Treatment of Low Biodegradability Leachates in a Serial System of Aged Refuse-Filled Bioreactors
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Characterization of AR
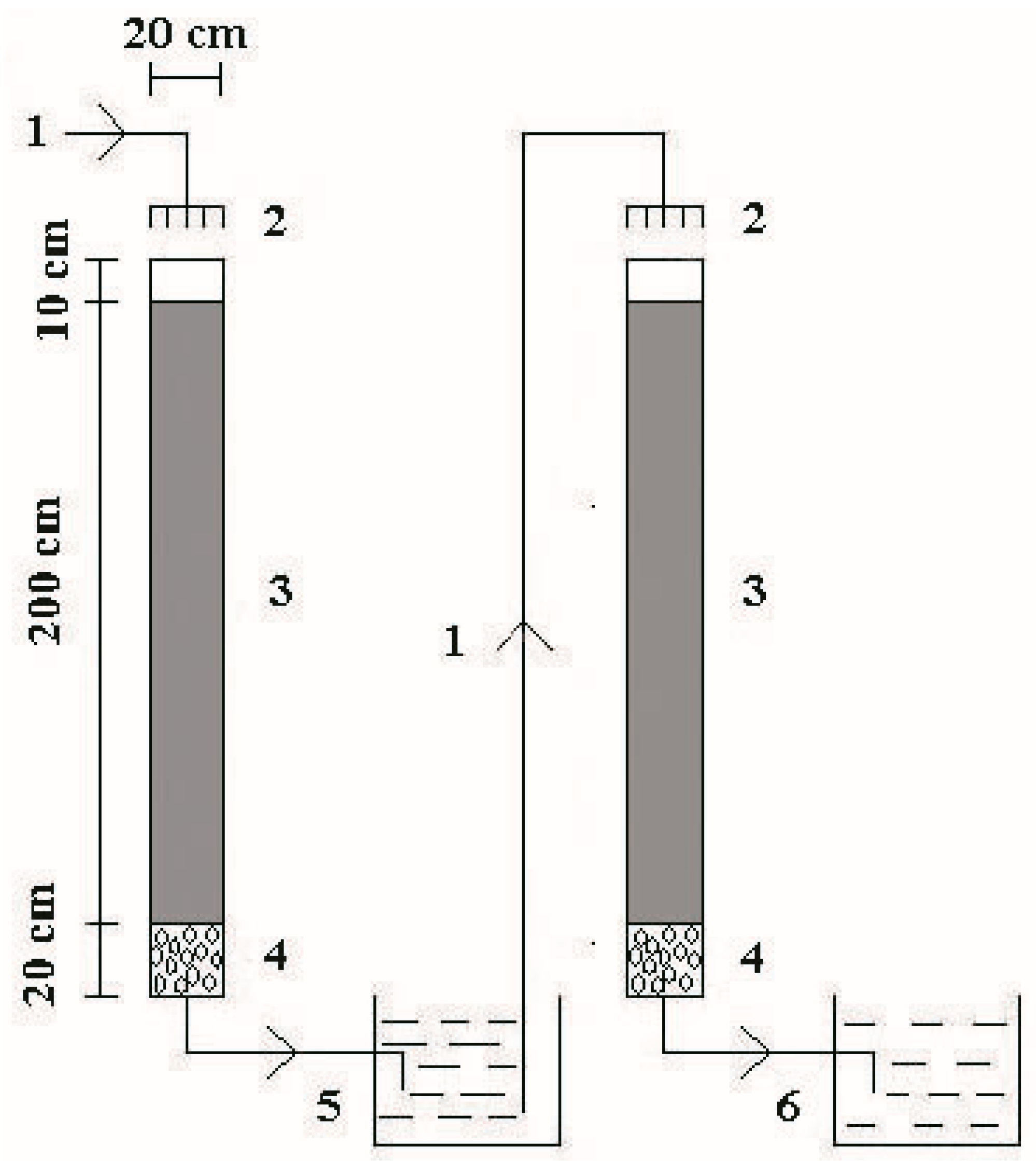
2.2. Aged Refuse Filled Bioreactors (ARFBs)
2.3. Sampling and Leachate Characterization
2.4. AR Microbiological Characterization
2.5. Operation and Monitoring of the ARFB System
2.6. Analysis of Experimental Data
3. Results and Discussion
3.1. Excavated Material Characterization
3.2. Characterization of Leachate in the Influent
3.3. Bacterial Characterization in the Packing Material
3.4. Operation and Monitoring of the ARFB System
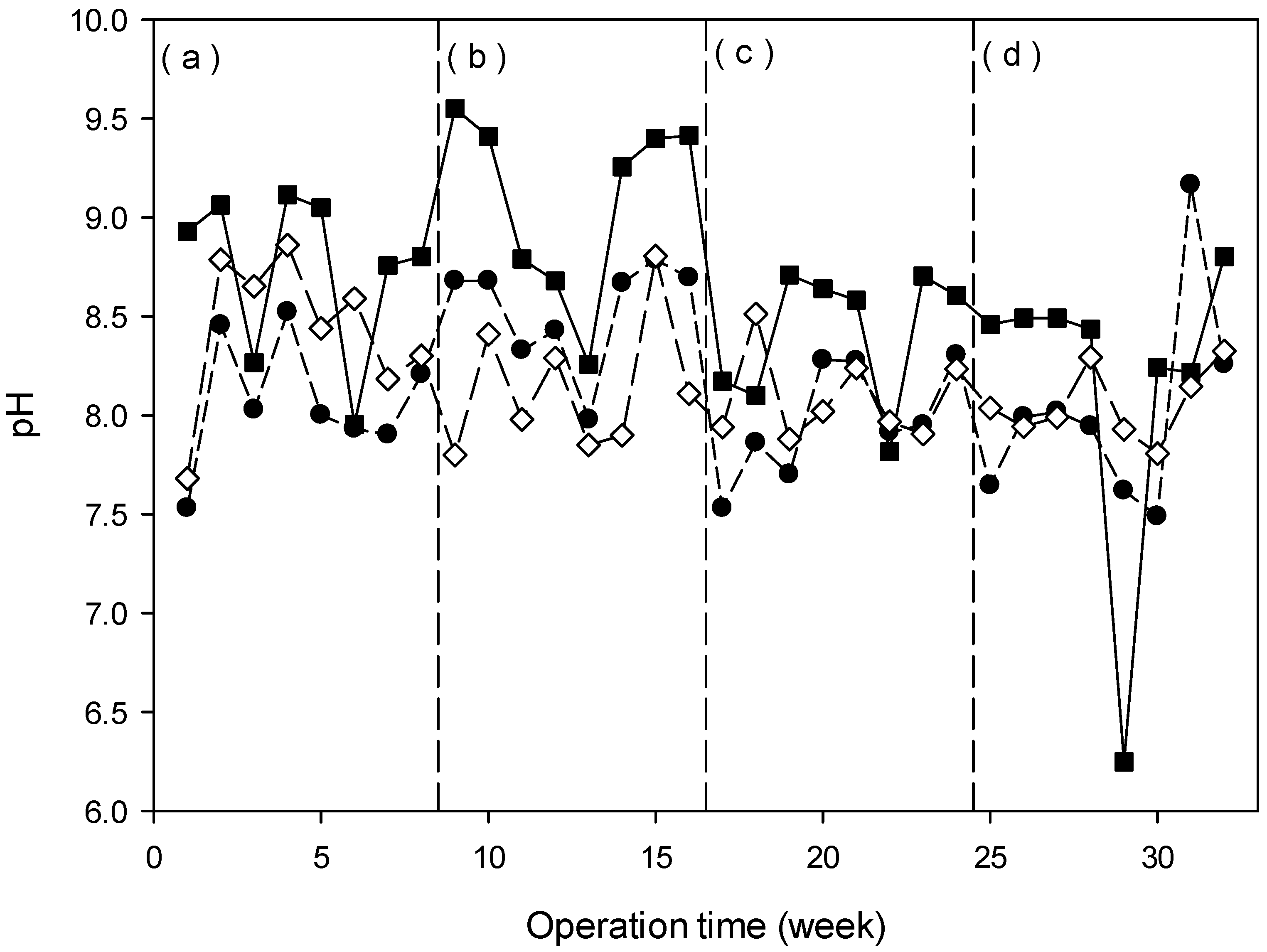
3.4.1. pH Behavior
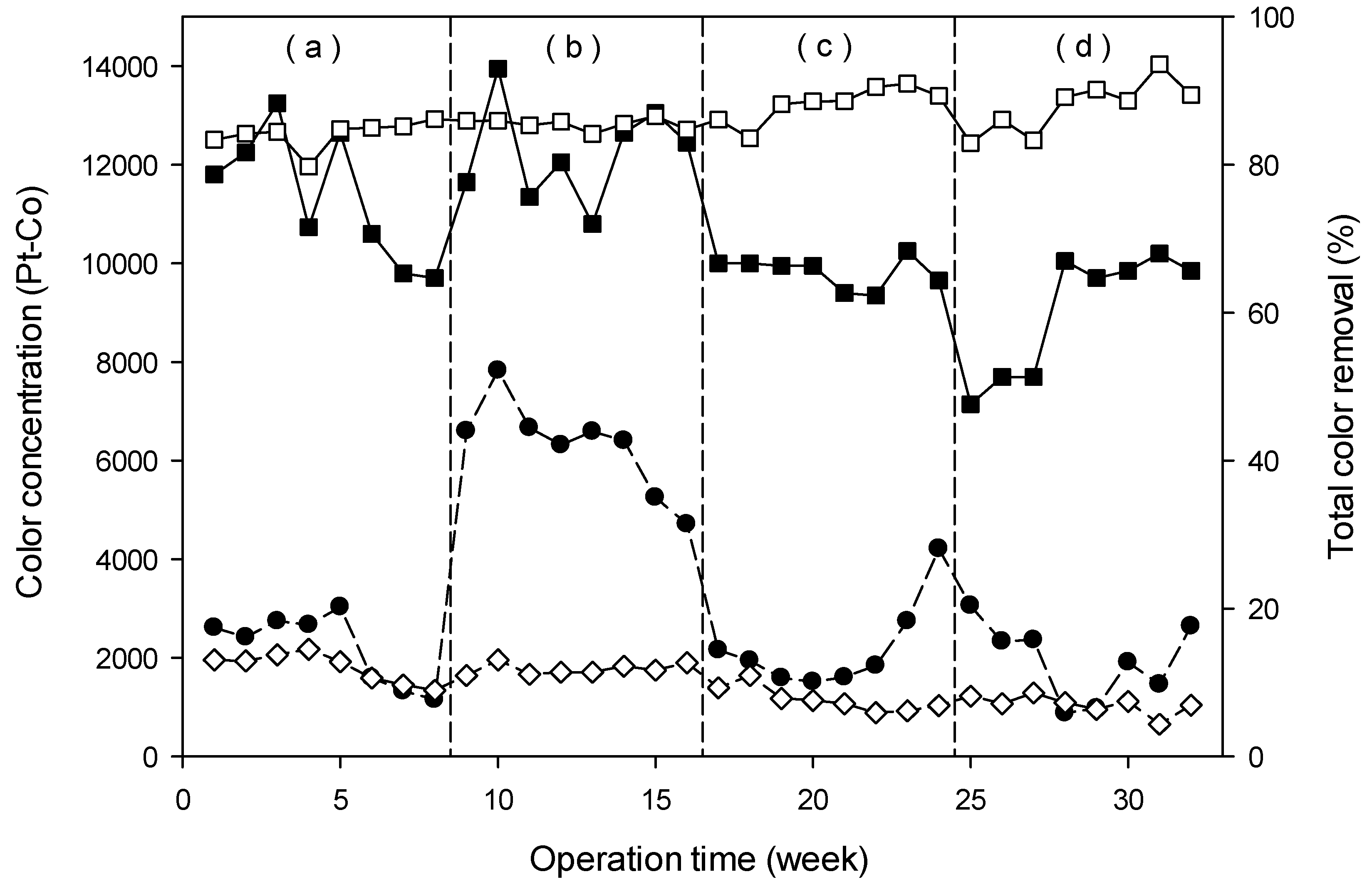
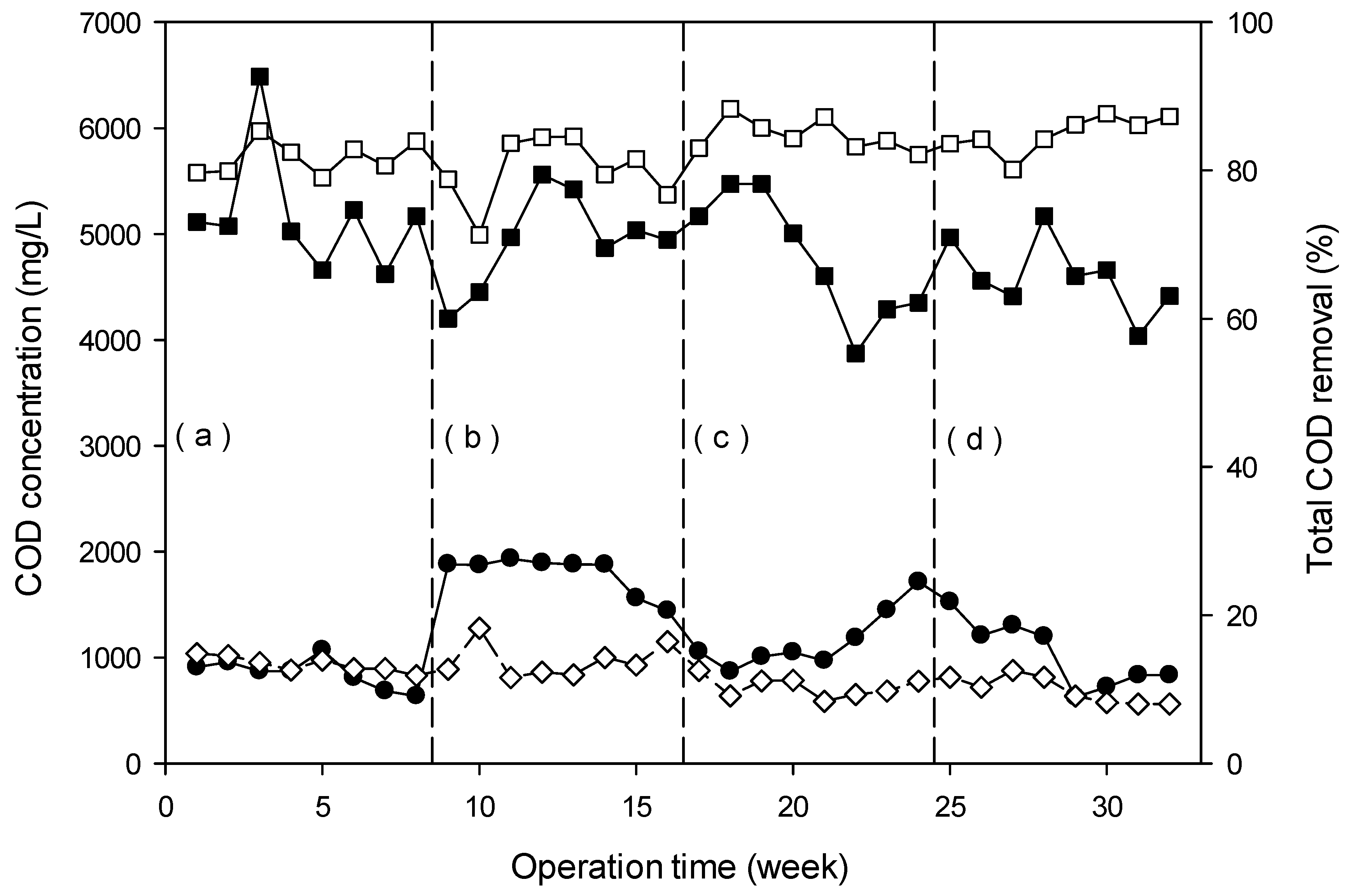
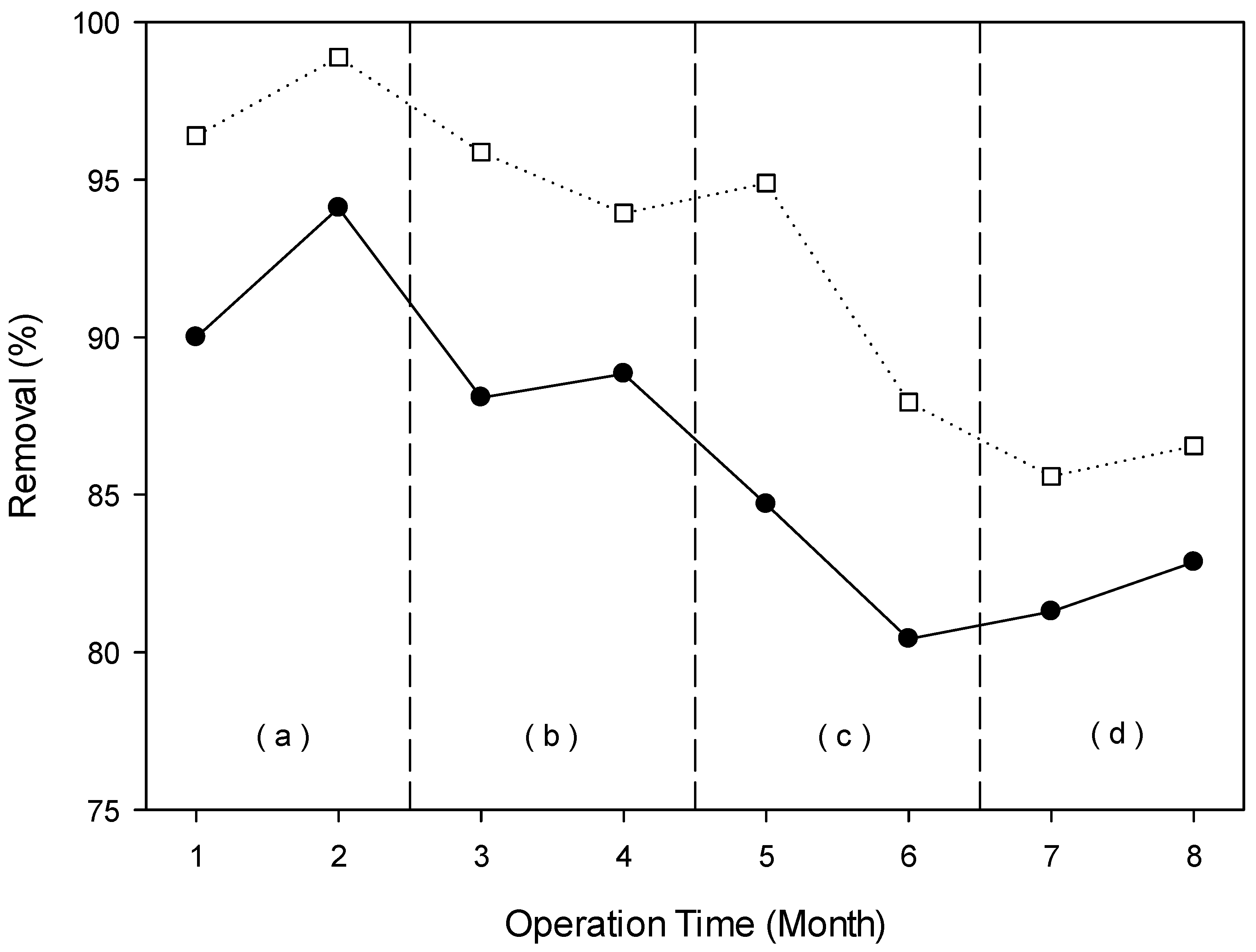
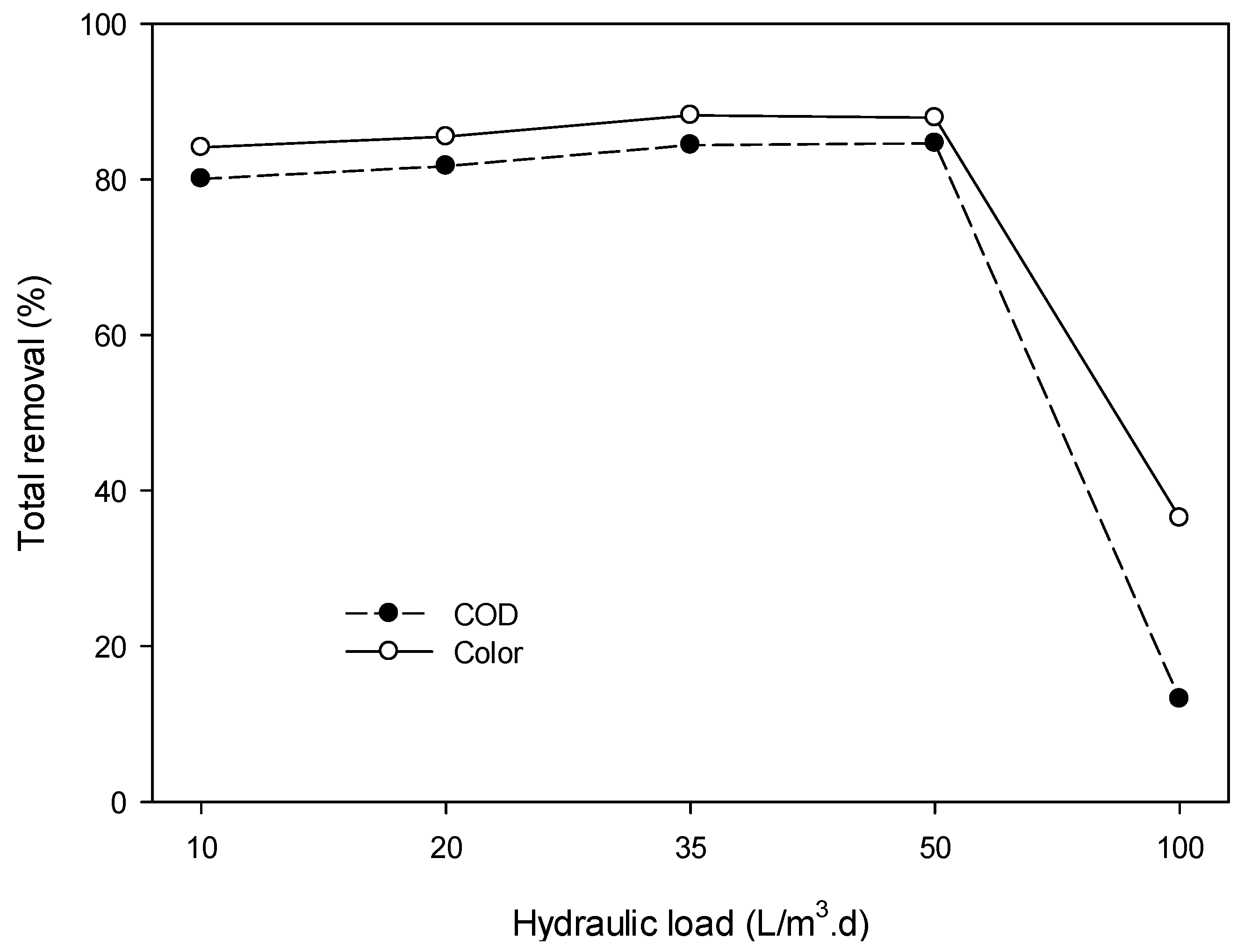
3.4.2. Removal of Organic Pollutants (Color and COD)
3.4.3. Removal of Biodegradable Matter (BOD) and Alkalinity
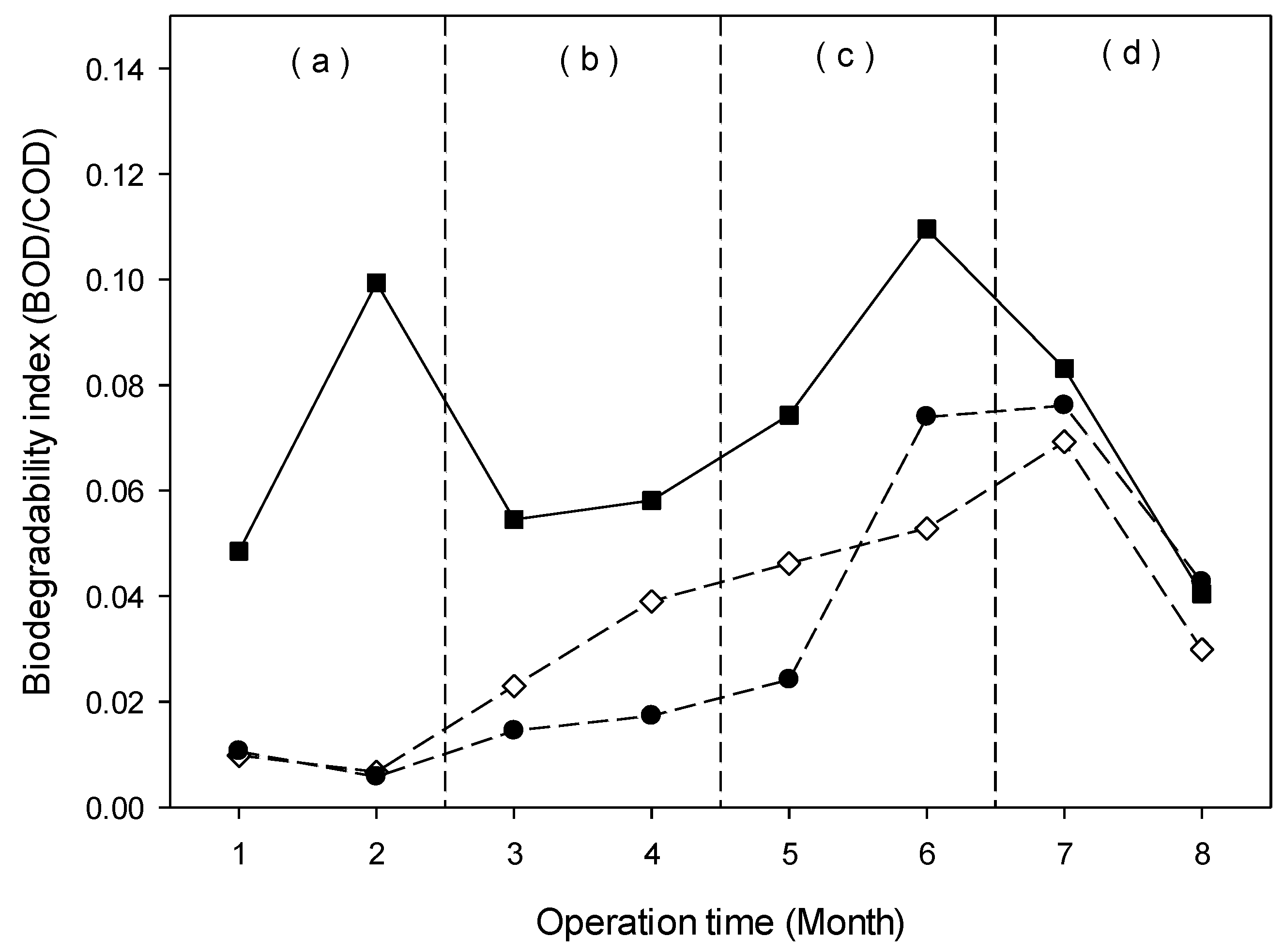
3.4.4. Behavior of the Biodegradability Index (BI)
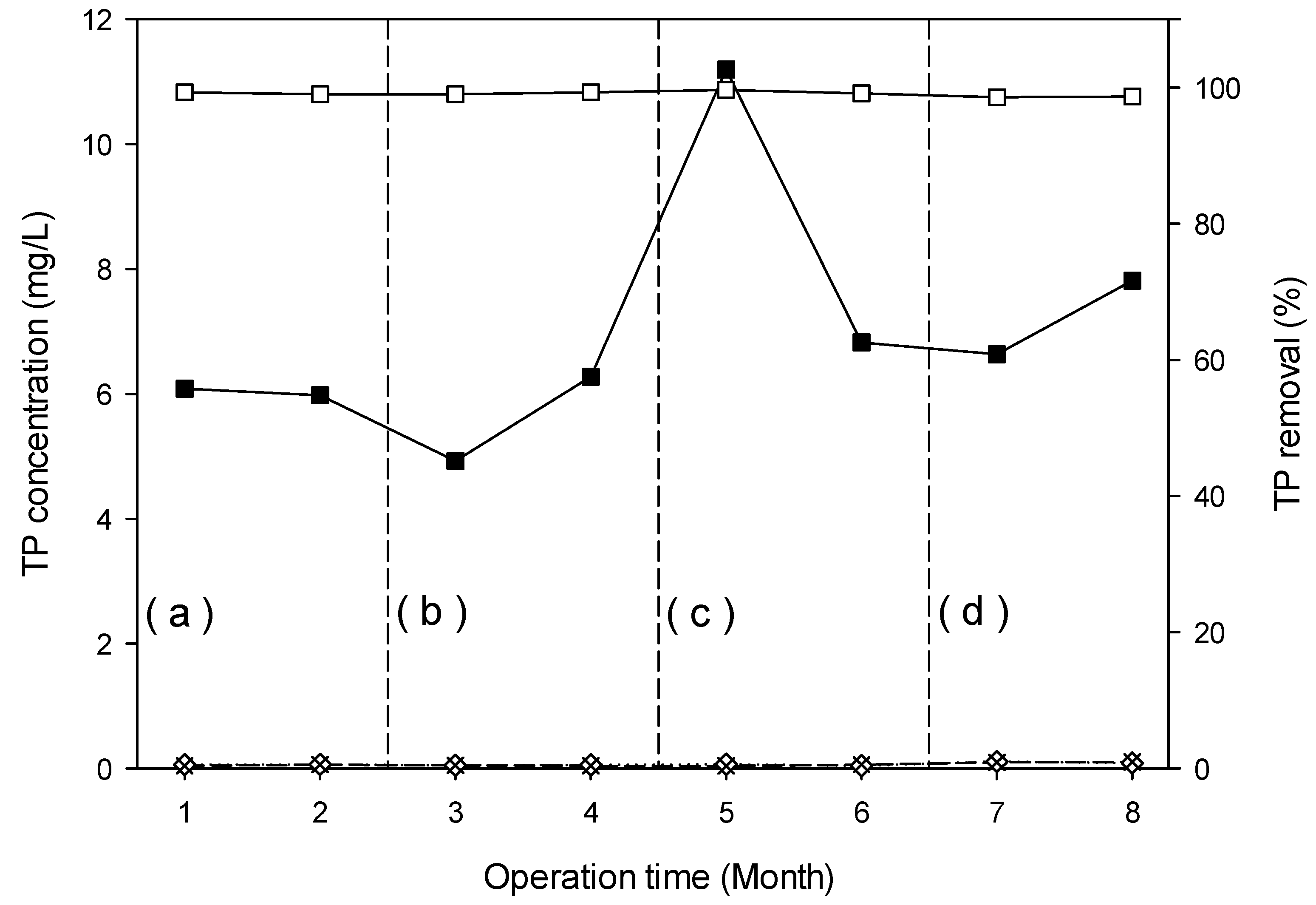
3.4.5. Total Phosphorus Removal
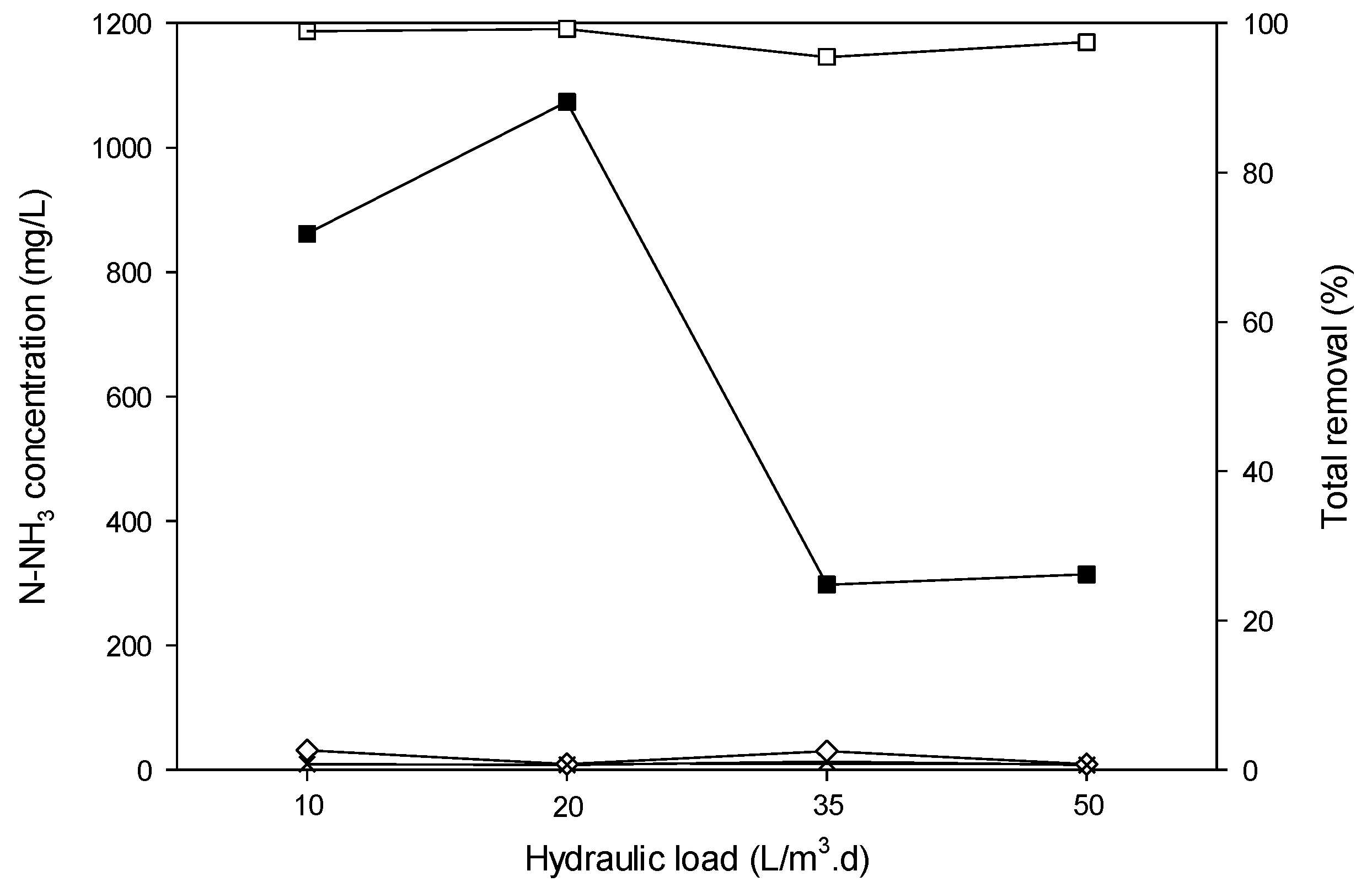
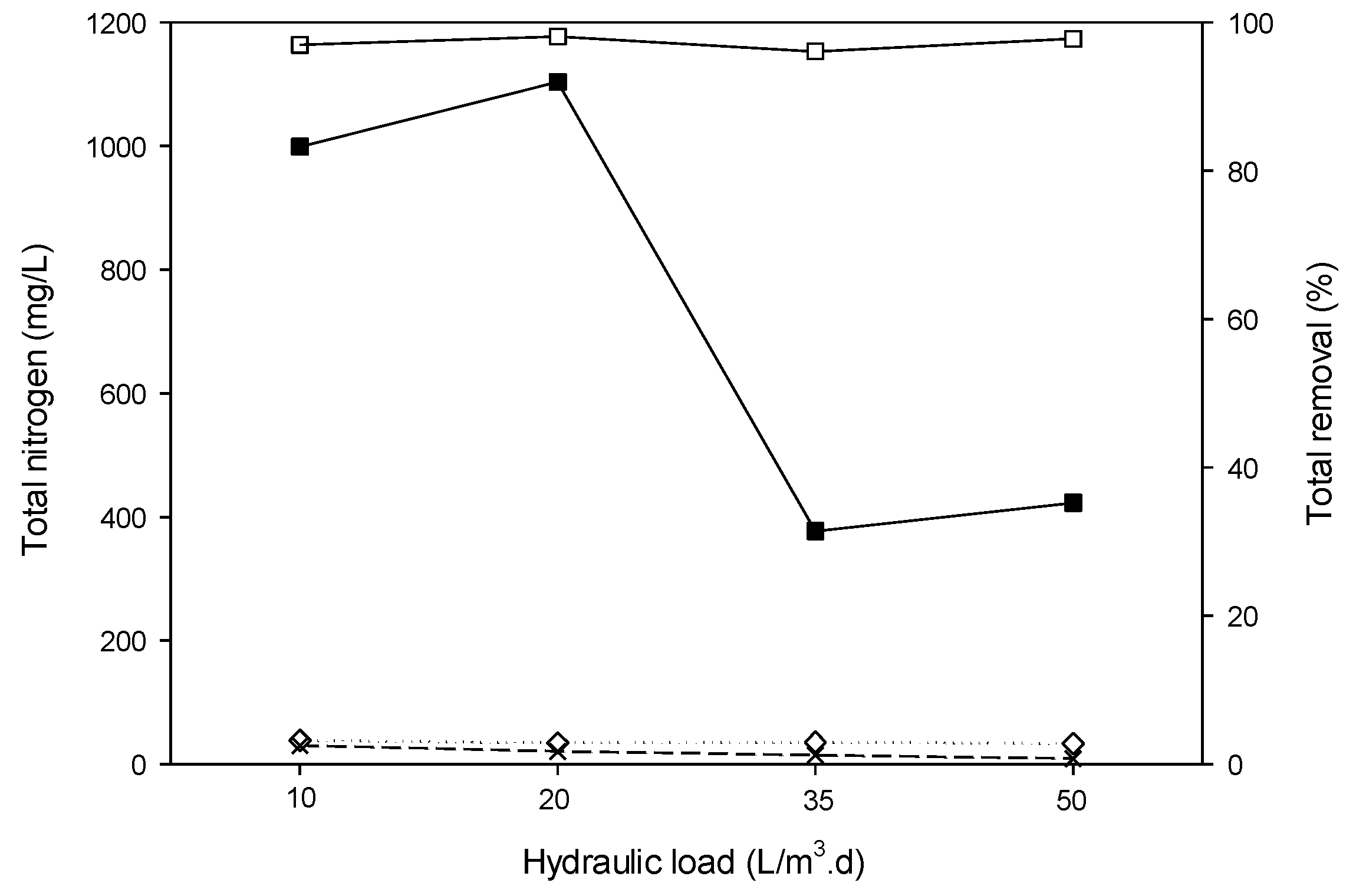
3.4.6. Removal of TN and N-NH3.
3.4.7. Physicochemical Quality of the Final Effluent
3.4.8. Behavior of the ARFB System in Series with Higher HL
3.4.9. Heavy Metal Removal
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Renou, S.; Givaudan, J.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: Review and oportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Pacheco, M.J.; Ciríaco, L.; Lopes, A. Review on the electrochemical processes for the treatment of sanitary landfill leachates: Present and future. Appl. Catal. B 2015, 176, 183–200. [Google Scholar] [CrossRef]
- Foo, K.; Hameed, B. An overview of landfill leachate treatment via activated carbon adsorption process. J. Hazard. Mater. 2009, 171, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Wiszniowski, J.; Robert, D.; Surmacz-Gorska, J.; Miksch, K.; Weber, J. Landfill leachate treatment methods: A review. Environ. Chem. Lett. 2006, 4, 51–61. [Google Scholar] [CrossRef]
- Abbas, A.A.; Jingsong, G.; Ping, L.Z.; Ya, P.Y.; Al-Rekabi, W.S. Review on Landfill Leachate Treatments. J. Appl. Sci. Res. 2009, 5, 534–545. [Google Scholar] [CrossRef]
- Loukidou, M.X.; Zouboulis, A.I. Comparison of two biological treatment processes using attached-growth biomass for sanitary landfill leachate treatment. Environ. Pollut. 2001, 111, 273–281. [Google Scholar] [CrossRef]
- Torretta, V.; Ferronato, N.; Katsoyiannis, I.; Tolkou, A.; Airoldi, M. Novel and conventional technologies for landfill leachates treatment: A review. Sustainability 2016, 9, 9. [Google Scholar] [CrossRef]
- Tatsi, A.; Zouboulis, A.; Matis, K.; Samaras, P. Coagulation–flocculation pretreatment of sanitary landfill leachates. Chemosphere 2003, 53, 737–744. [Google Scholar] [CrossRef]
- Gray, D.; Pollard, S.J.; Spence, L.; Smith, R.; Gronow, J.R. Spray irrigation of landfill leachate: Estimating potential exposures to workers and bystanders using a modified air box model and generalized source term. Environ. Pollut. 2005, 133, 587–599. [Google Scholar] [CrossRef][Green Version]
- Li, H.; Zhao, Y.; Shi, L.; Gu, Y. Three-stage aged refuse biofilter for the treatment of landfill leachate. J. Environ. Sci. 2009, 21, 70–75. [Google Scholar] [CrossRef]
- Li, H.; Gu, Y.; Zhao, Y.; Wen, Z. Leachate treatment using a demonstration aged refuse biofilter. J. Environ. Sci. 2010, 22, 1116–1122. [Google Scholar] [CrossRef]
- Xie, B.; Xiong, S.; Liang, S.; Hu, C.; Zhang, X.; Lu, J. Performance and bacterial compositions of aged refuse reactors treating mature landfill leachate. Bioresour. Technol. 2012, 103, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Shi, L.; Zhao, Y.; Li, H. Novel engineering controls to increase leachate contaminant degradation by refuse: From lab test to in situ engineering application. Ecol. Eng. 2011, 37, 1914–1919. [Google Scholar] [CrossRef]
- Erses, A.S.; Onay, T.T.; Yenigun, O. Comparison of aerobic and anaerobic degradation of municipal solid waste in bioreactor landfills. Bioresour. Technol. 2008, 99, 5418–5426. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Shen, Z.; Huang, R.; Wang, W. Treatment of landfill leachate by combined aged-refuse bioreactor and electro-oxidation. Water Res. 2007, 41, 2417–2426. [Google Scholar] [CrossRef]
- Erabee, I.; Ethaib, S. Treatment of contaminated Landfill Leachate using Aged Refuse Biofilter Medium. Orient. J. Chem. 2018, 34, 1441–1450. [Google Scholar] [CrossRef]
- Xie, B.; Lv, Z.; Lv, B.; Gu, Y. Treatment of mature landfill leachate by biofilters and fenton oxidation. Waste Manag. 2010, 30, 2108–2112. [Google Scholar] [CrossRef]
- Ding, W.; Zeng, X.; Hu, X.; Deng, Y.; Hossain, M.; Chen, L. Characterization of Dissolved Organic Matter in Mature Leachate during Ammonia Stripping and Two-Stage Aged-Refuse Bioreactor Treatment. J. Environ. Eng. 2017, 144, 04017082. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- Zhao, Y.; Lou, Z.; Guo, Y.; Xu, G. Treatment of sewage using an aged-refuse-based bioreactor. J. Environ. Manag. 2007, 88, 32–38. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, L.; Huang, R.; Song, L.; Li, X. Recycling of aged refuse from a closed landfill. Waste Manag. Res. 2007, 25, 130–138. [Google Scholar] [CrossRef]
- Ziyang, L.; Wang, L.; Zhu, N.; Youcai, Z. Martial recycling from renewable landfill and associated risks: A review. Chemosphere 2015, 131, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Hashisho, J.; El-Fadel, M.; Al-Hindi, M.; Salam, D.; Alameddine, I. Hollow fiber vs. flat sheet MBR for the treatment of high strength stabilized landfill leachate. Waste Manag. 2016, 55, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Kulikowska, D. Usability of powdered activated carbon for landfill leachate treatment—Continued research. Desalin. Water Treat. 2016, 57, 28560–28569. [Google Scholar] [CrossRef]
- Ganguli, A.; Tripathi, A. Bioremediation of toxic chromium from electroplating effluent by chromate-reducing Pseudomonas aeruginosa A2Chr in two bioreactors. Appl. Microbiol. Biotechnol. 2002, 58, 416–420. [Google Scholar] [PubMed]
- Klonowska, A.; Clark, M.; Thieman, S.; Giles, B.; Wall, J.; Fields, M. Hexavalent chromium reduction in Desulfovibrio vulgaris Hildenborough causes transitory inhibition of sulfate reduction and cell growth. Appl. Microbiol. Biotechnol. 2008, 78, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Li, X.; Liu, H.; Miller, S.; Wang, G.; Rensing, C. Characterization and genomic analysis of a highly chromate resistant and reducing bacterial strain Lysinibacillus fusiformis ZC1. J. Hazard. Mater. 2011, 185, 682–688. [Google Scholar] [CrossRef] [PubMed]
- De Vegasa, E.; Nieves, B. Acinetobacter spp.: Aspectos microbiológicos, clínicos y epidemiológicos. Rev. Soc. Ven. Microbiol. 2005, 25, 64–71. [Google Scholar]
- Benslama, O.; Boulahrouf, A. High-quality draft genome sequence of Enterobacter sp. Bisph2, a glyphosate-degrading bacterium isolated from a sandy soil of Biskra, Algeria. Genom. Data 2016, 8, 61–66. [Google Scholar] [CrossRef]
- Mujtaba, G.; Rizwan, M.; Lee, K. Removal of nutrients and COD from wastewater using symbiotic co-culture of bacterium Pseudomonas putida and immobilized microalga Chlorella vulgaris. J. Ind. Eng. Chem. 2017, 49, 145–151. [Google Scholar] [CrossRef]
- Demir, A.; Arisoy, M. Biological and chemical removal of Cr (VI) from waste water: Cost and benefit analysis. J. Hazard. Mater. 2007, 147, 275–280. [Google Scholar] [CrossRef]
- Şahin, Y.; Öztürk, A. Biosorption of chromium (VI) ions from aqueous solution by the bacterium Bacillus thuringiensis. Process Biochem. 2005, 40, 1895–1901. [Google Scholar] [CrossRef]
- Shi, T.; Ge, Y.; Zhao, N.; Hu, X.; Yuan, Z. Polyphosphate kinase of Lysinibacillus sphaericus and its effects on accumulation of polyphosphate and bacterial growth. Microbiol. Res. 2015, 172, 41–47. [Google Scholar] [CrossRef]
- He, P.J.; Xue, J.F.; Shao, L.M.; Li, G.J.; Lee, D.J. Dissolved organic matter (DOM) in recycled leachate of bioreactor landfill. Water Res. 2006, 40, 1465–1473. [Google Scholar] [CrossRef]
- Gao, W.J.; Lin, H.J.; Leung, K.T.; Liao, B.Q. Influence of elevated pH shocks on the performance of a submerged anaerobic membrane bioreactor. Process Biochem. 2010, 45, 1279–1287. [Google Scholar] [CrossRef]
- Tadkaew, N.; Sivakumar, M.; Khan, S.; McDonald, J.; Nghiem, L. Effect of mixed liquor pH on the removal of trace organic contaminants in a membrane bioreactor. Bioresour. Technol. 2010, 101, 1494–1500. [Google Scholar] [CrossRef]
- Hassan, M.; Xie, B. Use of aged refuse-based bioreactor/biofilter for landfill leachate treatment. Appl. Microbiol. Biotechnol. 2014, 98, 6543–6553. [Google Scholar] [CrossRef]
- He, Y.; Li, D.; Zhao, Y.; Huang, M.; Zhou, G. Assessment and analysis of aged refuse as ammonium-removal media for the treatment of landfill leachate. Waste Manag. Res. 2017, 35, 1168–1174. [Google Scholar] [CrossRef]
- Chen, Y.; Li, B.; Ye, L.; Peng, Y. The combined effects of COD/N ratio and nitrate recycling ratio on nitrogen and phosphorus removal in anaerobic/anoxic/aerobic (A2/O)-biological aerated filter (BAF) systems. Biochem. Eng. J. 2015, 93, 235–242. [Google Scholar] [CrossRef]
- SEMARNAT. Mexican Official Norm NOM-001-SEMARNAT-1996, that establishes the maximum allowable pollutant in wastewater discharges in national waters (in Spanish). In Official Journal of the Federation; SEMARNAT: Mexico City, Mexico, 2003. [Google Scholar]
- Wang, F.; Sun, Y.; Zhou, R. Experimental study on the treatment of chromium-containing wastewater by aged refuse. Procedia Environ. Sci. 2012, 16, 598–605. [Google Scholar] [CrossRef]
- Bonilla, J.O.; Callegari, E.A.; Delfini, C.D.; Estevez, M.C.; Villegas, L.B. Simultaneous chromate and sulfate removal by Streptomyces sp. MC1. Changes in intracellular protein profile induced by Cr (VI). J. Basic Microbiol. 2016, 56, 1212–1221. [Google Scholar] [CrossRef]
| Aged Refuse | Plastics and Other Materials in the Excavated Refuse (%) | Particle Distribution of the Fine Fraction (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Rigid Plastics | Plastic Bags | Others | Fine Fraction | Total | Diameter >40 mm | Diameter 15–40 mm | Diameter <15 mm | Total | |
| WB | 10.9 | 13 | 14 | 62.1 | 100 | --- 15.05 | --- 24.14 | --- 60.82 | --- 100 |
| DB | 10.2 | 11.1 | 21.3 | 57.4 | 100 | ||||
| Parameter | Average | Concentration Range |
|---|---|---|
| pH | 8.58 ± 0.60 | 6.3–9.6 |
| Alkalinity (mg CaCO3/L) | 2809 ± 1054 | 1400–5060 |
| COD (mg/L) | 4868 ± 509 | 3874–6486 |
| BOD5 (mg/L) | 347 ± 108 | 179–477 |
| BOD5/COD | 0.07 ± 0.02 | 0.04–0.10 |
| Color (Pt-Co) | 10,638 ± 1593 | 7150–13,950 |
| TSS (mg/L) | 0.037 ± 0.017 | 0.024–0.062 |
| TN (mg/L) | 725.7 ± 378 | 377–1104 |
| NH3-N (mg/L) | 636.7 ± 391.8 | 297–1073 |
| TP (mg/L) | 6.9 ± 1.9 | 4.9–11.2 |
| Cadmium (mg/L) | 0.14 ± 0.07 | 0.1–0.24 |
| Chrome (mg/L) | 0.55 ± 0.02 | 0.53–0.58 |
| Nickel (mg/L) | 0.40 ± 0.02 | 0.38–0.42 |
| Lead (mg/L) | ND | ND |
| Zinc (mg/L) | ND | ND |
| Microorganisms at the Species Level | Function | Reference |
|---|---|---|
| Acinetobacter sp. | Possible nitrifier | [28] |
| Enterobacter sp. | Possible denitrifying and phosphate reduction | [29] |
| Pseudomonas putida | Nitrifier, denitrifier and Cr (VI) and phosphate reduction. | [27,30] |
| Bacillus thuringiensis | Biological adsorption of Cr | [31,32] |
| Desulfovibrio vulgaris | Cr (VI) and phosphate reduction | [26] |
| Lysinibacillus sphaericus | Phosphate reduction | [33] |
| Lysinibacillus fusiformis | Reduction of Cr (VI) to Cr (III) | [27] |
| Pseudomonas aeruginosa | Nitrifier and Cr (VI) reduction | [25] |
| Leachate | BIo | BIf | Applied System | Reference |
|---|---|---|---|---|
| Young | 0.5 | 0.03 | ARB in series | [15] |
| Young | 0.5 | 0.03 | ARB (aerobic) | [14] |
| Young | 0.7 | 0.05 | ARB (anaerobic) | |
| Intermediate | 0.2 | 0.03 | ARB in series | [11] |
| Mature | 0.1 | 0.04 | ARB-Fenton | [17] |
| Parameter | Units | Final Effluent Concentration | Mexican Regulations |
|---|---|---|---|
| BOD5 | mg/L | 39 | 150 |
| TSS | mg/L | <1 | 150 |
| TP | mg/L | 0.1 | 20 |
| TN | mg/L | 9.4 | 40 |
| HL | Sample | Cadmium (mg/L) | Chromium (mg/L) | Nickel (mg/L) | Lead (mg/L) | Zinc (mg/L) |
|---|---|---|---|---|---|---|
| 10 L/m3·d | *Inf | <LOQ | 0.55 | 0.38 | <LOQ | <LOQ |
| *E1 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | |
| *E2 | <LOQ | 0.54 | 0.38 | <LOQ | <LOQ | |
| 20 L/m3·d | Inf | 0.11 | 0.53 | 0.41 | <LOQ | <LOQ |
| E1 | <LOQ | 0.13 | 0.40 | <LOQ | <LOQ | |
| E2 | <LOQ | 0.79 | 0.38 | <LOQ | <LOQ | |
| 35 L/m3·d | Inf | <LOQ | 0.55 | 0.42 | <LOQ | <LOQ |
| E1 | <LOQ | 0.27 | 0.43 | <LOQ | <LOQ | |
| E2 | <LOQ | 0.16 | 0.41 | <LOQ | <LOQ | |
| 50 L/m3·d | Inf | 0.24 | 0.58 | 0.42 | <LOQ | <LOQ |
| E1 | <LOQ | <LOQ | 0.40 | <LOQ | <LOQ | |
| E2 | 0.21 | <LOQ | 0.44 | <LOQ | <LOQ | |
| Limit of Quantification (LOQ) | 0.10 | 0.12 | 0.31 | 0.5 | 5 | |
| Maximum permissible limit mg/L (NOM-001-SEMARNAT-1996) | 0.1 | 0.5 | 2 | 0.2 | 10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nájera-Aguilar, H.A.; Gutiérrez-Hernández, R.F.; Bautista-Ramírez, J.; Martínez-Salinas, R.I.; Escobar-Castillejos, D.; Borraz-Garzón, R.; Rojas-Valencia, M.N.; Giácoman-Vallejos, G. Treatment of Low Biodegradability Leachates in a Serial System of Aged Refuse-Filled Bioreactors. Sustainability 2019, 11, 3193. https://doi.org/10.3390/su11113193
Nájera-Aguilar HA, Gutiérrez-Hernández RF, Bautista-Ramírez J, Martínez-Salinas RI, Escobar-Castillejos D, Borraz-Garzón R, Rojas-Valencia MN, Giácoman-Vallejos G. Treatment of Low Biodegradability Leachates in a Serial System of Aged Refuse-Filled Bioreactors. Sustainability. 2019; 11(11):3193. https://doi.org/10.3390/su11113193
Chicago/Turabian StyleNájera-Aguilar, Hugo A., Rubén F. Gutiérrez-Hernández, Jesús Bautista-Ramírez, Rebeca I. Martínez-Salinas, Daisy Escobar-Castillejos, Rocío Borraz-Garzón, María N. Rojas-Valencia, and Germán Giácoman-Vallejos. 2019. "Treatment of Low Biodegradability Leachates in a Serial System of Aged Refuse-Filled Bioreactors" Sustainability 11, no. 11: 3193. https://doi.org/10.3390/su11113193
APA StyleNájera-Aguilar, H. A., Gutiérrez-Hernández, R. F., Bautista-Ramírez, J., Martínez-Salinas, R. I., Escobar-Castillejos, D., Borraz-Garzón, R., Rojas-Valencia, M. N., & Giácoman-Vallejos, G. (2019). Treatment of Low Biodegradability Leachates in a Serial System of Aged Refuse-Filled Bioreactors. Sustainability, 11(11), 3193. https://doi.org/10.3390/su11113193




