nutrient and chemical characteristics of Robinia Pseudoacia L. plantation forestland in Loess Plateau. J. Soil Water Conserv. 2008, 22, 91–95. [Google Scholar]
- Song, N.P.; Yang, X.G.; He, X.Z.; He, T.H.; Li, Y.; Liu, X.Y. Soil nutrient effect of desert steppe reconstructed by artificial Caragana Microphylla stand. Bull. Soil Water Conserv. 2012, 32, 21–26. [Google Scholar]
- Yang, Y.; Liu, B.R.; Yang, X.G.; Han, C.C. Soil stoichiometry characteristics of articifial Caragana Korshinskii shrubs with different density in desert steppe. Bull. Soil Water Conserv. 2014, 34, 67–73. [Google Scholar]
- Uselman, S.M.; Qualls, R.G.; Thomas, R.B. Effects of increased atmospheric CO2, temperature, and soil N availability on root exudation of dissolved organic carbon by a N-fixing tree (Robinia pseudoacacia L.). Plant Soil 2000, 222, 191–202. [Google Scholar] [CrossRef]
- Chen, C.R.; Xu, Z.H.; Zhang, S.L.; Keay, P. Soluble organic nitrogen pools in forest soils of Subtropical Australia. Plant Soil 2005, 277, 285–297. [Google Scholar] [CrossRef]
- Yang, Z.A. A Study on Root Characteristics and Nutrients of Different-Aged Chinese Fir Plantations. Master’s Thesis, Northwest A&F University, Xianyang, China, 2014. [Google Scholar]
- Wu, Y.L.; Wang, B.; Zhao, C.; Dai, W.; Li, P. Comprehensive evaluation of soil fertility in different developing stages of Chinese Fir Plantations. J. Northwest A&F Univ. 2011, 39, 69–75. [Google Scholar]
- Li, X.L.; Yu, Q.Y.; Chen, S.Y.; Shen, X.Y. The distribution and effect of phosphorus on Anhui soil. J. Anhui Agrotech. Teach. Coll. 2001, 15, 12–15. [Google Scholar]
- An, S.S.; Huang, Y.M. Study on the ameliorate benefits of Caragana korshinskii shrubwood to soil properties in loess hilly area. Sci. Silvae Sin. 2006, 42, 70–74. [Google Scholar]
- Jin, L.; Zhou, J.M.; Wang, H.Y.; Chen, X.Q.; Du, C.W. Vertical diffusion and transformation of diammonium phosphate in acidic soil. J. Ecol. Rural. Environ. 2008, 24, 45–50. [Google Scholar]
- Li, A.; Wang, X.; Fan, H.L. Effects of four soil conditioners on alleviating aluminum toxicity in acid red soil. Soil Fertil. Sci. China 2014, 19, 7–11. [Google Scholar]
- Dong, Y.H.; Wang, H.Y.; Zhou, J.M.; Ren, Z.W. Preliminary study on potassium leaching characteristics of different soils. Soils 2014, 46, 225–231. [Google Scholar]
- Li, X.K.; Lu, J.W.; Wu, L.S. Advance on mechanisms of soil potassium fixation and release. Hubei Agric. Sci. 2008, 47, 473–477. [Google Scholar]
- Zhan, L.P.; Li, X.K.; Lu, J.W.; Wang, J.; Liao, Z.W. Research advances on influence factors of soil potassium movement. Soils 2012, 44, 548–553. [Google Scholar]
- Li, G.L.; Liu, Y.; Yu, H.Q.; Lv, R.H.; Li, R.S. Response of the undergrowth development to the growth rhythm of Chinese Pine plantation. Acta Ecol. Sin. 2009, 39, 1264–1275. [Google Scholar]
- Wang, J.S.; Fan, X.H.; Fan, J.; Zhang, C.Y.; Xia, F.C. Effect of aboveground competition on biomass partitioning of understory Korean pine (Pinus koraiensis). Acta Ecol. Sin. 2012, 32, 2447–2457. [Google Scholar]
- Zhang, Y.F.; Yin, G.T.; Yang, J.C.; Li, S.W.; Li, R.S.; Zou, W.T. Effects of planting densities on production and dynamics of litter of Mytilaria laosensis plantation. Bull. Bot. Res. 2017, 37, 768–777. [Google Scholar]
- Zhang, L.H.; Qi, Q.J.; Li, T.T.; Yu, S.Y.; Zhang, X.Y.; Zhang, R.; F, H.J. Effects of stand density on understory plant diversity and biomass in a Pinus massoniana plantation in Wenfeng Mountain, Xinjin County. Acta Ecol. Sin. 2019, 39. [Google Scholar]
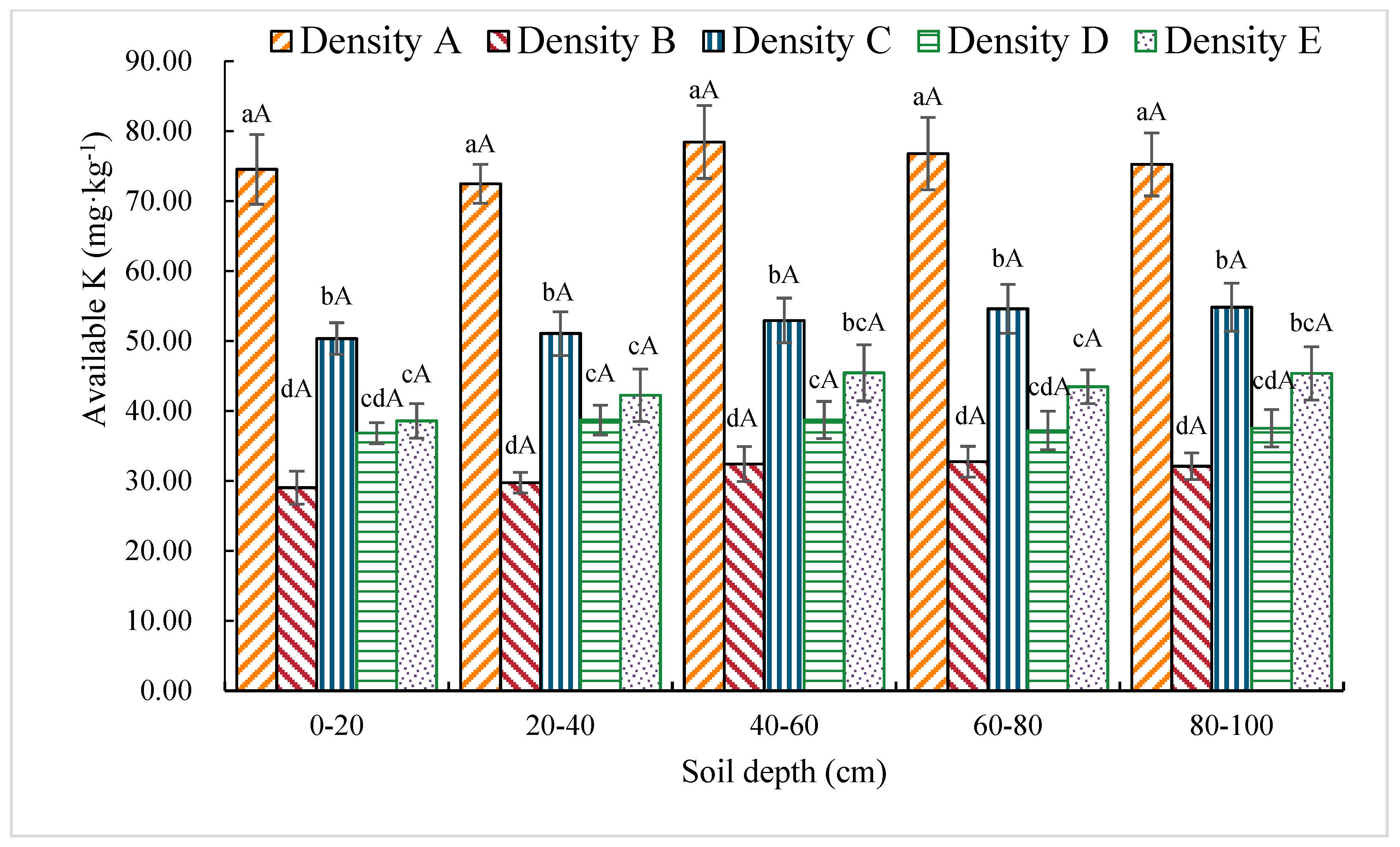
| pH | Organic Matter | Total N | Hydrolytic N | Total P | Available P | Total K | Available K | Total Ca | Total Fe | Total Mg | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | –0.813** | –0.779** | –0.777** | –0.275 | –0.286 | 0.566** | –0.04 | 0.126 | 0.531** | 0.502* |
| Organic Matter | 1 | 0.949** | 0.913** | 0.513** | 0.077 | –0.198 | –0.263 | –0.01 | –0.305 | –0.091 | |
| Total N | 1 | 0.867** | 0.583** | 0.097 | –0.177 | –0.157 | –0.133 | –0.178 | –0.073 | ||
| Hydrolytic N | 1 | 0.509** | 0.1 | –0.264 | –0.387 | 0.206 | –0.520** | –0.094 | |||
| Total P | 1 | –0.07 | 0.422* | 0.079 | –0.255 | –0.072 | 0.073 | ||||
| Available P | 1 | –0.430* | 0.386 | –0.349 | 0.005 | –0.444* | |||||
| Total K | 1 | –0.184 | –0.023 | 0.445* | 0.548** | ||||||
| Available K | 1 | –0.798** | 0.236 | –0.499* | |||||||
| Total Ca | 1 | –0.469* | 0.501* | ||||||||
| Total Fe | 1 | 0.394 | |||||||||
| Total Mg | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, J.; Du, H.; Duan, A.; Zhang, J. Effect of Stand Density and Soil Layer on Soil Nutrients of a 37-year-old Cunninghamia lanceolata Plantation in Naxi, Sichuan Province, China. Sustainability 2019, 11, 5410. https://doi.org/10.3390/su11195410
Lei J, Du H, Duan A, Zhang J. Effect of Stand Density and Soil Layer on Soil Nutrients of a 37-year-old Cunninghamia lanceolata Plantation in Naxi, Sichuan Province, China. Sustainability. 2019; 11(19):5410. https://doi.org/10.3390/su11195410
Chicago/Turabian StyleLei, Jie, Hailun Du, Aiguo Duan, and Jianguo Zhang. 2019. "Effect of Stand Density and Soil Layer on Soil Nutrients of a 37-year-old Cunninghamia lanceolata Plantation in Naxi, Sichuan Province, China" Sustainability 11, no. 19: 5410. https://doi.org/10.3390/su11195410
APA StyleLei, J., Du, H., Duan, A., & Zhang, J. (2019). Effect of Stand Density and Soil Layer on Soil Nutrients of a 37-year-old Cunninghamia lanceolata Plantation in Naxi, Sichuan Province, China. Sustainability, 11(19), 5410. https://doi.org/10.3390/su11195410





