Pyrolysis and Thermogravimetric Study to Elucidate the Bioenergy Potential of Novel Feedstock Produced on Poor Soils While Keeping the Environmental Sustainability Intact
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Production and Cost Estimation
2.2. Proximate and Ultimate Analyses
2.3. High Heating Values (MJ kg−1)
2.4. Coupled Thermogravimetric Analysis and Differential Scanning Calorimetry
2.5. Mathematical Model Applied
2.6. Calculating the Kinetic Parameters
3. Results and Discussion
3.1. Biomass Yields and Cost of Production
3.2. Physicochemical Properties of the Grasses
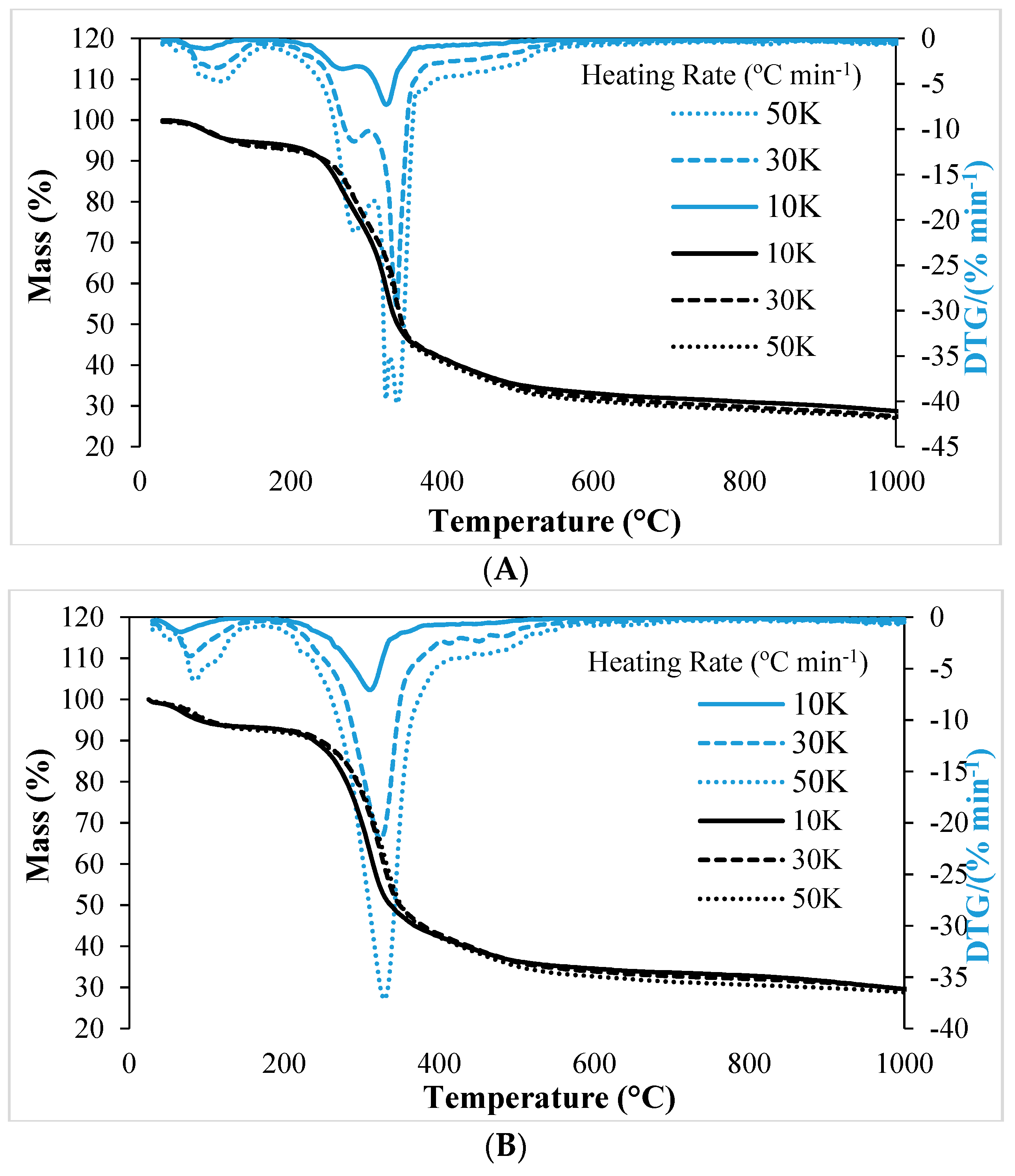
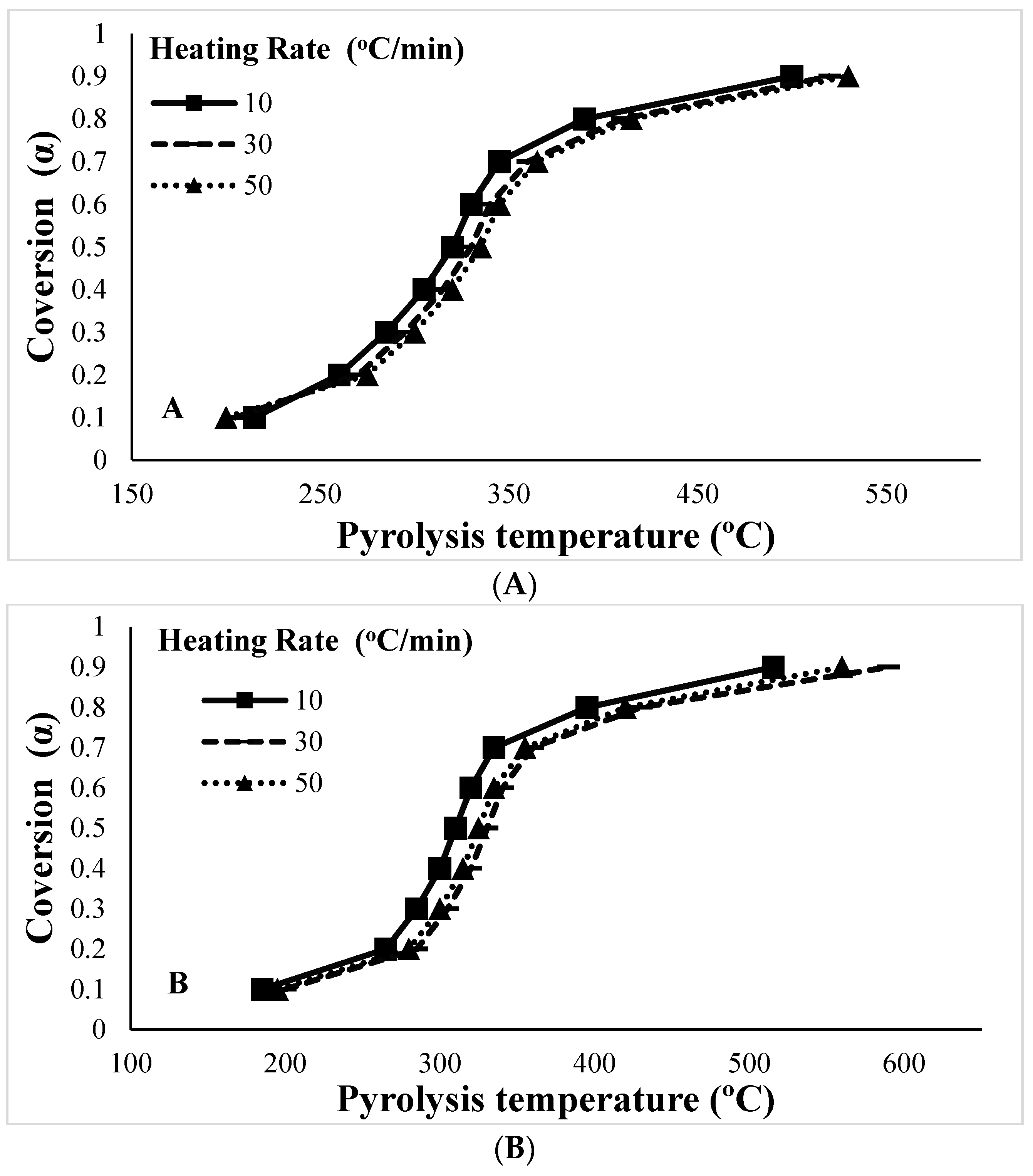
3.3. The Pyrolytic Behavior and Product Formation
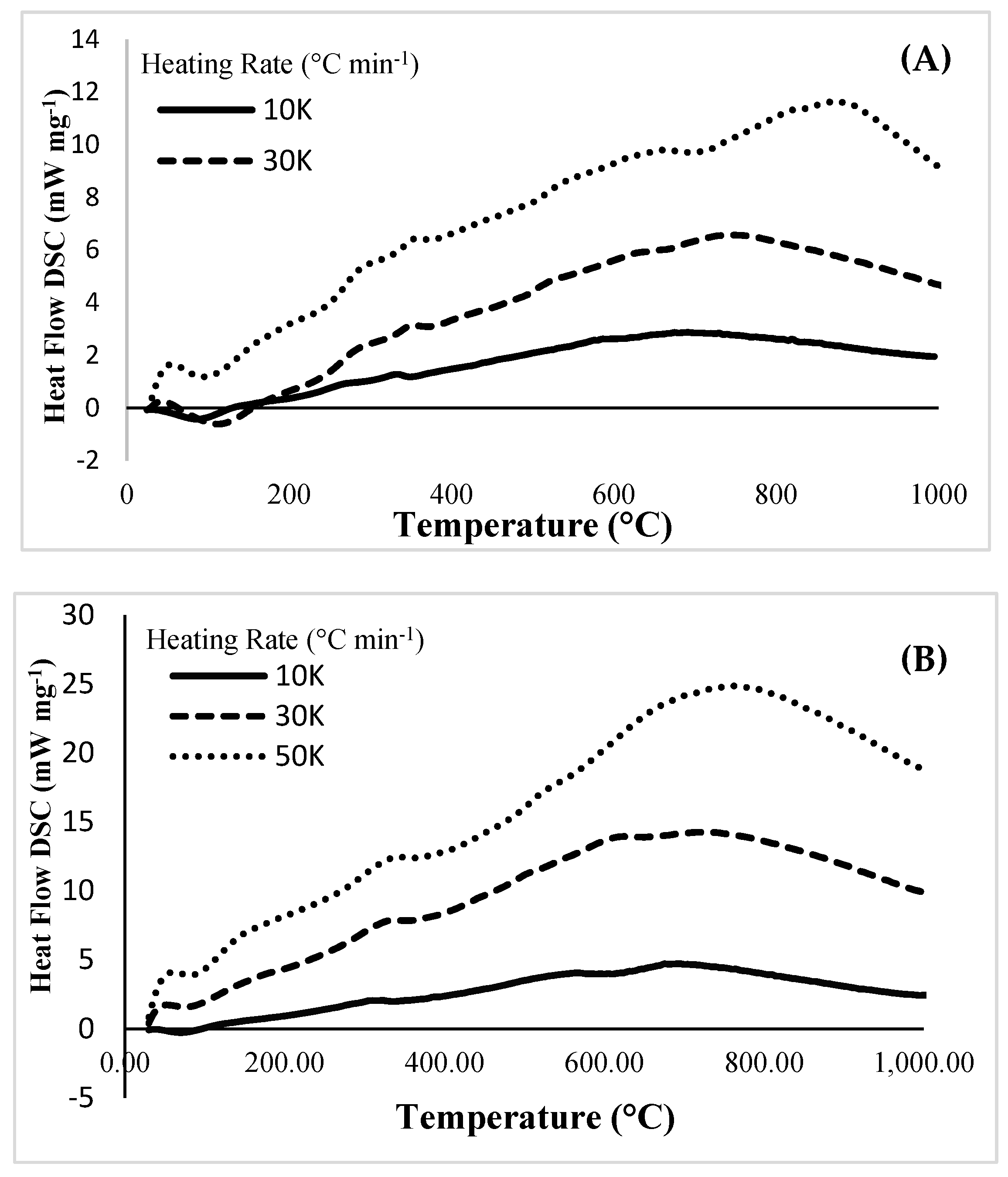
3.4. Heat Flow Measurement during Pyrolysis Reaction
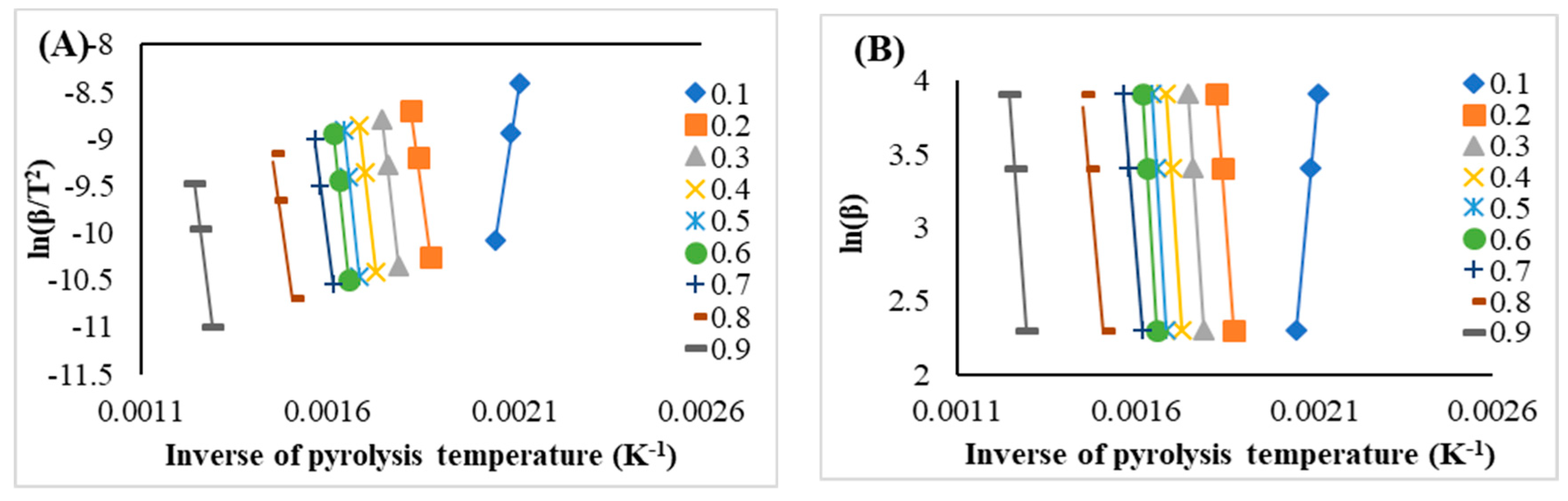
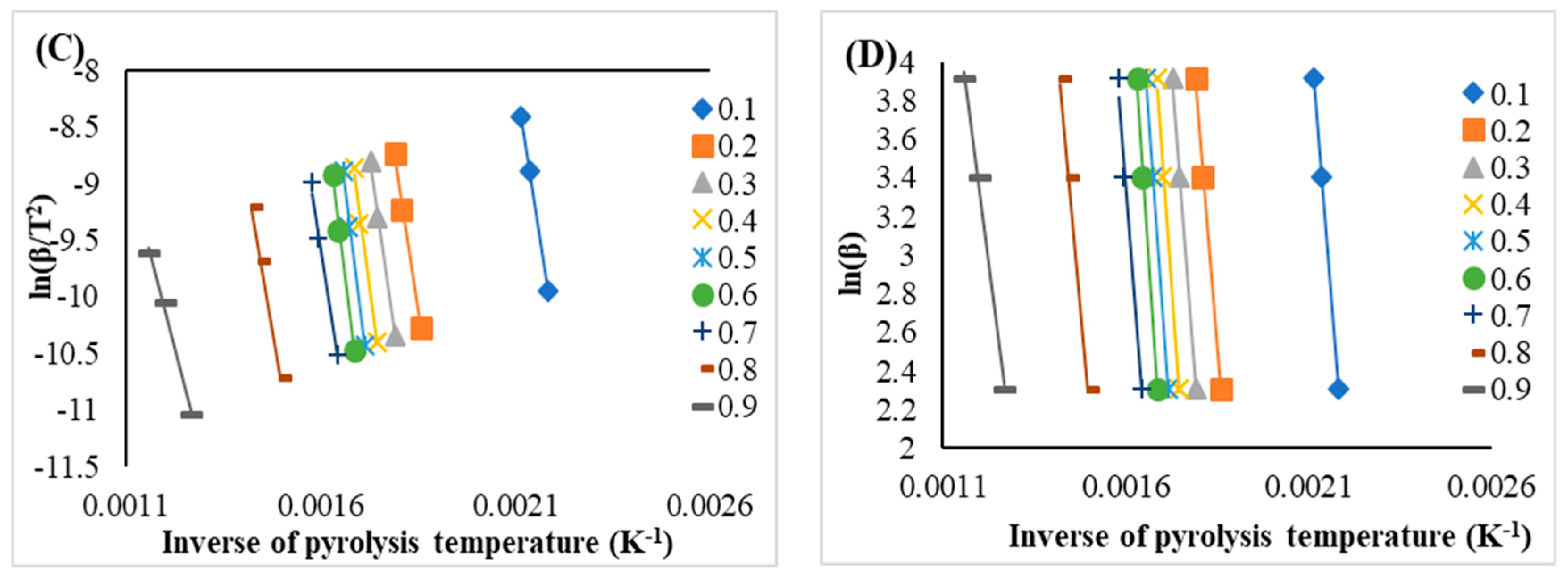
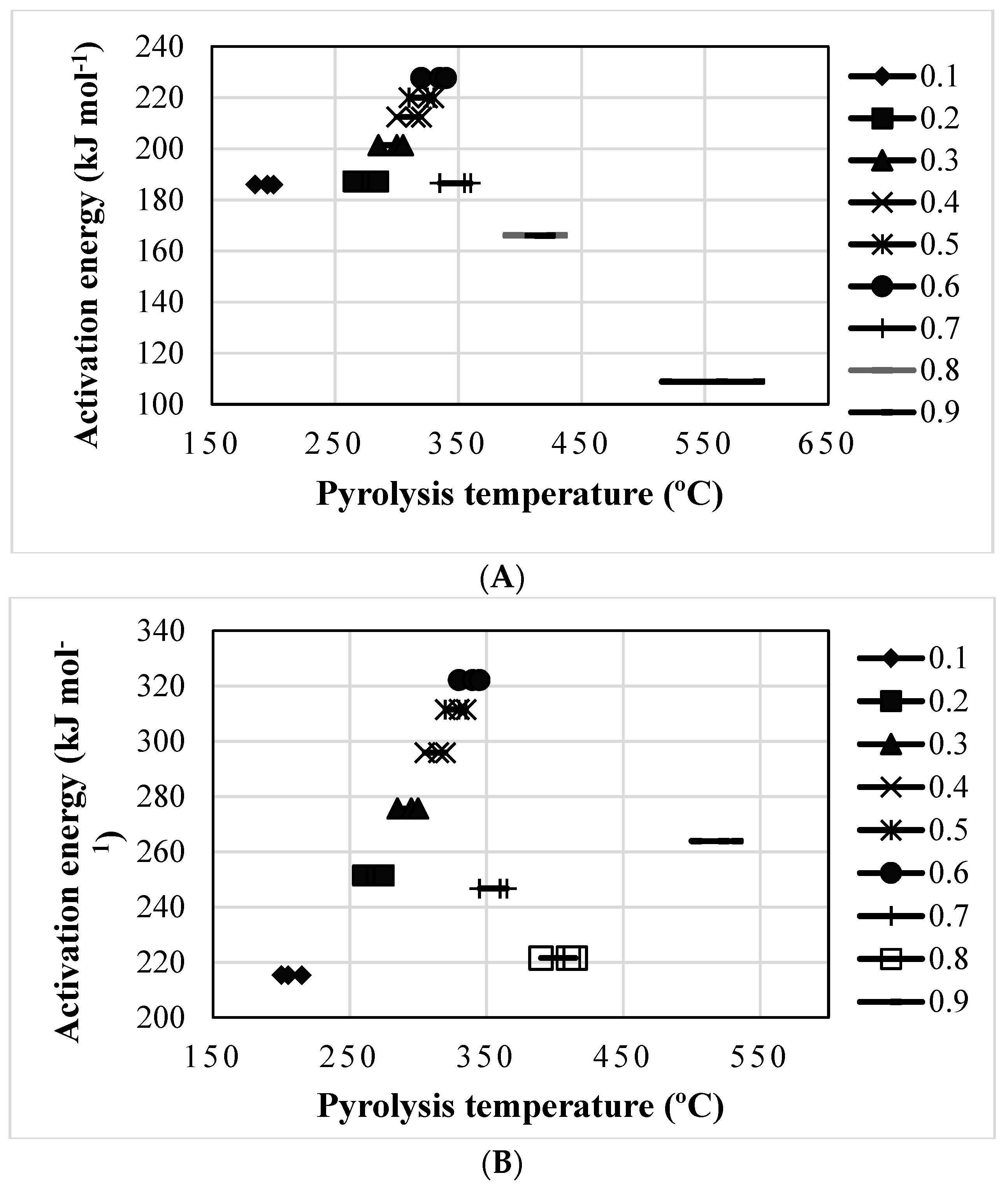
3.5. The Activation Energies and Reaction Enthalpies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Initial mass | |
| Change in mass | |
| Residual mass | |
| Pre-exponential factor (s−1) | |
| Velocity constant | |
| Differential coefficient mechanism function | |
| Heating rate (°C min−1) | |
| Ea | Activation energy (kJ mol−1) |
| Function of the conversion degree (α) | |
| Universal gas constant (8.314 J K−1 mol−1) | |
| Pyrolysis temperature (K) | |
| Boltzmann constant ( JK−1) | |
| Plank’s constant Js) | |
| DTG peak temperature (K) | |
| Polynomial Quantity | |
| Enthalpy (kJ mol−1) | |
| K | Kelvin |
| kJ | Kilo Joule |
| mol | Moles |
| α | Conversion |
References
- Saqib, A.; Tabbssum, M.R.; Rashid, U.; Ibrahim, M.; Gill, S.S.; Mehmood, M.A. Marine macro algae Ulva: A potential feed-stock for bio-ethanol and biogas production. Asian J. Agri. Biol. 2013, 1, 155–163. [Google Scholar]
- Mehmood, M.A.; Ibrahim, M.; Rashid, U.; Nawaz, M.; Ali, S.; Hussain, A.; Gull, M. Biomass production for bioenergy using marginal lands. Sustain. Prod. Consum. 2017, 9, 3–21. [Google Scholar] [CrossRef]
- Liao, J.C.; Mi, L.; Pontrelli, S.; Luo, S. Fuelling the future: Microbial engineering for the production of sustainable biofuels. Nat. Rev. Microbiol. 2016, 14, 288–304. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.S.; Mehmood, M.A.; Al Ayed, O.S.; Ye, G.; Luo, H.; Ibrahim, M.; Rashid, U.; Nehdi, I.A.; Qadir, G. Kinetic analyses and pyrolytic behavior of Para grass (Urochloa mutica) for its bioenergy potential. Bioresour. Technol. 2017, 224, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Boni, M.R.; Chiavola, A.; Marzeddu, S. Application of biochar to the remediation of Pb-contaminated solutions. Sustainability 2018, 10, 4440. [Google Scholar] [CrossRef]
- Graham, R.; Bergougnou, M.; Overend, R. Fast pyrolysis of biomass. J. Anal. Appl. Pyrolysis 1984, 6, 95–135. [Google Scholar] [CrossRef]
- Patel, M.; Zhang, X.; Kumar, A. Techno-economic and life cycle assessment on lignocellulosic biomass thermochemical conversion technologies: A review. Renew. Sustain. Energy Rev. 2016, 53, 1486–1499. [Google Scholar] [CrossRef]
- Rogers, J.; Brammer, J. Estimation of the production cost of fast pyrolysis bio-oil. Biomass Bioenergy 2012, 36, 208–217. [Google Scholar] [CrossRef]
- Pennsylvania State University. 2013. Available online: https://extension.psu.edu/natural-resources/energy (accessed on 25 May 2019).
- Slopiecka, K.; Bartocci, P.; Fantozzi, F. Thermogravimetric analysis and kinetic study of poplar wood pyrolysis. Appl. Energy 2012, 97, 491–497. [Google Scholar] [CrossRef]
- Maia, A.A.D.; de Morais, L.C. Kinetic parameters of red pepper waste as biomass to solid biofuel. Bioresour. Technol. 2016, 204, 157–163. [Google Scholar] [CrossRef]
- Heo, H.S.; Park, H.J.; Park, Y.-K.; Ryu, C.; Suh, D.J.; Suh, Y.W.; Yim, J.H.; Kim, S.S. Bio-oil production from fast pyrolysis of waste furniture sawdust in a fluidized bed. Bioresour. Technol. 2010, 101, S91–S96. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.M.; Melo, D.M.; Aquino, F.M.; Freitas, J.C.; Melo, M.A.; Barros, J.M.; Fontes, M.S. Characterization and comparative study of pyrolysis kinetics of the rice husk and the elephant grass. J. Therm. Anal. Calorim. 2014, 115, 1915–1920. [Google Scholar] [CrossRef]
- Wu, W.; Mei, L.; Zhang, Y.; Liu, R.; Cai, J. Kinetics and reaction chemistry of pyrolysis and combustion of tobacco waste. Fuel 2015, 156, 71–80. [Google Scholar] [CrossRef]
- Mehmood, M.A.; Ye, G.; Luo, H.; Liu, C.G.; Malik, S.; Afzal, I.; Xu, J.; Ahmad, M.S. Pyrolysis and kinetic analyses of Camel grass (Cymbopogon schoenanthus) for bioenergy. Bioresour. Technol. 2017, 228, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Mohomane, S.M.; Motaung, T.E.; Revaprasadu, N. Thermal degradation kinetics of sugarcane bagasse and soft wood cellulose. Materials 2017, 10, 1246. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, L.; Ma, C.; Zhang, Y. Thermal behavior of sweet potato starch by non-isothermal thermogravimetric analysis. Materials 2019, 12, 699. [Google Scholar] [CrossRef] [PubMed]
- Khiari, B.; Moussaoui, M.; Jeguirim, M. Tomato-processing by-product combustion: Thermal and kinetic analyses. Materials 2019, 12, 553. [Google Scholar] [CrossRef] [PubMed]
- Volpe, M.; Anna, C.; Messineo, S.; Volpe, R.; Messineo, A. Sustainable production of bio-combustibles from pyrolysis of agro-industrial wastes. Sustainability 2014, 6, 7866–7882. [Google Scholar] [CrossRef]
- Adkins, S.; Shabbir, A. Biology, ecology and management of the invasive parthenium weed (Parthenium hysterophorus L.). Pest Manag. Sci. 2014, 70, 1023–1029. [Google Scholar] [CrossRef]
- Navie, S.; Panetta, F.; McFadyen, R.; Adkins, S. Behaviour of buried and surface-sown seeds of Parthenium. Weed Res. 1998, 38, 335–341. [Google Scholar] [CrossRef]
- Bilal, M.; Younas, M.; Ahmad, S. Yield of Mott grass as affected by varying levels of nitrogen and farmyard manure application. Int. J. Agri. Biol. 2001, 3, 73–74. [Google Scholar]
- Nhuchhen, D.R.; Salam, P.A. Estimation of higher heating value of biomass from proximate analysis: A new approach. Fuel 2012, 99, 55–63. [Google Scholar] [CrossRef]
- Coats, A.; Redfern, J. Kinetic parameters from thermogravimetric data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. A quick, direct method for the determination of activation energy from thermogravimetric data. J. Polym. Sci. B 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Akahira, T.; Sunose, T. Transactions of joint convention of four electrical institutes. J. Sci. Educ. Technol. 1971, 16, 22–31. [Google Scholar]
- Doyle, C. Kinetic analysis of thermogravimetric data. J. Appl. Polym. Sci. 1961, 5, 285–292. [Google Scholar] [CrossRef]
- Jeguirim, M.; Dorge, S.; Trouve, G. Thermogravimetric analysis and emission characteristics of two energy crops in air atmosphere: Arundo donax and Miscanthus giganthus. Bioresour. Technol. 2010, 101, 788–793. [Google Scholar] [CrossRef]
- Paulrud, S.; Nilsson, C. Briquetting and combustion of spring-harvested reed canary-grass: Effect of fuel composition. Biomass Bioenergy 2001, 20, 25–35. [Google Scholar] [CrossRef]
- Howaniec, N.; Smolinski, A. Steam gasification of energy crops of high cultivation potential in Poland to hydrogen-rich gas. Int. J. Hydrogen Energy 2011, 36, 2038–2043. [Google Scholar] [CrossRef]
- Monti, A.; Di Virgilio, N.; Venturi, G. Mineral composition and ash content of six major energy crops. Biomass Bioenergy 2008, 32, 216–223. [Google Scholar] [CrossRef]
- Yan, H.L.; Zong, Z.M.; Li, Z.K.; Kong, J.; Zheng, Q.X.; Li, Y.; Wei, X.Y. Sweet sorghum stalk liquefaction in supercritical methanol: Effects of operating conditions on product yields and molecular composition of soluble fraction. Fuel Process. Technol. 2016, 155, 42–50. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, B. Investigation of thermodynamic parameters in the pyrolysis conversion of biomass and manure to biochars using thermogravimetric analysis. Bioresour. Technol. 2013, 146, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Biney, P.O.; Gyamerah, M.; Shen, J.; Menezes, B. Kinetics of the pyrolysis of arundo, sawdust, corn stover and switch grass biomass by thermogravimetric analysis using a multi-stage model. Bioresour. Technol. 2015, 179, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.H.; Çakman, G.; Goldfarb, J.L.; Topcu, Y.; Naqvi, S.R.; Ceylan, S. Demonstrating the suitability of Canola residue biomass to biofuel conversion via pyrolysis through reaction Kinetics, thermodynamics and evolved gas analyses. Bioresour. Technol. 2019, 279, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.H.; Zhao, Z.; Ren, J.; Rasool, T.; Naqvi, S.R. Thermo-kinetics and gaseous product analysis of banana peel pyrolysis for its bioenergy potential. Biomass Bioenergy 2019, 122, 193–201. [Google Scholar] [CrossRef]
- Chong, C.T.; Mong, G.R.; Ng, J.H.; Chong, W.W.F.; Ani, F.N.; Lam, S.S.; Ong, H.C. Pyrolysis characteristics and kinetic studies of horse manure using thermogravimetric analysis. Energy Convers. Manag. 2019, 180, 1260–1267. [Google Scholar] [CrossRef]
- Li, D.; Chen, L.; Chen, S.; Zhang, X.; Chen, F.; Ye, N. Comparative evaluation of the pyrolytic and kinetic characteristics of a macroalga (Sargassum thunbergii) and a freshwater plant (Potamogeton crispus). Fuel 2012, 96, 185–191. [Google Scholar] [CrossRef]
- Sanchez-Jimenez, P.E.; Perez-Maqueda, L.A.; Pereon, A.; Criado, J.M. Generalized master plots as a straightforward approach for determining the kinetic model: The case of cellulose pyrolysis. Thermochim. Acta 2013, 552, 54–59. [Google Scholar] [CrossRef]
- Vlaev, L.; Georgieva, V.; Genieva, S. Products and kinetics of non-isothermal decomposition of vanadium (IV) oxide compounds. J. Therm. Anal. Calorim. 2007, 88, 805–812. [Google Scholar] [CrossRef]
| Soil Properties | Normal Soil | Salt-Affected Soil (Used in this Study) |
|---|---|---|
| pHs | 7.4–7.8 | 8.41 |
| Electrical Conductivity (ECe) dS m−1 | 2.78 | 4.23 |
| Sodium Adsorption Ratio (SAR) (mmol L−1)1/2 | 12.13 | 18.56 |
| Grasses | Proximate Analyses | Elemental Composition (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VM (%) | Moisture (%) | Ash (%) | FC (%) | HHV (MJ kg−1) | C | H | O | N | S | |
| Carrot grass | 69.03 ± 0.75 | 7.66 ± 0.15 | 5.41 ± 0.08 | 17.90 ± 0.17 | 18.25 | 44.70 ± 0.56 | 5.99 ± 0.23 | 47.77 ± 0.71 | 1.20 ± 0.04 | 0.34 ± 0.02 |
| Mott grass | 70.03 ± 0.53 | 5.83 ± 0.15 | 3.18 ± 0.17 | 20.96 ± 0.13 | 18.63 | 47.32 ± 0.75 | 6.19 ± 0.16 | 45.17 ± 0.56 | 1.01 ± 0.05 | 0.31 ± 0.03 |
| Heating Rate (°C min−1) | Temperature (°C) | ||||
| T1 | T2 | T3 | T4 | T5 | |
| Carrot Grass | |||||
| 10 | 185 | 267 | 296 | 326peak | 470 |
| 30 | 195 | 283 | 306 | 338peak | 493 |
| 50 | 200 | 284 | 310 | 341peak | 504 |
| Mott Grass | |||||
| 10 | 174 | 310peak | 380 | -- | -- |
| 30 | 179 | 324peak | 384 | -- | -- |
| 50 | 184 | 330peak | 394 | -- | -- |
| Stages | Heating Rate (°C min−1) | |||
| 10 | 30 | 50 | ||
| Carrot Grass | ||||
| Stage-I, Mass loss (%) | 5.96 | 7.07 | 7.57 | |
| Stage-II, Mass loss (%) | Zone-I | 19.83 | 19.22 | 20.5 |
| Zone-II | 37.49 | 38.91 | 38.41 | |
| Stage-III, Mass loss (%) | 8.48 | 8.14 | 7.14 | |
| Residual mass (%) at 1000 °C | 28.23 | 26.66 | 26.38 | |
| Mott Grass | ||||
| Stage-I, Mass loss (%) | 6.93 | 7.09 | 7.8 | |
| Stage-II, Mass loss (%) | 49.12 | 48.53 | 49.54 | |
| Stage-III, Mass loss (%) | 15.04 | 17.92 | 14.5 | |
| Residual mass (%) at 1000 °C | 28.91 | 26.46 | 28.16 | |
| Conversion Points (α) | Ea kJ mol−1 | R2 | ΔH kJ mol−1 | Ea kJ mol−1 | R2 | ΔH kJmol−1 |
| FWO Method | KAS Method | |||||
| Carrot Grass | ||||||
| 0.1 | 215.43 | 0.99 | 210.34 | 197.18 | 0.99 | 192.09 |
| 0.2 | 251.47 | 0.98 | 246.38 | 247.57 | 0.99 | 242.48 |
| 0.3 | 275.72 | 0.98 | 270.63 | 271.02 | 0.99 | 265.93 |
| 0.4 | 295.92 | 0.98 | 290.83 | 290.55 | 0.99 | 285.46 |
| 0.5 | 311.55 | 0.99 | 306.46 | 305.63 | 0.99 | 300.55 |
| 0.6 | 322.18 | 0.99 | 317.10 | 315.91 | 0.99 | 310.82 |
| 0.7 | 246.73 | 0.99 | 241.64 | 244.44 | 0.99 | 239.35 |
| 0.8 | 221.58 | 0.99 | 216.50 | 221.29 | 0.98 | 216.20 |
| 0.9 | 263.88 | 0.99 | 258.79 | 263.27 | 0.99 | 258.19 |
| Avg. | 267.16 | -- | 262.07 | 261.87 | -- | 256.78 |
| Mott Grass | ||||||
| 0.1 | 185.96 | 0.99 | 180.95 | 184.12 | 0.99 | 179.10 |
| 0.2 | 187.16 | 0.99 | 182.14 | 186.55 | 0.99 | 181.54 |
| 0.3 | 201.43 | 0.99 | 196.42 | 200.44 | 0.99 | 195.43 |
| 0.4 | 212.49 | 0.99 | 207.48 | 211.19 | 0.99 | 206.17 |
| 0.5 | 220.01 | 0.98 | 215.00 | 218.50 | 0.98 | 213.49 |
| 0.6 | 227.68 | 0.99 | 222.67 | 225.95 | 0.99 | 220.93 |
| 0.7 | 186.53 | 0.99 | 181.52 | 187.10 | 0.99 | 182.09 |
| 0.8 | 166.09 | 0.99 | 161.08 | 168.70 | 0.99 | 163.68 |
| 0.9 | 108.83 | 0.99 | 103.82 | 116.46 | 0.99 | 111.45 |
| Avg. | 188.46 | -- | 183.45 | 188.78 | -- | 183.77 |
| Conversion Range (α) | Temperature Range (°C) | Thermal Transformations | The Response of Activation Energies (kJ mol−1) |
| Carrot Grass | |||
| α ≤ 0.1 | 25–200 | Retained/trapped water was released along with the possible degradation simple/small sugars | Was shown to be increased from the starting point to 215 |
| 0.2 ≤ α ≤ 0.6 | 220–345 | Thermal transformation of polysaccharides, mainly hemicellulose, cellulose followed by lignin breakdown | Was shown to be increased from 215 to 322 |
| 0.6 ≤ α | 345–410 | Char formation and degradation of any residual lignin | A decreasing trend was observed from 322 to 263 |
| Mott Grass | |||
| α ≤ 0.1 | 25–195 | Retained/trapped water was released along with the possible degradation simple/small sugars | Was shown to be increased from the starting point to 185 |
| 0.2 ≤ α ≤ 0.6 | 195–340 | Thermal transformation of polysaccharides, mainly hemicellulose, cellulose, followed by lignin breakdown | Was shown to be increased from 185 to 227 |
| 0.6 ≤ α | 340–560 | Char formation and degradation of any residual lignin | A decreasing trend was observed from 227 to 108 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, M.S.; Mehmood, M.A.; Luo, H.; Shen, B.; Latif, M.; Ghani, W.A.W.A.K.; Alkhattabi, N.A.; Aloqbi, A.A.; Jambi, E.J.; Gull, M.; et al. Pyrolysis and Thermogravimetric Study to Elucidate the Bioenergy Potential of Novel Feedstock Produced on Poor Soils While Keeping the Environmental Sustainability Intact. Sustainability 2019, 11, 3592. https://doi.org/10.3390/su11133592
Ahmad MS, Mehmood MA, Luo H, Shen B, Latif M, Ghani WAWAK, Alkhattabi NA, Aloqbi AA, Jambi EJ, Gull M, et al. Pyrolysis and Thermogravimetric Study to Elucidate the Bioenergy Potential of Novel Feedstock Produced on Poor Soils While Keeping the Environmental Sustainability Intact. Sustainability. 2019; 11(13):3592. https://doi.org/10.3390/su11133592
Chicago/Turabian StyleAhmad, Muhammad Sajjad, Muhammad Aamer Mehmood, Huibo Luo, Boxiong Shen, Muhammad Latif, Wan Azlina Wan Ab Karim Ghani, Nuha Abdulhamid Alkhattabi, Akram Ahmed Aloqbi, Ebtihaj Jamaluddin Jambi, Munazza Gull, and et al. 2019. "Pyrolysis and Thermogravimetric Study to Elucidate the Bioenergy Potential of Novel Feedstock Produced on Poor Soils While Keeping the Environmental Sustainability Intact" Sustainability 11, no. 13: 3592. https://doi.org/10.3390/su11133592
APA StyleAhmad, M. S., Mehmood, M. A., Luo, H., Shen, B., Latif, M., Ghani, W. A. W. A. K., Alkhattabi, N. A., Aloqbi, A. A., Jambi, E. J., Gull, M., & Rashid, U. (2019). Pyrolysis and Thermogravimetric Study to Elucidate the Bioenergy Potential of Novel Feedstock Produced on Poor Soils While Keeping the Environmental Sustainability Intact. Sustainability, 11(13), 3592. https://doi.org/10.3390/su11133592








