3.1. Struvite Recovery from Swine Wastewater
Table 5 shows the performance of the struvite recovery process. The mean removal efficiencies of O-P and NH
4-N were approximately 73.96% and 21.97%, respectively. The removal efficiency of O-P is associated with struvite recovery [
22]. Due to struvite crystallization and air stripping, NH
4-N removal efficiency is higher than the theoretical value of 7%. The struvite recovery efficiency is highly dependent on the waste source type and experimental conditions such as pH and ionic molar ratios [
28,
29]. Previous studies reported that very pure forms of struvite can be recovered from swine wastewater at pH 8.0 to 9.0 and an Mg:P molar ratio of 0.8:1 to 1.2:1 [
22,
30,
31]. The pH of the influent used in this study was 8.69 and therefore no additional chemical was used to control the pH during the recovery process. In this study, the struvite was recovered at pH 8.52 and an Mg:P molar ratio of 1.2:1. The mean removal efficiencies of TS and SS were 42.67% and 77.86%, respectively. A small amount of heavy metals (very low compared to the Korean standard for fertilizer raw materials) was detected in the recovered struvite (
Table 6). All the ingredients in the struvite recovered from animal wastewater originated from the animal feedstock containing low heavy metal and the recovered struvite therefore needs lesser efforts to purify for target use such as fertilizer and in chemical industries [
21].
The swine wastewater management system in Korea is quite different compared to other countries. Due to rapid economic return, the swine farming system in Korea is becoming more intensive. Intensification in swine farms resulted in an increase of the swine population from around 3.6 million in 1983 to 11.3 million in 2018 as well as wastewater generation [
32]. The Korean Government has therefore imposed some strict regulations including the prohibition of the direct use of swine wastewater in agricultural fields and stringent nutrient concentration limits for swine wastewater discharge to protect the environment. The government has also established more than 200 centralized swine wastewater treatment plants all over Korea with a treatment capacity of more than 7.3 million ton per year and the number is still increasing [
33,
34]. The mass production of struvite from the centralized wastewater treatment plants might therefore be economically viable, help maintaining social equity, and protect the environment, thus ensuring sustainability.
3.2. Pre-Treatment of Recovered Struvite
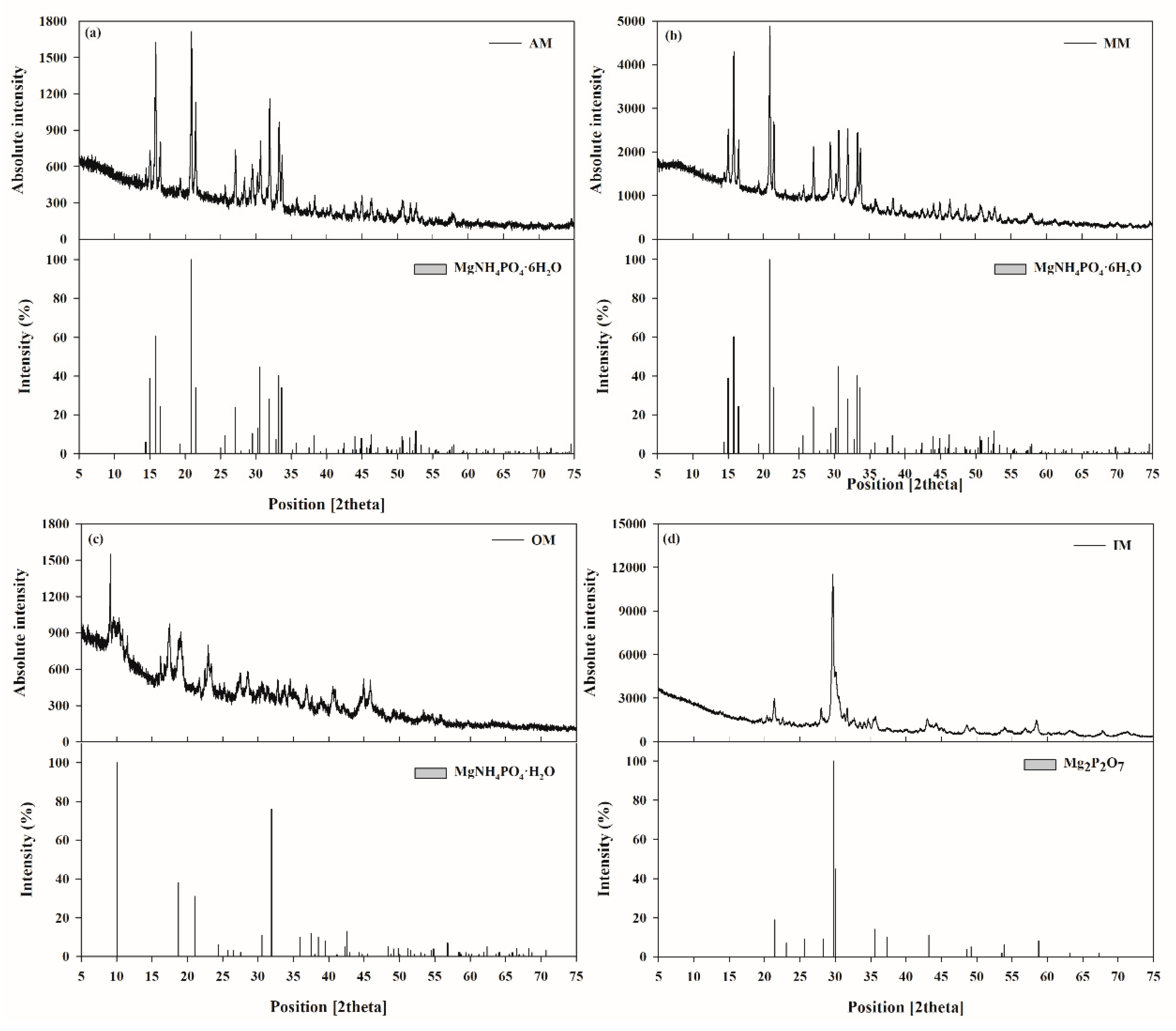
The recovered struvite from the swine wastewater was precipitated in the reactor as dense fine particles. Therefore, the settled struvite precipitates were dried to evaporate the moisture and obtain struvite in powder form for further use. Among the pre-treatment techniques, air dry is simple and economical, but time consuming [
35]. Whereas, microwave irradiation is a dielectric heating process where electromagnetic waves are produced by a magnetic and an electric field component. As a drying pre-treatment, it requires less time and ensures uniform drying of the material [
36]. On the other hand, incineration and over dry are the conventional drying methods using high temperature. To study the effect of drying pre-treatments on the chemical composition of the recovered struvites, XRD analysis was performed. The XRD pattern (position and peaks intensity) generated from the pre-treated materials were matched with the reference values. Among the pre-treated struvites, AM showed the characteristic struvite structuration (magnesium ammonium phosphate (MAP), MgNH
4PO
4·6H
2O) (
Figure 1a), while MM showed several peaks indicative of MAP (
Figure 1b). OM produced a thermally more stable product dittmarite, MgNH
4PO
4·H
2O (
Figure 1c). Whereas, IM denatured the struvite to magnesium pyrophosphate (Mg
2P
2O
7) (
Figure 1d) with the removal of ammonium and hydrates.
Nutrient contents of struvite vary significantly depending on the type of material used for struvite recovery. Struvite has N:P
2O
5:K
2O:Mg content ratio of 5.7:29:0:16.4 [
37]. N and P content in struvite usually ranges from 56–70 g kg
−1 and 56–260 g kg
−1, respectively [
19,
20,
21]. The results of nutrient contents analysis reveal that drying pre-treatment can change the nutrient contents of struvite (
Table 7). After pre-treatment, a decrease in N content of the pre-treated struvites was observed, whereas P content was well within range.
To ascertain the fertilizer potential of the pre-treated stuvites, the solubility experiment was conducted. The solubility of struvites generally varies between 15–40% [
20].
Table 7 shows the solubility of the pre-treated struvites at different pH levels. Among the pre-treated materials, AM and IM showed low solubility, while the solubility of MM and OM were similar to chemical fertilizer (60–95% soluble) [
20]. Based on solubility test results and crystalline nature, AM and IM were selected for further experiments. Although IM did not show the characteristic struvite structuration, due to low solubility, the nutrient leaching loss and agronomic performance of IM were investigated.
3.3. Nutrient Leaching in Soil Column
Nutrient leaching from control, FSP, AM, and IM in soil column is shown in
Figure 2a. Nutrient leaching from FSP reached the maximum value (3.72 mg O-P g
−1 d
−1) within 30 days after the onset of the experiment and gradually decreased to 2 mg O-P g
−1 d
−1 or less after 70 days. Whereas, AM gradually started to elute more than 2 mg O-P g
−1 d
−1 after 40 days due to an increase in leaching rate. After that, the leaching rate began to decrease and ranged between 1.85–2.56 mg O-P g
−1 d
−1 for about 2 months until the end of the experiment. In the case of IM, the average leaching rate was about 4.2 and 3.6 times lower than FSP and AM, respectively, and no decreasing pattern was observed during 18 weeks of the experiment. The average O-P leaching rate for control, FSP, AM, and IM was 0.19 ± 0.06, 2.07 ± 0.87, 1.78 ± 0.95, and 0.5 ± 0.31 mg O-P g
−1 d
−1, respectively. Although O-P leaching from FSP was faster than AM and IM (
Figure 2a), the average leaching rate of O-P for FSP and AM was significantly different from the control and IM, while no significant difference was observed between FSP and AM (
p > 0.05).
For the NH
4-N leaching test, IM was not considered as it was incinerated at a high temperature (550 °C) and most of the N was evaporated during incineration. However, a small fraction of N was present in the form of urea. Therefore, to determine the elution of NH
4-N from FSP and AM, the concentration of ammonia N leached by urea was excluded. The average NH
4-N leaching rate for the control, FSP, and AM were 1.25 ± 1.21, 5.59 ± 5.93, and 4.88 ± 5.47 mg O-P g
−1 d
−1, respectively. As shown in
Figure 2(b), the leaching profiles of N contained in commercial FSP did not differ significantly from that of N in AM (
p > 0.05).
The results of the soil column nutrient leaching experiments support earlier findings, which reported that nutrient leaching from struvite is lower than chemical fertilizers. Rahman et al. applied 30–40 kg ha
−1 struvite-Urea and FSP-Urea in a soil column and observed a P leaching of 0.03–0.37% and 0.23–0.25% for struvite and FSP, respectively [
22]. Latifian et al. conducted a nutrient release experiment in a dialysis bag for 105 days and found that the commercial NPK fertilizer released 72% O-P in just one day and reached a maximum release of 88% within 20 days, while struvite slowly released O-P and even after 105 days, the elution for struvite recovered from anaerobic digestion effluent was around 12% [
38]. Sharma et al. applied di-ammonium phosphate (DAP) in a packed box experiment with sandy and loamy soils and reported a P leaching of 7–57% in sandy soils and 27–67% of P leaching in loamy soils. They found that over 90% of P losses in sandy soils were via runoff and leaching in the form of dissolved unreactive P (DURP) and particulate P (PP), while dissolved reactive P (DRP) showed a minor contribution [
39]. In sandy soils, P leached by vertical flow while leaching was governed by through flow in loamy soils. The relatively higher affinity of DRP with soil results in less mobility compared to other forms of P [
39]. A critical limit of 0.1 mg-P L
−1 for leaching set by USEPA to control eutrophication can therefore be easily maintained using struvite due to its slow-releasing characteristics.
Ryu and Lee reported the slow-releasing character of struvite recovered from swine wastewater compared to complex fertilizer by applying 100 mg-N kg
−1 in a 40 h soil column experiment [
40]. Maximum NH
4-N leaching from complex fertilizer was reported within 2 h compared to struvite. In another 48 h column leaching experiment, Ryu et al. observed that within a short period of time (<10 h), complex fertilizer reached the steady-state, while struvite recovered from semiconductor wastewater required more than 40 h and the cumulative leached N mass from complex fertilizer and struvite was about 32.9% and 17.6% at the steady state, respectively [
41]. Latifian et al. also observed a quick release of NPK fertilizer in the dialysis bag. They found that 57% of NH
4-N was released within one day from commercial NPK fertilizer, whereas struvite released only 11% NH
4-N [
38]. In another study, Rahman et al. observed a minute release of NH
4-N from struvite (1.9–2.0%) compared to commercial FSP fertilizer (7.8–6.4%) when 30–40 kg ha
−1 fertilizer was applied in a soil column [
22]. The results in this study are well in line with previous studies conducted using struvites and confirm that pre-treated struvite as a fertilizer source is more eco-friendly compared to chemical fertilizers by slowly releasing nutrients into soil.
3.4. Effects of Test Materials on Sudan Grass Production
The use of struvite as a fertilizer was first proposed by Sir James Murray in 1858 [
19]. The struvite is generally applied as P fertilizer, therefore supplementation of N needs to be considered to fulfill the nutrient requirements of any specific crop [
42]. In this study, urea was applied as a supplementary N source to meet N deficiency in pot trials of Sudan grass. No significant difference was observed in the germination rate (average 66.3%) for all the test materials including the control (
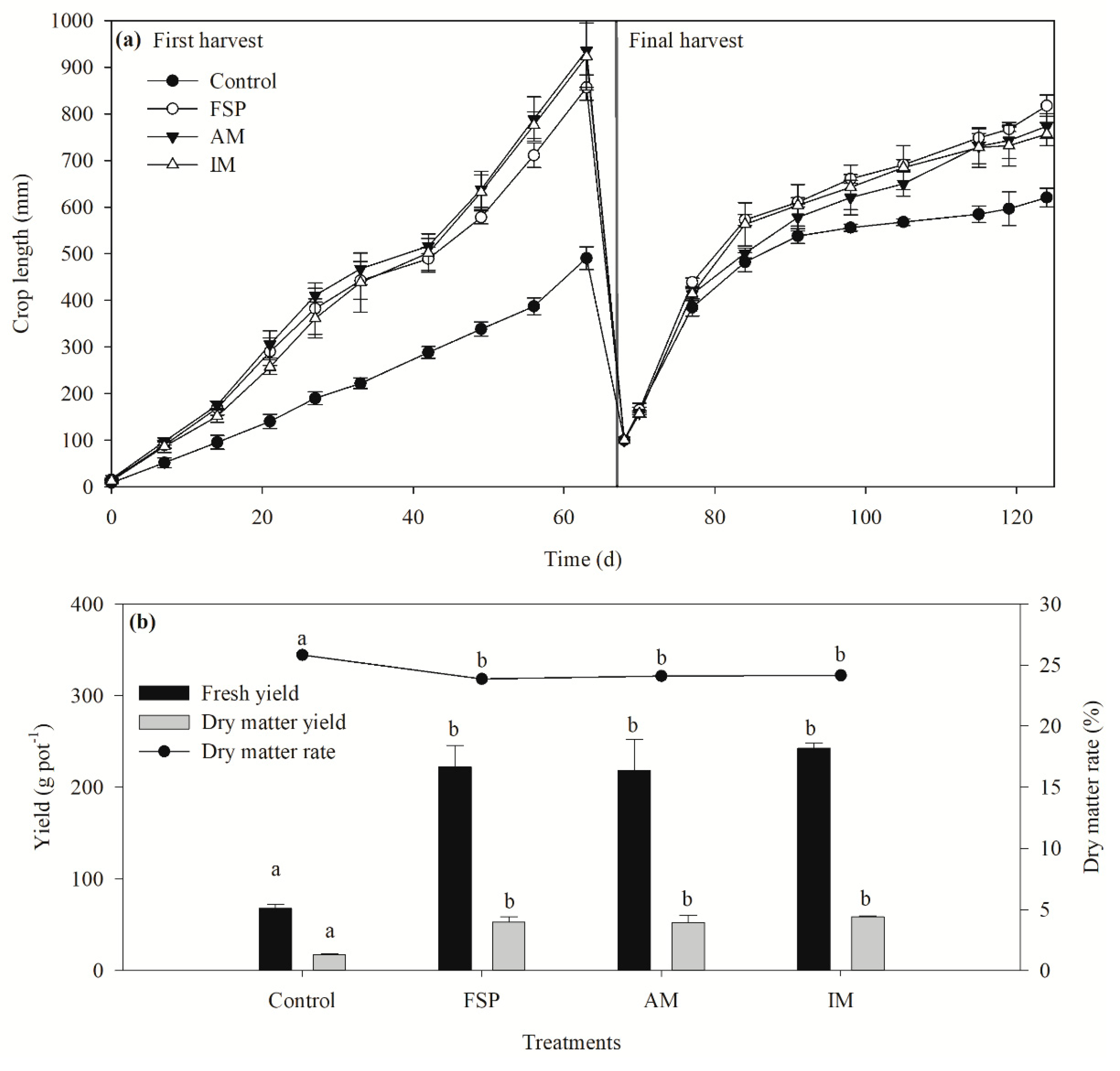
p > 0.05) (data not shown). The crop growth was visually compared during a 124 day experiment.
Figure 3a shows that the effects of FSP, AM, and IM on Sudan grass growth was similar except for the control. The growth rate of Sudan grass using the control, FSP, AM, and IM treatments were calculated as 8.1 ± 0.1, 12.6 ± 0.4, 12.8 ± 0.7, and 12.6 ± 0.5 mm d
−1, respectively. The growth rates were not significantly different among test materials except for the control (
p > 0.05) (
Figure 3a). These results are highly consistent with previous studies. Li and Zhao observed no significant difference in the growth rate of the leaf or stalk length of cabbage, Chinese chard, water spinach, and water convolvulus when they were treated with struvite and chemical fertilizer [
43]. However, Ryu et al. reported that the leaf length of struvite fertilized lettuce was almost double that of complex fertilizer [
44]. In another study, Ryu et al. assessed the quality of semiconductor wastewater recovered struvite as a fertilizer and observed that leaf length and nutrient content in the tissues of struvite fertilized lettuce were higher than the complex fertilizer [
41]. Liu et al. reported significantly higher leaf area with struvite treated maize compared to FSP [
30].
Sudan grass is usually harvested after primary growth and is then allowed to regrow from the remaining portion.
Figure 3a shows the secondary growth of the Sudan grass after its first harvesting. Initially, the growth was rapid for around two weeks and then it plateaued. The mean fresh yield for FSP, AM, and IM were 222.3 ± 23.4, 218.5 ± 33.9, and 242.3 ± 5.8 g pot
−1, respectively; while the mean dry matter yield for FSP, AM, and IM were 53.1 ± 5.8, 52.6 ± 7.9, and 58.5 ± 1.1 g pot
−1, respectively (
Figure 3b). For fresh and dry matter yield, a significant difference between the control and test materials was observed, but no significant difference among treatments (FSP, AM, IM) was found (
p > 0.05). Previous studies are in good agreement with this general finding. Johnston and Richards compared the effectiveness of several recovered struvite with commercial monocalcium phosphate for ryegrass growth and total dry matter yield and found no significant difference among the treatments [
19]. Li and Zhao did not find any significant difference in fresh or dry yield for Chinese flowering cabbage, Chinese chard, water spinach, and water convolvulus with struvite and commercial fertilizer treatments [
43]. Plaza et al. also reported no significant difference in the dry matter yield of ryegrass with 9–44 mg-P kg
−1 struvite or superphosphate [
45].
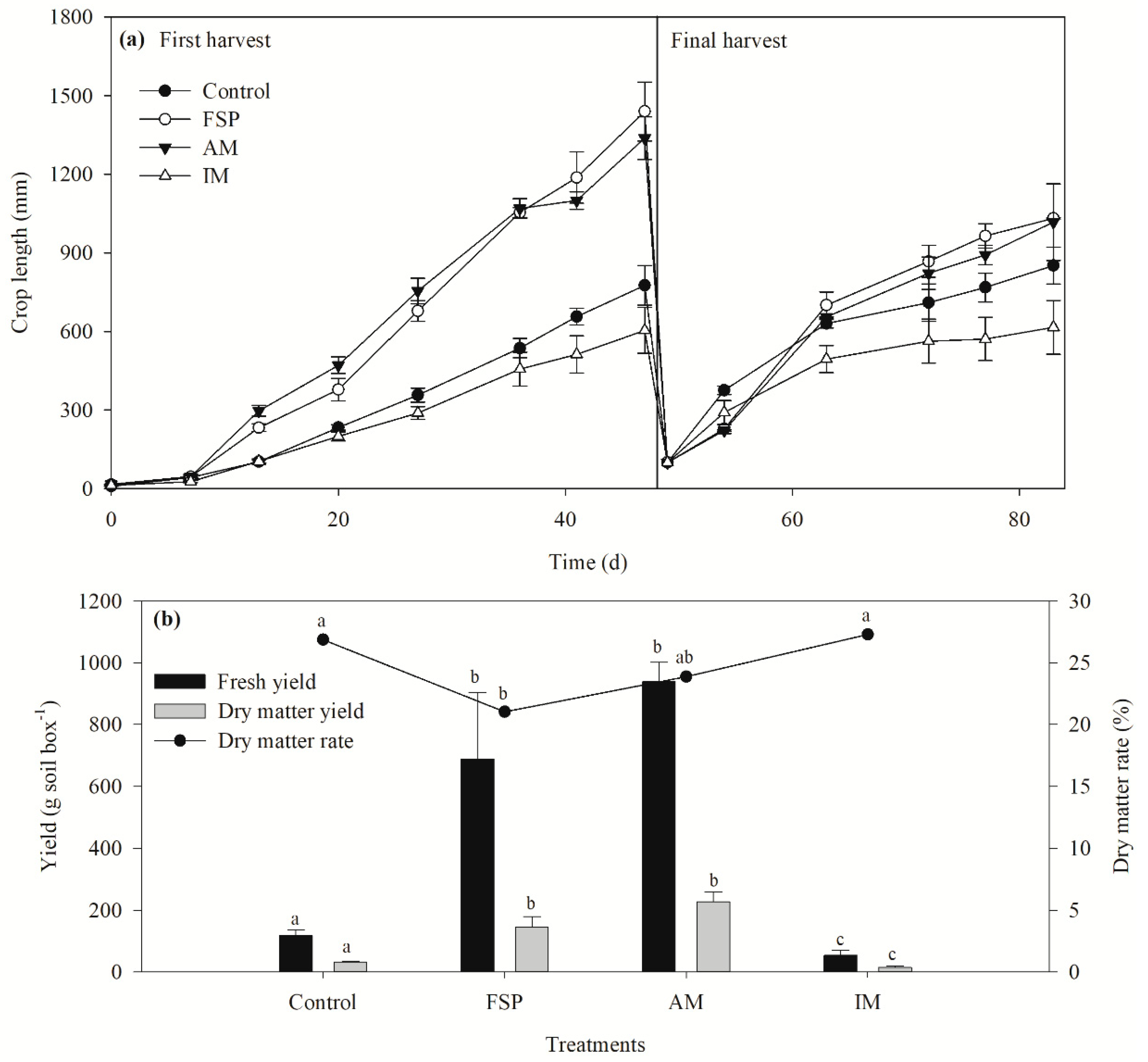
In soil box trials, the FSP, AM, and IM were applied 20 times higher than the required amount for Sudan grass to check their suitability when supplementary N was not supplied (
Table 4). No statistically significant difference was observed in the germination rate (average 70%) among the test materials (
p > 0.05) (data not shown). The growth rates were 28.4 ± 1.4, 27.1 ± 0.8, and 13.3 ± 2.2 mm d
−1 for FSP, AM, and IM treatments, respectively. As shown in
Figure 4a, the growth rate obtained with the IM treatment was lower than that of the FSP and AM treatments. However, FSP and AM did not show any statistically significant difference (
p > 0.05). The growth rate of Sudan grass using IM was less than 50% compared to FSP and AM treatments due to evaporation loss of N during incineration.
The fresh yield for FSP and AM were 688.9 ± 214.6 and 939.2 ± 64.1 g soil box
−1, respectively and were comparatively higher than IM treatment (53.6 ± 16.1 g soil box
−1). No significant difference (
p > 0.05) in fresh yield was found between FSP and AM treatments (
Figure 4b). However, the dry matter percentage was significantly lower in the FSP treatment (22.2%) (
p < 0.05). Our study revealed that increasing application of struvite as AM stimulates Sudan grass growth without any side effects. This finding is in line with other studies. Ryu et al. applied 0.1–0.6 and 0.3–1.3 g struvite kg
−1 soil for lettuce growth and observed an increase in the leaf length, fresh yield, and dry yield with increasing application of struvite [
44]. Li and Zhao applied 2–8 times higher doses of landfill leachate recovered struvite on water spinach and found no significant difference in the sprouts among the treatments without any burning effects [
43]. Ackerman et al. applied 9.5–47.5 mgP pot
−1 and observed that the biomass yield of canola increased from 5.4 to 9.6 g pot
−1 [
46]. Uysal et al. increased struvite doses up to 4 times higher than the standard and found an increase in the growth and nutrient uptake of maize and tomato plants with the increasing amount of struvite [
47]. Plaza et al. observed that increased struvite application in ryegrass did not affect N, K, and Ca uptake, while the P and Mg uptake increased significantly in both struvite and superphosphate [
45]. Mg could be the reason for comparatively higher growth of plants with higher struvite applications as Mg complements P uptake [
48]. The above-mentioned studies clarified that over-application of struvite as AM does not burn the roots as it is a slow-releasing fertilizer, but rather enhances the plant growth by higher P and Mg uptake.