In Vivo Cytotoxicity Induced by 60 Hz Electromagnetic Fields under a High-Voltage Substation Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. ELF-EMF Exposure
2.3.1. Switchyard Area
2.3.2. Magnetic Field Exposure Facility (Standardized Solenoid)
2.3.3. Micronucleus Test
2.4. Male Germ Cell Analysis
2.5. Statistical Analysis
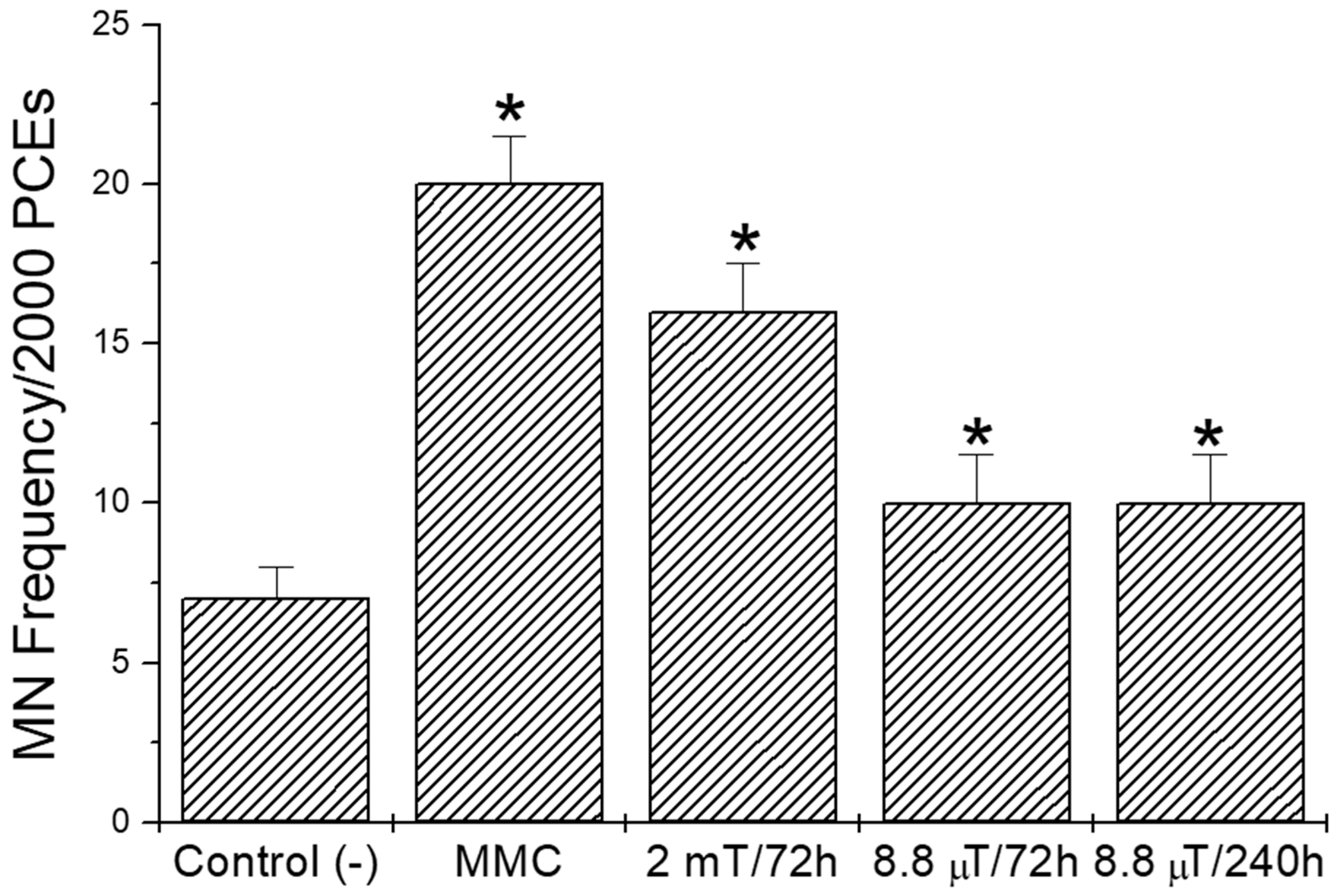
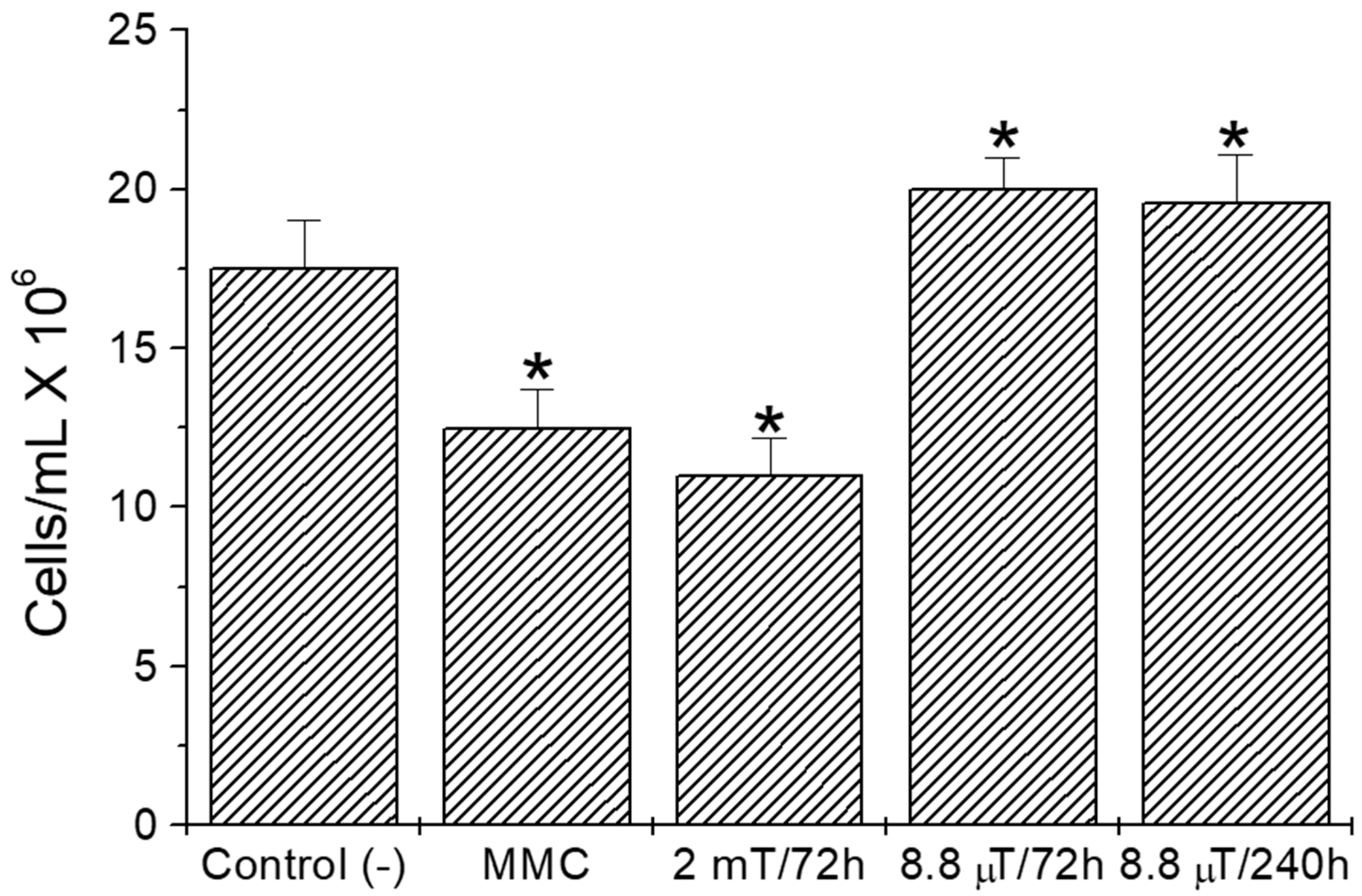
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gajšek, P.; Ravazzani, P.; Grellier, J.; Samaras, T.; Bakos, J.; Thuróczy, G. Review of Studies Concerning Electromagnetic Field (EMF) Exposure Assessment in Europe: Low Frequency Fields (50 Hz–100 kHz). Int. J. Environ. Res. Public Health 2016, 13, 875. [Google Scholar] [CrossRef] [PubMed]
- Cifra, M.; Fields, J.Z.; Farhadi, A. Electromagnetic cellular interactions. Prog. Biophys. Mol. Biol. 2011, 105, 223–246. [Google Scholar] [CrossRef] [PubMed]
- The International Agency for Research on Cancer. Non-Ionizing Radiation, Part I: Static and Extremely Low-Frequency (ELF) Electric and Magnetic Fields; The International Agency for Research on Cancer Press: Lyon, France, 2002; p. 80. Available online: https://www.ncbi.nlm.nih.gov/books/NBK390731/ (accessed on 3 August 2018).
- Ivancsits, S.; Pilger, A.; Diem, E.; Jahn, O.; Rüdiger, H.W. Cell type-specific genotoxic effects of intermittent extremely low-frequency electromagnetic fields. Mutat. Res. 2005, 583, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.L.; Singh, N.P.; Lai, H. Electromagnetic fields and DNA damage. Pathophysiology 2009, 16, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Focke, F.; Schuermann, D.; Kuster, N.; Schär, P. DNA fragmentation in human fibroblasts under extremely low frequency electromagnetic field exposure. Mutat. Res. 2010, 683, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Yakymenko, I.; Tsybulin, O.; Sidorik, E.; Henshel, D.; Kyrylenko, O.; Kyrylenko, S. Oxidative mechanisms of biological activity of low-intensity radiofrequency radiation. Electromagn. Biol. Med. 2016, 35, 186–202. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, D.W.; O’Neill, K.L. The effect of electromagnetic field exposure on the formation of DNA single strand breaks in human cells. Cell Mol. Biol. 1994, 40, 561–567. Available online: https://www.ncbi.nlm.nih.gov/pubmed/8061573 (accessed on 3 August 2018). [PubMed]
- Heredia-Rojas, J.A.; Caballero-Hernández, D.E.; Rodríguez-De la Fuente, A.O.; Ramos-Alfano, G.; Rodríguez-Flores, L.E. Lack of alterations on meiotic chromosomes and morphological characteristics of male germ cells in mice exposed to a 60 Hz and 2.0 mT magnetic field. Bioelectromagnetics 2004, 25, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Speit, G.; Schütz, P.; Hoffmann, H. Genotoxic effects of exposure to radiofrequency electromagnetic fields (RF-EMF) in cultured mammalian cells are not independently reproducible. Mutat. Res. 2007, 626, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Udroiu, I.; Antoccia, A.; Tanzarella, C.; Giuliani, L.; Pacchierotti, F.; Cordelli, E.; Eleuteri, P.; Villani, P.; Sgura, A. Genotoxicity induced by foetal and infant exposure to magnetic fields and modulation of ionizing radiation effects. PLoS ONE 2015, 10, e0142259. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Bennetts, L.E.; Sawyer, D.; Wiklendt, A.M.; King, B.V. Impact of radio frequency electromagnetic radiation on DNA integrity in the male germline. Int. J. Androl. 2005, 28, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Al-Akhras, M.A.; Darmani, H.; Elbetieha, A. Influence of 50 Hz magnetic field on sex hormones and other fertility parameters of adult male rats. Bioelectromagnetics 2006, 27, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Li, D.K.; Yan, B.; Li, Z.; Gao, E.; Miao, M.; Gong, D.; Weng, X.; Ferber, J.R.; Yuan, W. Exposure to magnetic fields and the risk of poor sperm quality. Reprod. Toxicol. 2010, 29, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Rojas, J.A.; Beltcheva, M.; Rodríguez-de la Fuente, A.O.; Heredia-Rodríguez, O.; Metcheva, R.; Rodríguez-Flores, L.E.; Santoyo-Stephano, M.A.; Castañeda-Garza, E. Evidence of genotoxicity induced by 60 Hz magnetic fields on mice bone marrow as assessed by in vivo micronucleus test. Acta Zool. Bulg. Suppl. 2017, 8, 69–75. Available online: http://www.acta-zoologica-bulgarica.eu/downloads/acta-zoologica-bulgarica/2017/supplement-8-69-75.pdf (accessed on 3 August 2018).
- Crumpton, M.J.; Collins, A.R. Are environmental electromagnetic fields genotoxic? DNA Repair 2004, 11, 1385–1387. Available online: https://www.ncbi.nlm.nih.gov/pubmed/15336633 (accessed on 13 April 2018). [CrossRef] [PubMed]
- Vijayalaxmi, R.L.; Obe, G. Controversial cytogenetic observations in mammalian somatic cells exposed to extremely low frequency electromagnetic radiation: A review and future research recommendations. Bioelectromagnetics 2005, 26, 412–430. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Rojas, J.A.; Rodríguez-de la Fuente, A.O.; Alcocer-González, J.M.; Rodríguez-Flores, L.E.; Rodríguez-Padilla, C.; Santoyo-Stephano, M.A.; Castañeda-Garza, E.; Tamez-Guerra, R.S. Effect of 60 Hz magnetic fields on the activation of hsp70 promoter in cultured INER-37 and RMA E7 cells. Vitro Cell Dev. Biol.-Anim. 2010, 46, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Schmid, W. The micronucleus test for cytogenetic analysis in Chemical Mutagens. In Chemical Mutagens: Principles and Methods for Their Detection; Hollaender, A., Ed.; Springer Science and Business Media: New York, NY, USA, 1975; Volume 4, pp. 31–35. [Google Scholar]
- Oyeyemi, M.O.; Ubiogoro, O. Spermiogram and morphological characteristics in testicular and epididymal spermatozoa of Large White Boar in Nigeria. Int. J. Morphol. 2005, 23, 235–239. Available online: https://pdfs.semanticscholar.org/3e86/3438a072d41d9d4959f7f5bfed28a96a6a7f.pdf?_ga=2.63533223.941245367.1527095052-1250387518.1527095052 (accessed on 3 August 2018). [CrossRef]
- Pant, N.; Srivastava, S.P. Testicular and spermatotoxic effect of quinaphos in rats. J. Appl. Toxicol. 2003, 23, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Wyrobeck, A.J. Changes in mammalian sperm morphology after X-ray and chemical exposures. Genetics 1979, 92, S105–S119. Available online: https://www.ncbi.nlm.nih.gov/pubmed/385431 (accessed on 3 August 2018).
- Feytching, M.; Ahlom, A.; Kheifets, L. EMF and health. Annu. Rev. Public Health 2005, 26, 165–189. [Google Scholar] [CrossRef]
- Bowman, J.D.; Tapas, K.R.; Park, R.M. Possible health benefits from reducing occupational magnetic fields. Am. J. Ind. Med. 2013, 56, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Simkó, M.; Kriehuber, R.; Weiss, D.G.; Luben, R.A. Effects of 50 Hz EMF exposure on micronucleus formation and apoptosis in transformed and non-transformed human cell lines. Bioelectromagnetics. 1998, 19, 85–91. [Google Scholar] [CrossRef]
- Celikler, S.; Aydemir, N.; Vatan, O.; Kurtuldu, S.; Bilaloglu, R. A biomonitoring study of genotoxic risk to workers of transformers and distribution line stations. Int. J. Environ. Health. Res. 2009, 19, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Winker, R.; Ivancsits, S.; Pilger, A.; Adlkofer, F.; Rüdiger, H.W. Chromosomal damage in human diploid fibroblasts by intermittent exposure to extremely low-frequency electromagnetic fields. Mutat. Res. 2005, 585, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Erdal, N.; Gürgül, S.; Celik, A. Cytogenetic effects of extremely low frequency magnetic field on Wistar rat bone marrow. Mutat. Res. 2007, 630, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Frahm, J.; Lantow, M.; Lupke, M.; Weiss, D.G.; Simkó, M. Alteration in cellular functions in mouse macrophages after exposure to 50 Hz magnetic fields. J. Cell Biochem. 2006, 99, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Okudan, N.; Celik, I.; Salbacak, A.; Cicekcibasi, A.E.; Buyukmumcu, M.; Gökbel, H. Effects of long-term 50 Hz magnetic field exposure on the micronucleated polychromatic erythrocyte and blood lymphocyte frequency and argyrophilic nucleolar organizer regions in lymphocytes of mice. Neuro Endocrinol. Lett. 2010, 31, 208–214. Available online: https://www.ncbi.nlm.nih.gov/pubmed/20424591 (accessed on 3 August 2018). [PubMed]
- Zhu, K.; Lv, Y.; Cheng, Q.; Hua, J.; Zeng, Q. Extremely low frequency magnetic fields do not induce DNA damage in human lens epithelial cells in vitro. Anat. Rec. 2016, 299, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Pithawala, M.; Gadhia, P. Cytogenetic studies of individuals with occupational exposures to extremely low frequency electromagnetic fields (ELF-EMF). Bull. Environ. Pharmacol. Life Sci. 2012, 1, 10–16. Available online: https://pdfs.semanticscholar.org/40f4/f972c991e25eb2ab5f1587843db5e1a333b9.pdf (accessed on 3 August 2018).
- Luceri, C.; De Filippo, C.; Giovanelli, L.; Blangiardo, M.; Cavalieri, D.; Aglietti, F.; Pampaloni, M.; Andreuccetti, D.; Pieri, L.; Bambi, F.; et al. Extremely low-frequency electromagnetic fields do not affect DNA damage and gene expression profiles of yeast and human lymphocytes. Radiat. Res. 2005, 164, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Valberg, P.A.; Kavet, R.; Rafferty, C.N. Can low level 50/60 Hz electric and magnetic fields cause biological effects? Radiat. Res. 1997, 148, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Giese, B. Electron transfer through DNA and peptides. Bioorg. Med. Chem. 2006, 14, 6139–6143. [Google Scholar] [CrossRef] [PubMed]
- Lambrozo, J.; Souques, M. Electricity and extremely low frequency electric and magnetic fields. In Electromagnetic Fields, Environment and Health; Perrin, A., Souques, M., Eds.; Springer: Berlin, Germany, 2012; p. 41. [Google Scholar]
- Nordstrom, S.; Birke, E.; Gustavsson, L. Reproductive hazards among workers at high voltage substations. Bioelectromagnetics 1983, 4, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Lundsberg, L.S.; Bracken, M.B.; Belanger, K. Occupationally related magnetic field exposure and male subfertility. Fertil. Steril. 1995, 63, 384–391. [Google Scholar] [CrossRef]
- Furuya, H.; Aikawa, H.; Hagino, T.; Yoshida, T.; Sakabe, K. Flow cytometric analysis of the effects of 50 Hz magnetic fields on mouse spermatogenesis. Nippon Eiseigaku Zasshi 1998, 53, 420–425. Available online: https://www.ncbi.nlm.nih.gov/pubmed/9757758 (accessed on 15 April 2018). [CrossRef] [PubMed]
- Narra, V.R.; Howell, R.W.; Goddu, S.M.; Rao, D.V. Effects of a 1.5-Tesla static magnetic field on spermatogenesis and embryogenesis in mice. Investig. Radiol. 1996, 31, 586–590. Available online: https://www.ncbi.nlm.nih.gov/pubmed/8877496 (accessed on 25 January 2018). [CrossRef]
- Ramadan, L.A.; Abd-Allah, A.R.; Aly, H.A.; Saad-El-Din, A.A. Testicular toxicity effects of magnetic field exposure and prophylactic role of coenzyme Q10 and L-carnitine in mice. Pharmacol. Res. 2002, 46, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, S.; Jedlicka, J.; Parkanyi, V.; Rafay, J.; Ondruska, L.; Massanyi, P.; Bulla, J. Influence of a 50 Hz extra low frequency electromagnetic field on spermatozoa motility and fertilization rates in rabbits. J. Environ. Sci. Health Part A Tox./Hazard. Subst. Environ. Eng. 2009, 44, 1041–1047. [Google Scholar] [CrossRef]
- Withers, H.R.; Mason, K.A.; Davis, C.A. MR effect on murine spermatogenesis. Radiology 1985, 156, 741–742. [Google Scholar] [CrossRef] [PubMed]
- Tablado, L.; Pérez-Sánchez, F.; Núñez, J.; Núñez, M.; Soler, C. Effects of exposure to static magnetic fields on the morphology and morphometry of mouse epididymal sperm. Bioelectromagnetics 1998, 19, 377–383. Available online: https://www.ncbi.nlm.nih.gov/pubmed/9738528 (accessed on 14 February 2018). [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heredia-Rojas, J.A.; Rodríguez-De la Fuente, A.O.; Gomez-Flores, R.; Heredia-Rodríguez, O.; Rodríguez-Flores, L.E.; Beltcheva, M.; Castañeda-Garza, M.E. In Vivo Cytotoxicity Induced by 60 Hz Electromagnetic Fields under a High-Voltage Substation Environment. Sustainability 2018, 10, 2789. https://doi.org/10.3390/su10082789
Heredia-Rojas JA, Rodríguez-De la Fuente AO, Gomez-Flores R, Heredia-Rodríguez O, Rodríguez-Flores LE, Beltcheva M, Castañeda-Garza ME. In Vivo Cytotoxicity Induced by 60 Hz Electromagnetic Fields under a High-Voltage Substation Environment. Sustainability. 2018; 10(8):2789. https://doi.org/10.3390/su10082789
Chicago/Turabian StyleHeredia-Rojas, J. Antonio, Abraham Octavio Rodríguez-De la Fuente, Ricardo Gomez-Flores, Omar Heredia-Rodríguez, Laura E. Rodríguez-Flores, Michaela Beltcheva, and Ma. Esperanza Castañeda-Garza. 2018. "In Vivo Cytotoxicity Induced by 60 Hz Electromagnetic Fields under a High-Voltage Substation Environment" Sustainability 10, no. 8: 2789. https://doi.org/10.3390/su10082789
APA StyleHeredia-Rojas, J. A., Rodríguez-De la Fuente, A. O., Gomez-Flores, R., Heredia-Rodríguez, O., Rodríguez-Flores, L. E., Beltcheva, M., & Castañeda-Garza, M. E. (2018). In Vivo Cytotoxicity Induced by 60 Hz Electromagnetic Fields under a High-Voltage Substation Environment. Sustainability, 10(8), 2789. https://doi.org/10.3390/su10082789




