Sustainable Irrigation Management of Ornamental Cordyline Fruticosa “Red Edge” Plants with Saline Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth Environment
2.2. Crop Conditions
2.3. Treatments
2.4. Experimental Design and Statistical Analysis
2.5. Sampling and Data Recording
- (a)
- Climatic conditions: For the whole experiment, the air and substrate temperature, radiation, and VPD were monitored on an hourly basis with an Onset HOBO LCD data logger model H8 RH/Temp/Light/External H08-004-02, with a probe TMC6-HA (substrate temperature) and a model PYR Apogee pyranometer sensor.
- (b)
- Night and day water uptake: For the whole experiment, night and day plant weights were measured in order to determine the transpiration of each replicate. A scale Dicsa MonoBloc incide PB602-S was used. The plants’ weekly water uptake was measured at the end of the four weeks of experiment with the aim of analyzing the evolution of the transpiration along the experiment. Night and day water uptakes were measured separately in order to assess how the salinity of the nutrient solution affects the transpiration of the plant during dark and light periods. From the measured data, average daily water uptakes for each week were calculated.
- (c)
- Transpiration dynamics: The weight of each plant was measured each hour for a period of 25 h in order to determine the hourly transpiration.
- (d)
- Leaf area index (LAI): it was measured via digitalization of the leaves with a scanner HP Deskjet 990CXI and the subsequent image treatment was done with the IDRISI software package for Windows version 2007. LAI was estimated from the leaf area measurement considering the plant density.
- (e)
- Aerial dry weight: Leaves and stems were jointly dried in a NüveE FN500 oven at 60 °C for 48 h at the end of the experiment to determine the dry weight with a scale Dicsa MonoBloc incide PB602-S.
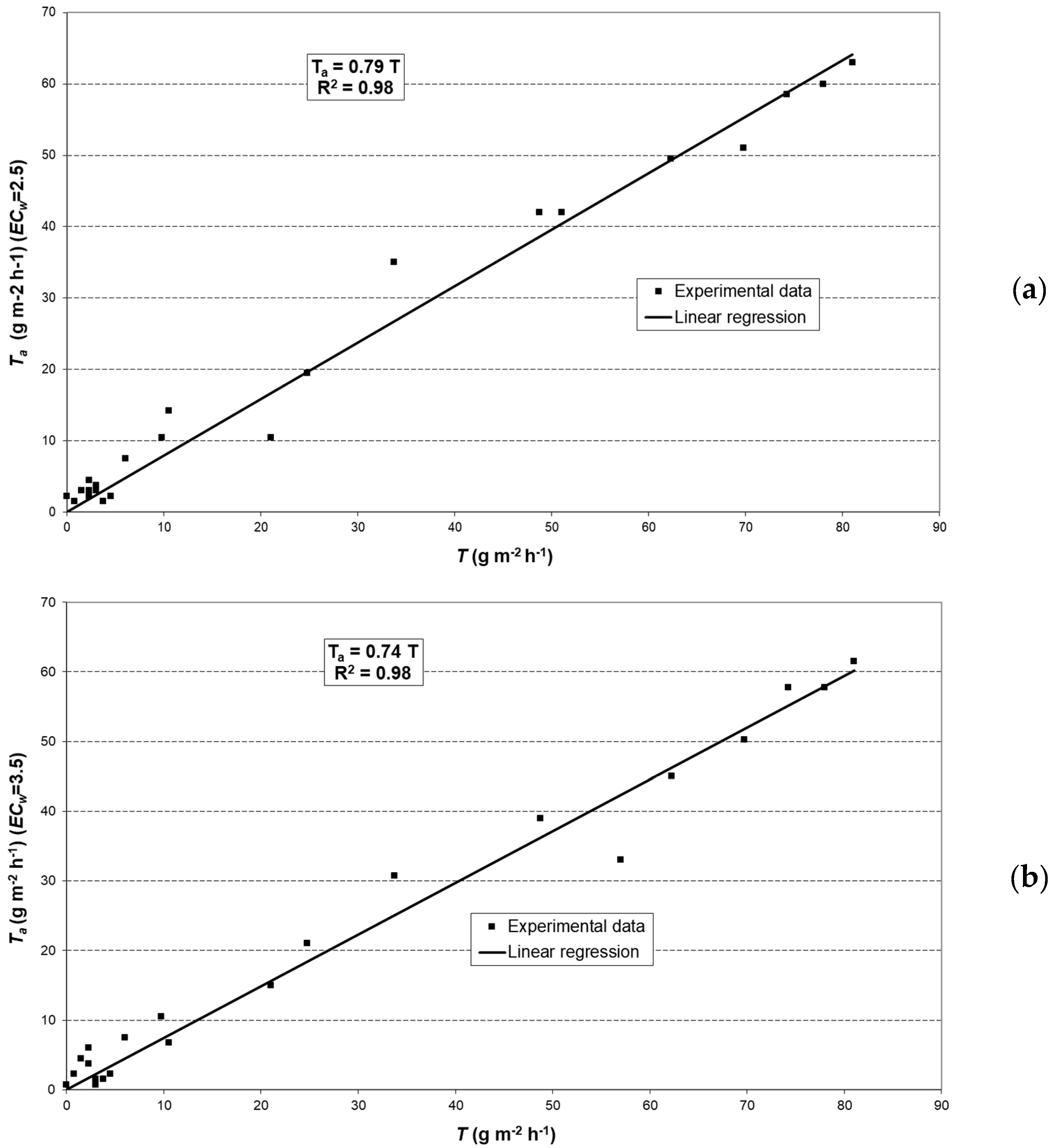
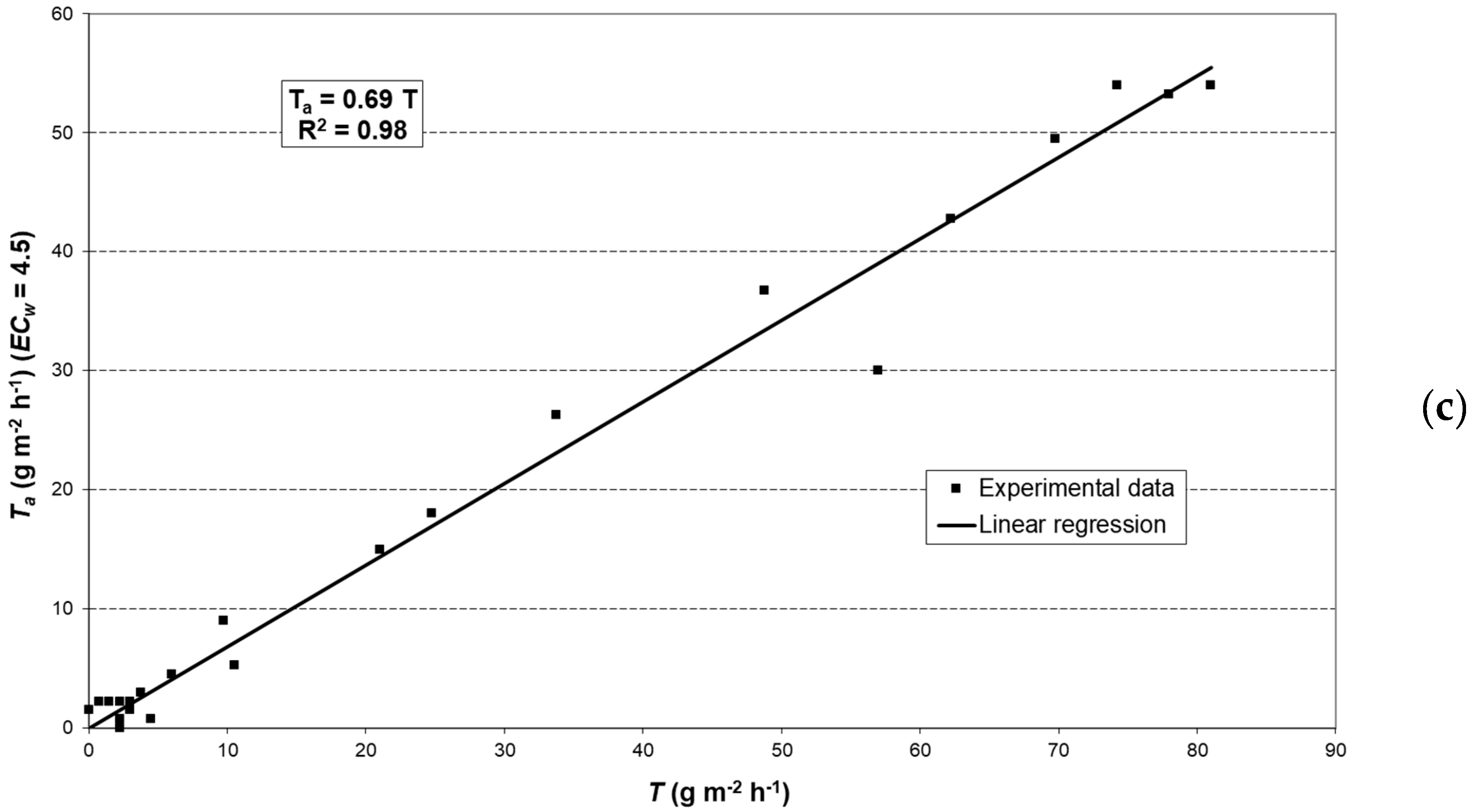
2.6. Modeling Actual Transpiration
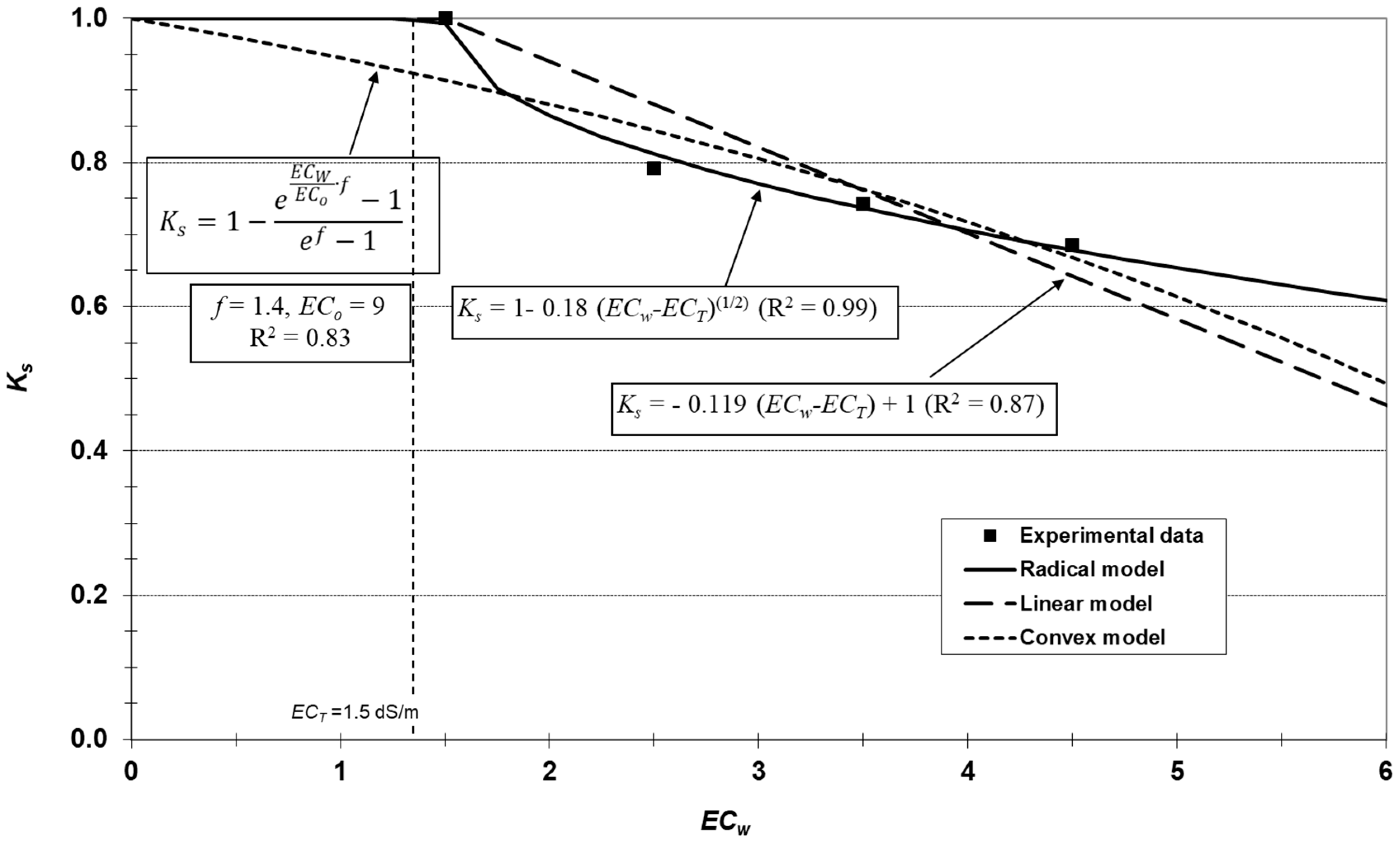
2.7. Saline Stress Curve
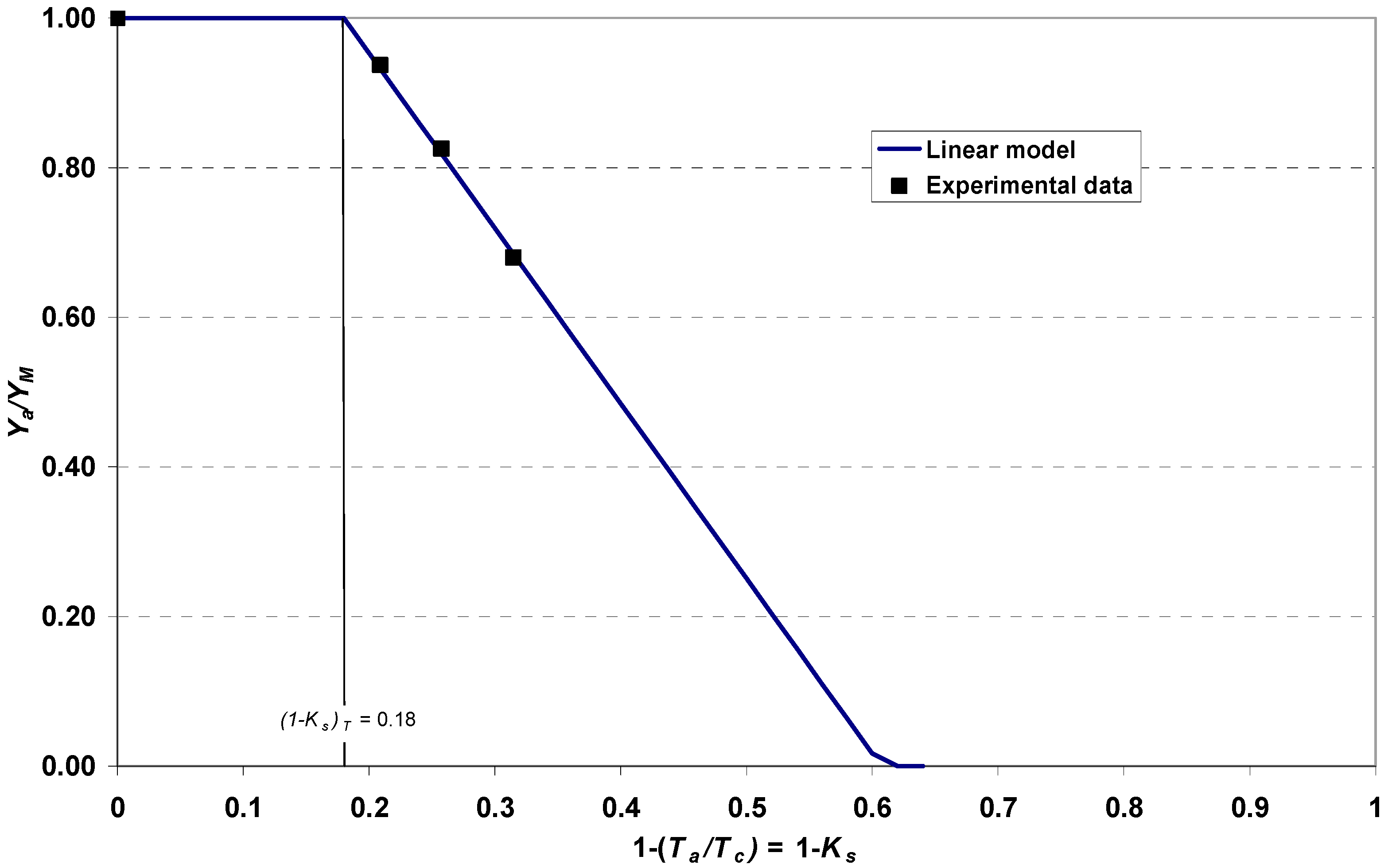
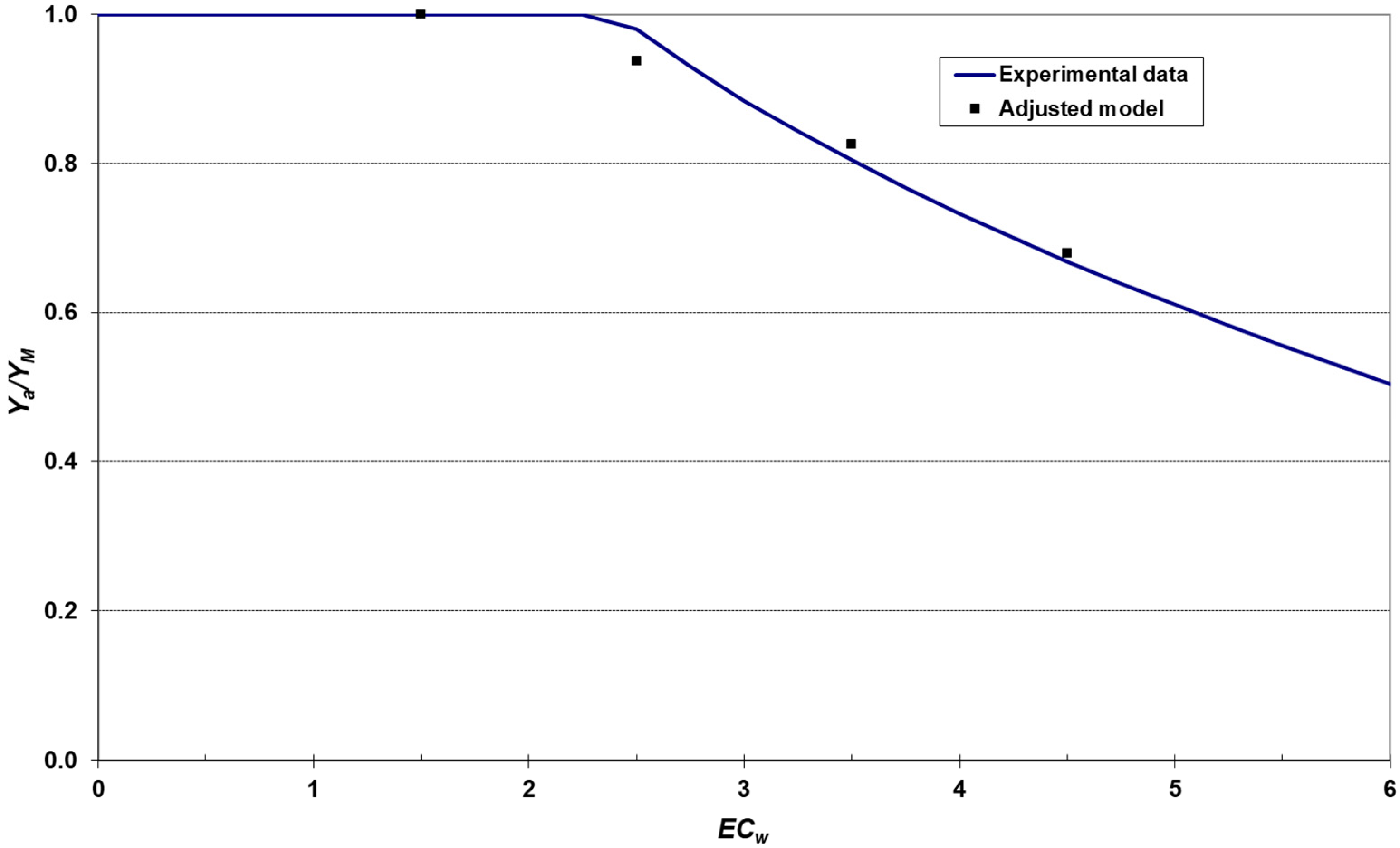
2.8. Modeling the Relationship between Salinity and Crop Yield
3. Results
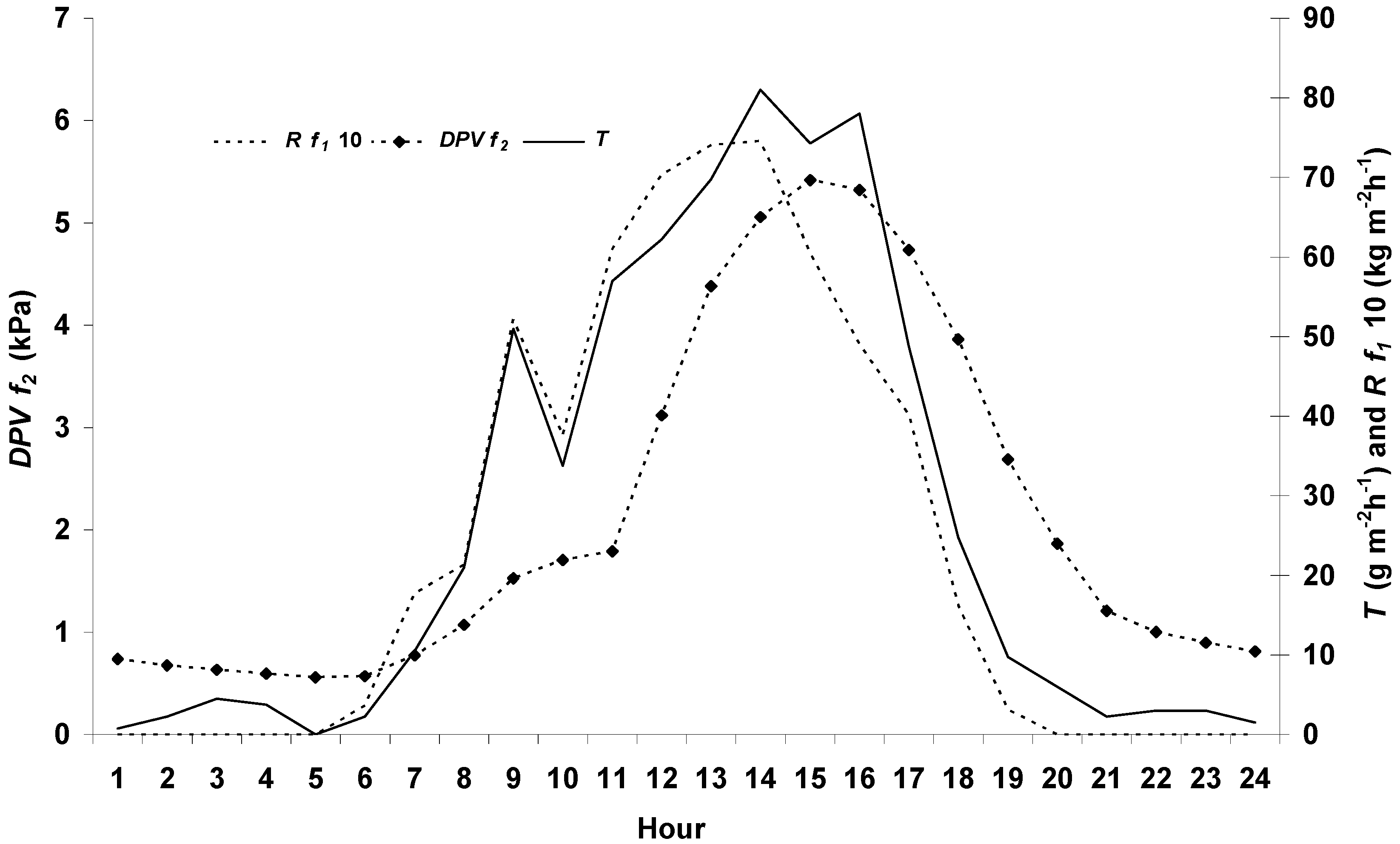
3.1. Environment Conditions
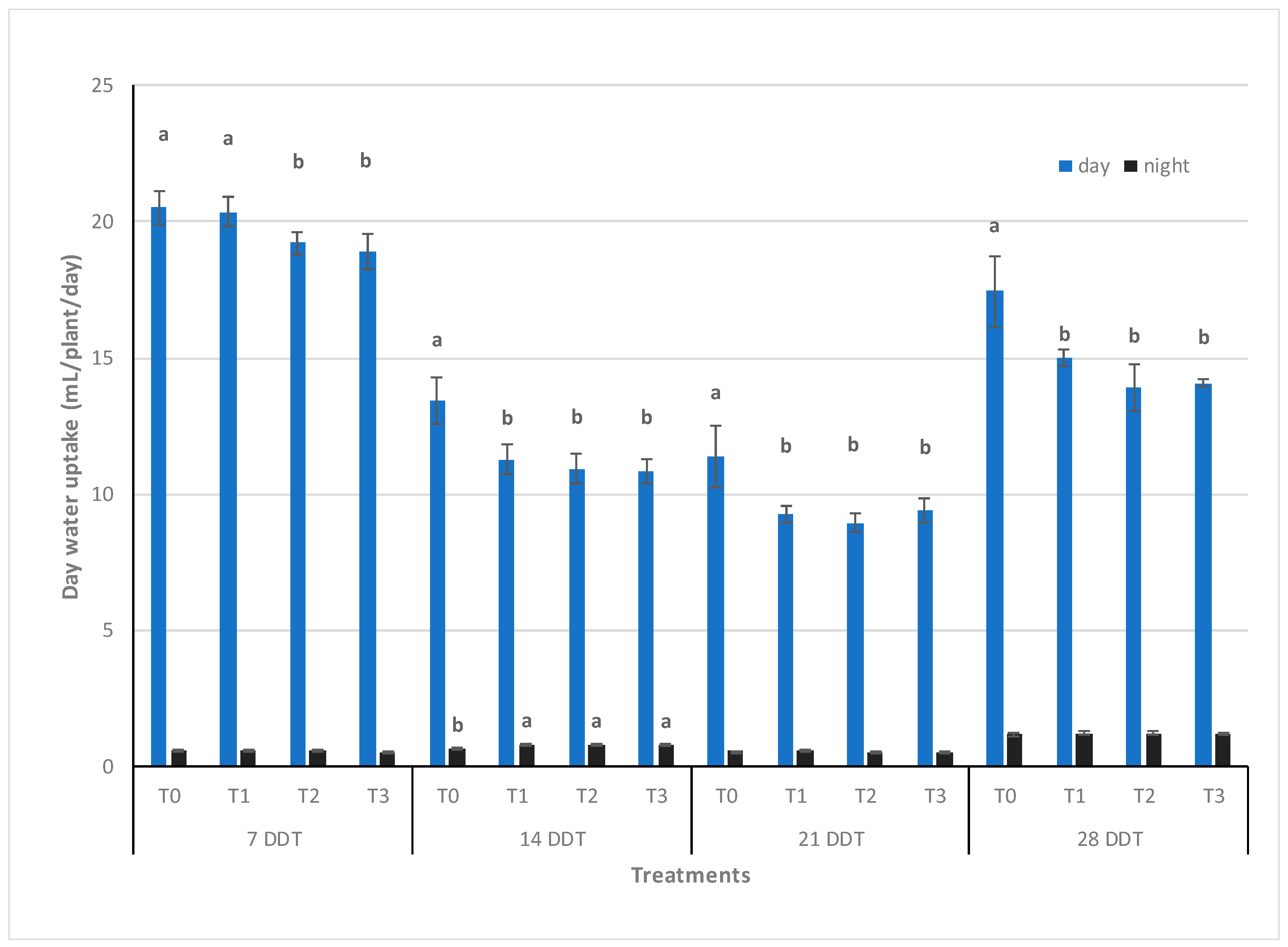
3.2. Water Uptake
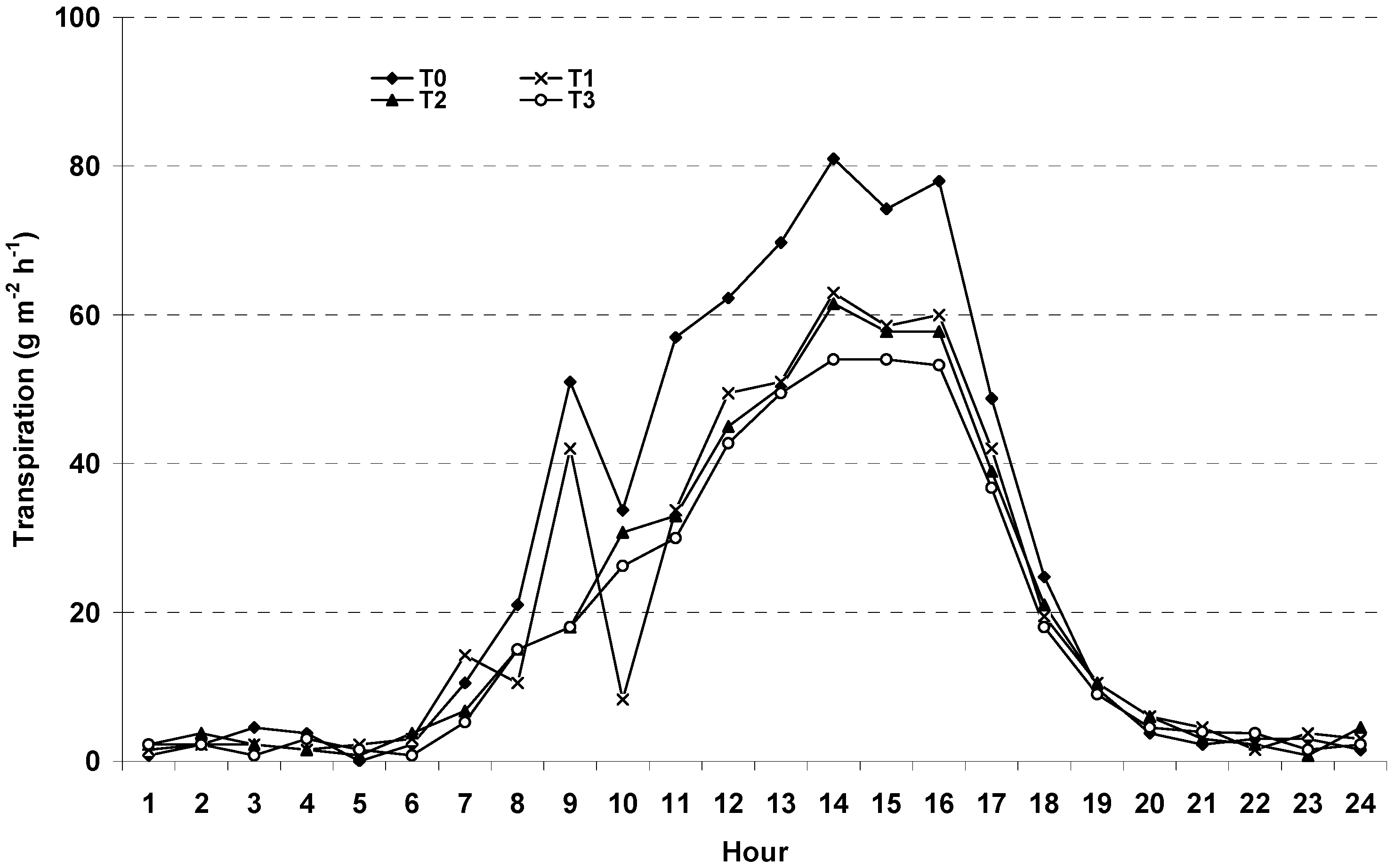
3.3. Potential Crop Transpiration (TC)
3.4. Transpiration Dynamics and Salinity
3.5. Relationship between Salinity and Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- MARM (Ministerio de Medio Ambiente y Medio Rural y Marino). Anuario de Estadística Agroalimentaria; MARM: Madrid, Spain, 2013.
- Martínez, J.; Reca, J. Water use efficiency of surface drip irrigation versus an alternative subsurface drip irrigation method. J. Irrig. Drain. Eng. 2014, 140, 04014030. [Google Scholar] [CrossRef]
- Sánchez, J.A.; Reca, J.; Martínez, J. Irrigation water management in a Mediterranean greenhouse district: Irrigation adequacy assessment. Irrig. Drain. 2015, 64, 299–313. [Google Scholar] [CrossRef]
- Sánchez, J.A.; Reca, J.; Martínez, J. Water productivity in a Mediterranean semi-arid greenhouse district. Water Res. Man. 2015, 29, 5395–5411. [Google Scholar] [CrossRef]
- EEA (European Environmental Agency). Agriculture and Environment in EU-15–The IRENA Indicator Report (N 6/2005); EEA: Copenhagen, Denmark, 2005. [Google Scholar]
- Tanji, K.K.; Kielen, N.C. Agricultural Drainage Water Management in Arid and Semi-Arid Areas; FAO: Rome, Italy, 2002; p. 61. [Google Scholar]
- García-Caparrós, P.; Llanderal, A.; Maksimovic, I.; Lao, M.T. Cascade Cropping System with Horticultural and Ornamental Plants under Greenhouse Conditions. Water 2018, 125, 125. [Google Scholar] [CrossRef]
- Garcia-Caparros, P.; Contreras, I.J.; Baeza, R.; Segura, L.M.; Lao, T.M. Integral Management of Irrigation Water in Intensive Horticultural Systems of Almería. Sustainability 2017, 9, 2271. [Google Scholar] [CrossRef]
- Simpson, R.C.; Franco, G.J.; King, R.S.; Volder, A. Intercropping Halophytes to Mitigate Salinity Stress in Watermelon. Sustainability 2018, 10, 681. [Google Scholar] [CrossRef]
- Plaza, B.M.; Jiménez, S.; Segura, M.L.; Contreras, J.I.; Lao, M.T. Physiological stress caused by salinity in Cordyline fruticosa and its indicators. Comm. Soil Sci. Plant Anal. 2009, 40, 473–484. [Google Scholar] [CrossRef]
- Plaza, B.M.; Jiménez, S.; Lao, M.T. Influence of salt stress on the nutritional state of Cordyline fruticosa var. Red Edge: Chloride, Nitrogen, and Phosphorus. Comm. Soil Sci. Plant Anal. 2012, 43, 226–233. [Google Scholar] [CrossRef]
- Lao, M.T.; Plaza, B.M.; Jiménez, S. Impact of salt stress on micronutrients in Cordyline fruticosa var. “Red Edge”. J. Plant Nutr. 2013, 36, 990–1000. [Google Scholar] [CrossRef]
- Plaza, B.M.; Lao, M.T. Water management in ornamental crops. In Floriculture and Ornamental Biotechnology: Advances and Tropical Issues; Teixeira-da-Silva, J.A., Ed.; Global Science Books: London, UK, 2006; Volume 3, pp. 149–160. [Google Scholar]
- Medrano, E.; Lorenzo, P.; Sánchez-Guerrero, M.C.; Montero, J.I. Evaluation and modelling of greenhouse cucumber-crop transpiration under high and low radiation conditions. Sci. Hort. 2005, 105, 163–175. [Google Scholar] [CrossRef]
- Mathers, H.M.; Case, L.T.; Yeager, T.H. Improving irrigation water use in container nurseries. Horttech 2005, 15, 8–12. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements; UN-FAO: Rome, Italy, 1998; p. 56. [Google Scholar]
- Bailey, B.; Montero, J.; Biel, C.; Wilkinson, D.; Antón, A.; Jolliet, O. Transpiration of Ficus benjamina: Comparison of measurements with predictions of the Penman–Monteith model and a simplified version. Agric. For. Meteorol. 1993, 65, 229–243. [Google Scholar] [CrossRef]
- Bakker, J.C. Leaf conductance of four glasshouse vegetable crop as affected by air humidity. Agric. For. Meteorol. 1991, 55, 23–36. [Google Scholar] [CrossRef]
- Baille, M.; Baille, A.; Laury, J.C. A simplified model for predicting evapotranspiration rate of nine ornamental species vs. climate factors and leaf area. Sci. Hort. 1994, 59, 217–232. [Google Scholar] [CrossRef]
- Montero, J.I.; Antón, A.; Muñoz, P.; Lorenzo, P. Transpiration from geranium grown under high temperatures and low humidities in greenhouses. Agric. For. Meteorol. 2001, 107, 323–332. [Google Scholar] [CrossRef]
- Fernández, M.D.; Orgaz, F.; Fereres, E.; López, J.C.; Céspedes, A.; Pérez-Parra, J.; Bonachela, S.; Gallardo, M. Programación Del Riego de Cultivos Hortícolas Bajo Invernadero, en el Sudeste Español; CAJAMAR (Caja Rural Intermediterránea): Almería, Spain, 2001; p. 78. [Google Scholar]
- Baille, M.; Baille, A.; Delmon, D. Microclimate and transpiration of greenhouse rose crops. Agric. For. Meteorol. 1994, 71, 83–97. [Google Scholar] [CrossRef]
- Katsoulas, N.; Baille, A.; Kittas, C. Effect of misting on transpiration and conductances of a greenhouse rose canopy. Agric. For. Meteorol. 2001, 106, 233–247. [Google Scholar] [CrossRef]
- Lorenzo, P.; Medrano, E.; García, M. Irrigation management in perlite. Acta Hort. 1993, 335, 429–434. [Google Scholar] [CrossRef]
- Lieth, J.H.; Burger, D.W. Growth of chrysanthemum using an irrigation system controlled by soil moisture tension. J. Am. Soc. Hort. Sci. 1989, 114, 387–392. [Google Scholar]
- Dudley, L.M.; Ben-Gal, A.; Shani, U. Influence of plant, soil and water properties on the leaching fraction. Vadose Zone. J. 2008, 7, 420–425. [Google Scholar] [CrossRef]
- Maas, E.V.; Hoffman, G.J. Crop salt tolerance-current assessment. J. Irrig. Drain. Div. 1977, 103, 115–134. [Google Scholar]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; FAO: Rome, Italy, 1985; p. 29. [Google Scholar]
- Tanji, K.K. Irrigation with marginal quality waters: Issues. J. Irrig. Drain. Eng. 1997, 123, 165–169. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Z.; Li, Y.; He, K. The Effects of Saline Stress on the Growth of Two Shrub Species in the Qaidam Basin of Northwestern China. Sustainability 2019, 11, 838. [Google Scholar] [CrossRef]
- Hossain, M.M.; Nonami, H. Effect of salt stress on physiological response of tomato fruit grown in hydroponic culture system. Hort. Sci. 2012, 39, 26–32. [Google Scholar]
- Karlberg, L.; Ben-Gal, A.; Jansson, P.E.; Shani, U. Modelling transpiration and growth in salinity-stressed tomato under different climatic conditions. Ecol. Model. 2006, 190, 15–40. [Google Scholar] [CrossRef]
- Steduto, P.; Hsiao, T.C.; Fereres, E.; Raes, D. Crop Yield Response to Water; FAO: Rome, Italy, 2012; p. 66. [Google Scholar]
- Hsiao, T.C.; Steduto, P.; Fereres, E. A systematic and quantitative approach to improve water use efficiency in agriculture. Irrig. Sci. 2007, 25, 209–231. [Google Scholar] [CrossRef]
- Steduto, P.; Hsiao, T.C.; Raes, D.; Fereres, E. Aquacrop—The FAO Crop Model to Simulate Yield Response to Water: I. Concepts and Underlying Principles. Agron. J. 2009, 101, 426–437. [Google Scholar] [CrossRef]
- Grant, R.F. Salinity, water use and yield of maize—testing of the mathematical model Ecosys. Plant Soil. 1995, 172, 309–322. [Google Scholar] [CrossRef]
- Van Dam, J.C.; Groenendijk, P.; Hendriks, R.F.A.; Kroes, J.G. Advances of modeling water flow in variably saturated soils with SWAP. Vadose Zone J. 2008, 7, 640–653. [Google Scholar] [CrossRef]
- Lao, M.T.; Jiménez, S.; Fernández, E.J.; Camacho, F. Climatic parameters of solar buried greenhouse (Insole) under Mediterranean conditions. Acta Hort. 2003, 614, 453–460. [Google Scholar]
- USSL (United States Salinity Laboratory Staff). Diagnosis and Improvement of Saline and Alkali Soils; U.S. Dept. of Agriculture: Washington, DC, USA, 1954; Volume 60, p. 160.
- Urban, I.; Brun, R.; Urban, L. Influence of electrical conductivity, relative humidity and seasonal variations on the behaviour of cut roses produced in soilless culture. Acta Hort. 1995, 408, 101–107. [Google Scholar] [CrossRef]
- Correia, P.J.; Gama, F.; Pestana, M.; Martins-Loução, M.A. Tolerance of young (Ceratonia siliqua L.) carob rootstock to NaCl. Agric. Water Man. 2010, 97, 910–916. [Google Scholar] [CrossRef]
- Doorenbos, J.; Kassam, A.H. Yield Response to Water: FAO Irrigation and Drainage paper nº 33; FAO: Rome, Italy, 1979; p. 33. [Google Scholar]
- Sharma, N.; Gupta, N.K.; Gupta, S.; Hasegawa, H. Effect of NaCl salinity on photosynthetic rate, transpiration rate, and oxidative stress tolerance in contrasting wheat genotypes. Photosynthetica 2005, 43, 609–613. [Google Scholar] [CrossRef]
- Chen, S.; Li, J.; Wang, S.; Fritz, E.; Huttermann, A.; Altman, A. Effects of NaCl on shoot growth, transpiration, ion compartmentation, and transport in regenerated plants of Populus euphratica and Populus tomentosa. Can. J. For. Res. 2003, 33, 967–975. [Google Scholar] [CrossRef]
- Li, Y. Effect of transpiration control under high salinity in a greenhouse on tomato yield. Trans. Chin. Soc. Agric. Eng. 2001, 17, 85–89. [Google Scholar]
- Klepper, B. Root growth and water uptake. In Irrigation of Agriculture Crops; Nielsen, D.R., Ed.; American Society of Agronomy, Inc., Crop Science of America, Inc., Soil Science of America, Inc.: Madison, WI, USA, 1990; pp. 281–322. [Google Scholar]
- Van-Ieperen, W. Dynamic effects of changes in electric conductivity on transpiration and growth of greenhouse-grown tomato plants. J. Hortic. Sci. 1996, 71, 481–496. [Google Scholar] [CrossRef]
- Silberbush, M.; Ben-Asher, J. Simulation study of nutrient uptake by plants from soilless cultures as affected by salinity buildup and transpiration. Plant Soil. 2001, 233, 59–69. [Google Scholar] [CrossRef]
- Masuda, M.; Tanaka, T.; Matsunari, S. Uptake of water and minerals during the day and the night in tomato and cucumber plants. J. Jpn. Soc. Hortic. Sci. 1990, 58, 951–957. [Google Scholar] [CrossRef]
- Cachorro, P.; Martinez, R.; Ortiz, A.; Cerdá, A. Abscisic Acid and Osmotic Relations in Phaseolus Vulgaris L. Shoots under Salt Stress. J. Plant Growth Reg. New York 1995, 14, 99–104. [Google Scholar] [CrossRef]
- Al-Hamdani, S.H. Influence of variable NaCl concentrations on selected physiological responses of kudzu. Asian J. Plant Sci. 2004, 3, 114–119. [Google Scholar]
- Leidi, E.O.; Silberbush, M.; Lips, S.H. Wheat growth as affected by nitrogen type, pH and salinity. II. Photosynthesis and transpiration. J. Plant Nutr. 1991, 14, 247–256. [Google Scholar] [CrossRef]
- Romero-Aranda, R.; Soria, T.; Cuartero, J. Greenhouse mist improves yield of tomato plants grown under saline conditions. J. Am. Soc. Hortic. Sci. 2002, 127, 644–648. [Google Scholar] [CrossRef]
- Fricke, W.; Akhiyarova, G.; Veselov, D.; Kudoyarova, G. Rapid and tissue-specific changes in ABA and in growth rate in response to salinity in barley leaves. J. Exp. Bot. 2004, 55, 1115–1123. [Google Scholar] [CrossRef]
- Tattini, M.; Montagni, G.; Traversi, M.L. Gas exchange, water relations and osmotic adjustment in Phillyrea latifolia grown at various salinity concentrations. Tree Physiol. 2002, 22, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.K.; Clifford, S.C.; Wanek, W.; Jones, H.G.; Popp, M. Physiological and morphological adaptations of the fruit tree Ziziphus rotundifolia in response to progressive drought stress. Tree Physiol. 2001, 21, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Marigo, G.; Peltier, J.P. Analysis of the diurnal change in osmotic potential in leaves of Fraxinus excelsior L. J. Exp. Bot. 1996, 47, 763–769. [Google Scholar] [CrossRef]
- Nativ, R.; Ephrath, J.; Berliner, P.; Saranga, Y. Drought resistance and water use efficiency in Acacia saligna. Aust. J. Bot. 1999, 47, 577–586. [Google Scholar] [CrossRef]
- Mori, M.; Amato, M.; Di-Mola, I.; Caputo, R.; Quaglietta-Chiarandà, F.; Di-Tommaso, T. Productive behaviour of “cherry”—Type tomato irrigated with saline water in relation to nitrogen fertilisation. Eur. J. Agron. 2008, 29, 135–143. [Google Scholar] [CrossRef]
| EC. | ψ0 | Na+ | Cl− | |
|---|---|---|---|---|
| T0 | 1.5 | −54 | 14.3 | 11.6 |
| T1 | 2.5 | −90 | 22.2 | 22.4 |
| T2 | 3.5 | −126 | 32.7 | 31.8 |
| T3 | 4.5 | −162 | 38.2 | 37.4 |
| Climatic Conditions | Root Conditions | |||
|---|---|---|---|---|
| Global Radiation (W m−2) | Temperature (°C) | VPD (kPa) | Temperature (°C) | |
| Average of max. | 550 | 35.46 | −3.56 | 34.31 |
| Average of min. | 0 | 19.46 | −0.60 | 20.38 |
| Average of aver. | 170 | 26.76 | −1.80 | 26.75 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plaza, B.M.; Reca, J.; Martínez, J.; Alex, F.; Lao, M.T. Sustainable Irrigation Management of Ornamental Cordyline Fruticosa “Red Edge” Plants with Saline Water. Sustainability 2019, 11, 3751. https://doi.org/10.3390/su11133751
Plaza BM, Reca J, Martínez J, Alex F, Lao MT. Sustainable Irrigation Management of Ornamental Cordyline Fruticosa “Red Edge” Plants with Saline Water. Sustainability. 2019; 11(13):3751. https://doi.org/10.3390/su11133751
Chicago/Turabian StylePlaza, Blanca M., Juan Reca, Juan Martínez, Francisco Alex, and Maria Teresa Lao. 2019. "Sustainable Irrigation Management of Ornamental Cordyline Fruticosa “Red Edge” Plants with Saline Water" Sustainability 11, no. 13: 3751. https://doi.org/10.3390/su11133751
APA StylePlaza, B. M., Reca, J., Martínez, J., Alex, F., & Lao, M. T. (2019). Sustainable Irrigation Management of Ornamental Cordyline Fruticosa “Red Edge” Plants with Saline Water. Sustainability, 11(13), 3751. https://doi.org/10.3390/su11133751






