Re-Evaluation of the Impacts of Dietary Preferences on Macroinvertebrate Trophic Sources: An Analysis of Seaweed Bed Habitats Using the Integration of Stable Isotope and Observational Data
Abstract
1. Introduction
2. Materials and Methods
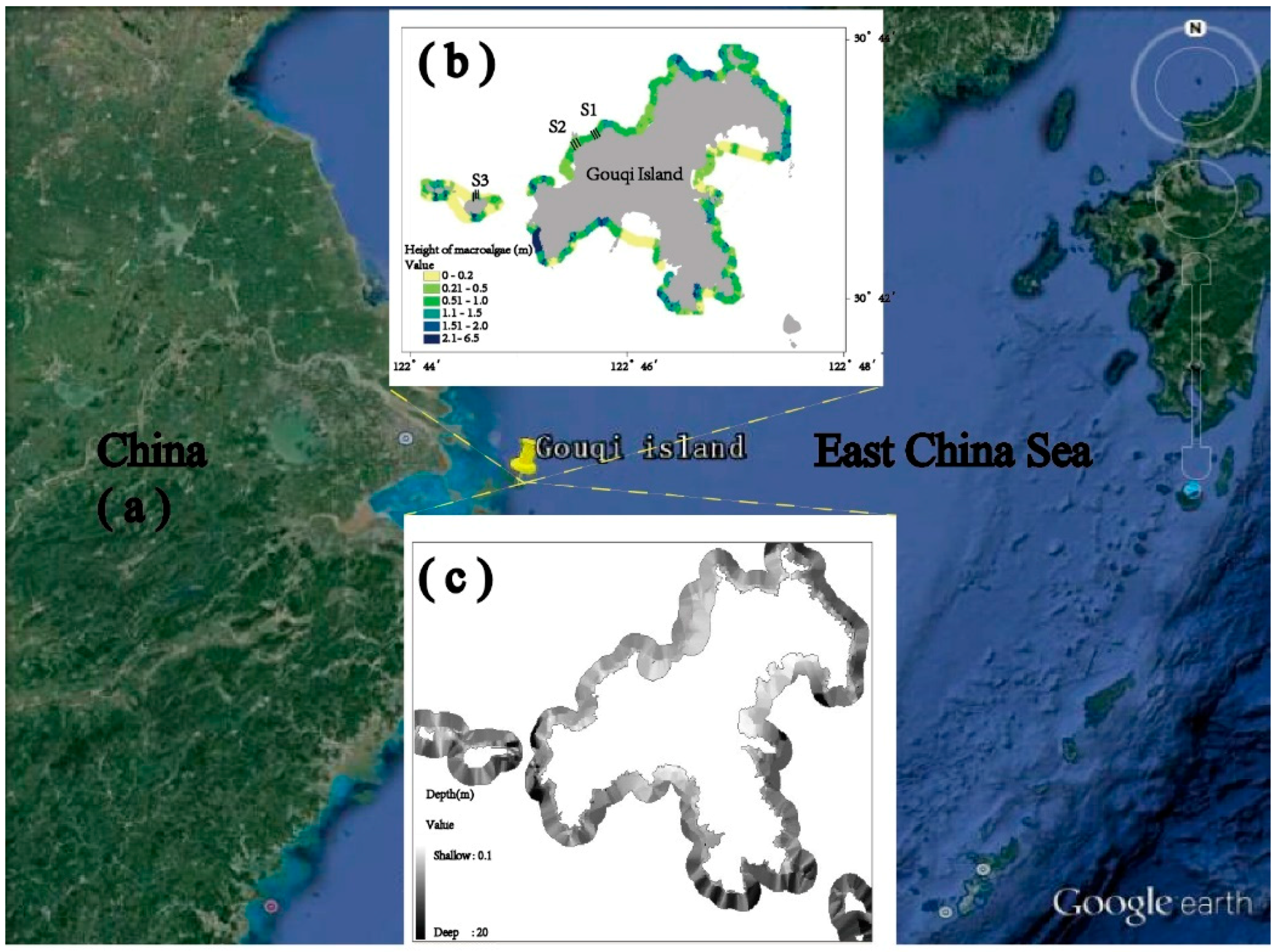
2.1. Study Site
2.2. Sampling
2.2.1. Potential Food Sources
2.2.2. Consumers
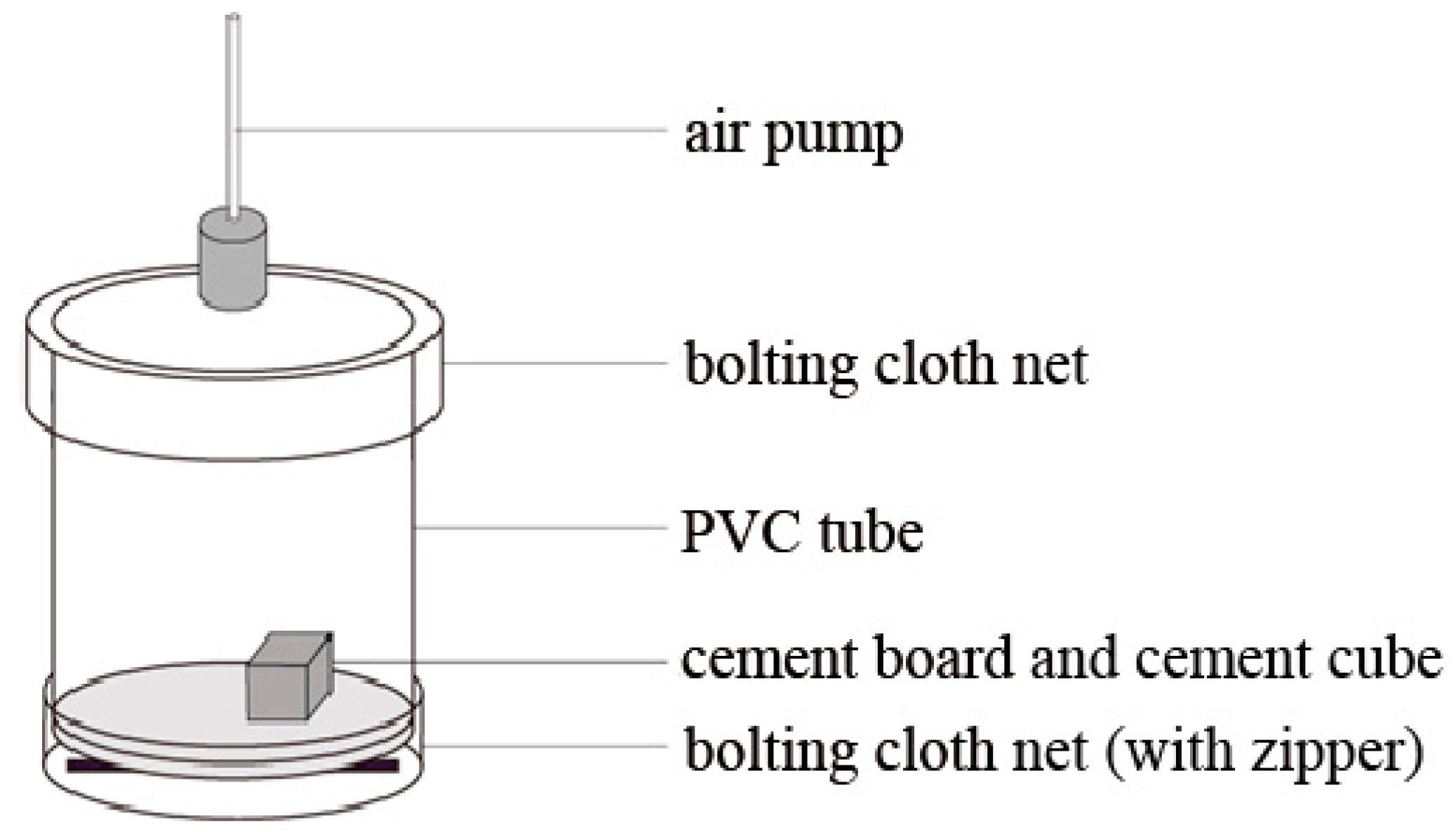
2.3. Species-Specific Diet Selection Observation
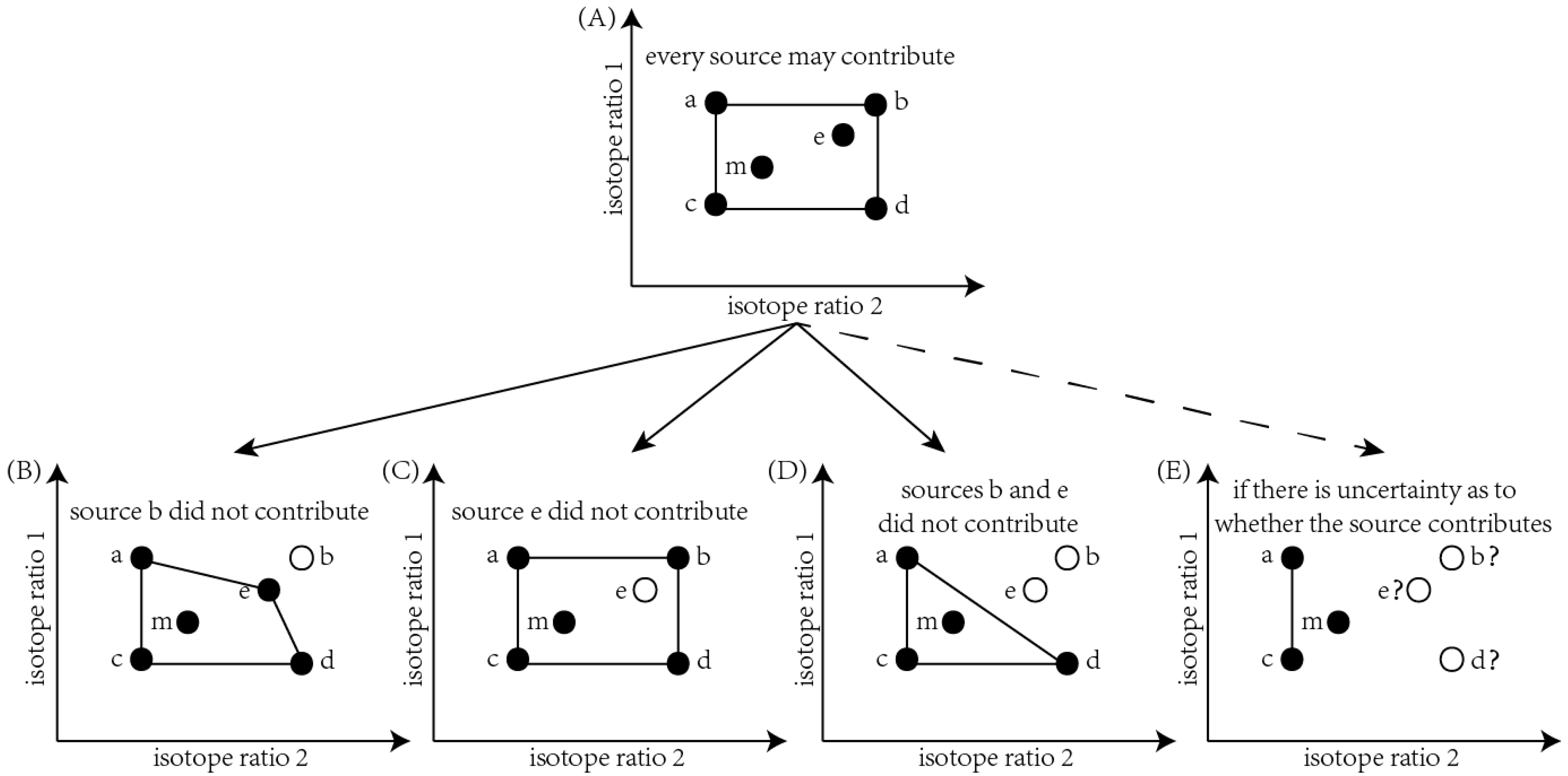
2.4. Estimation of Discrimination between Diets and Macroinvertebrates
2.5. Stable Isotope Analysis
2.6. Data Analysis
3. Results
3.1. Dietary Observations: Mobility, Mode and Preference
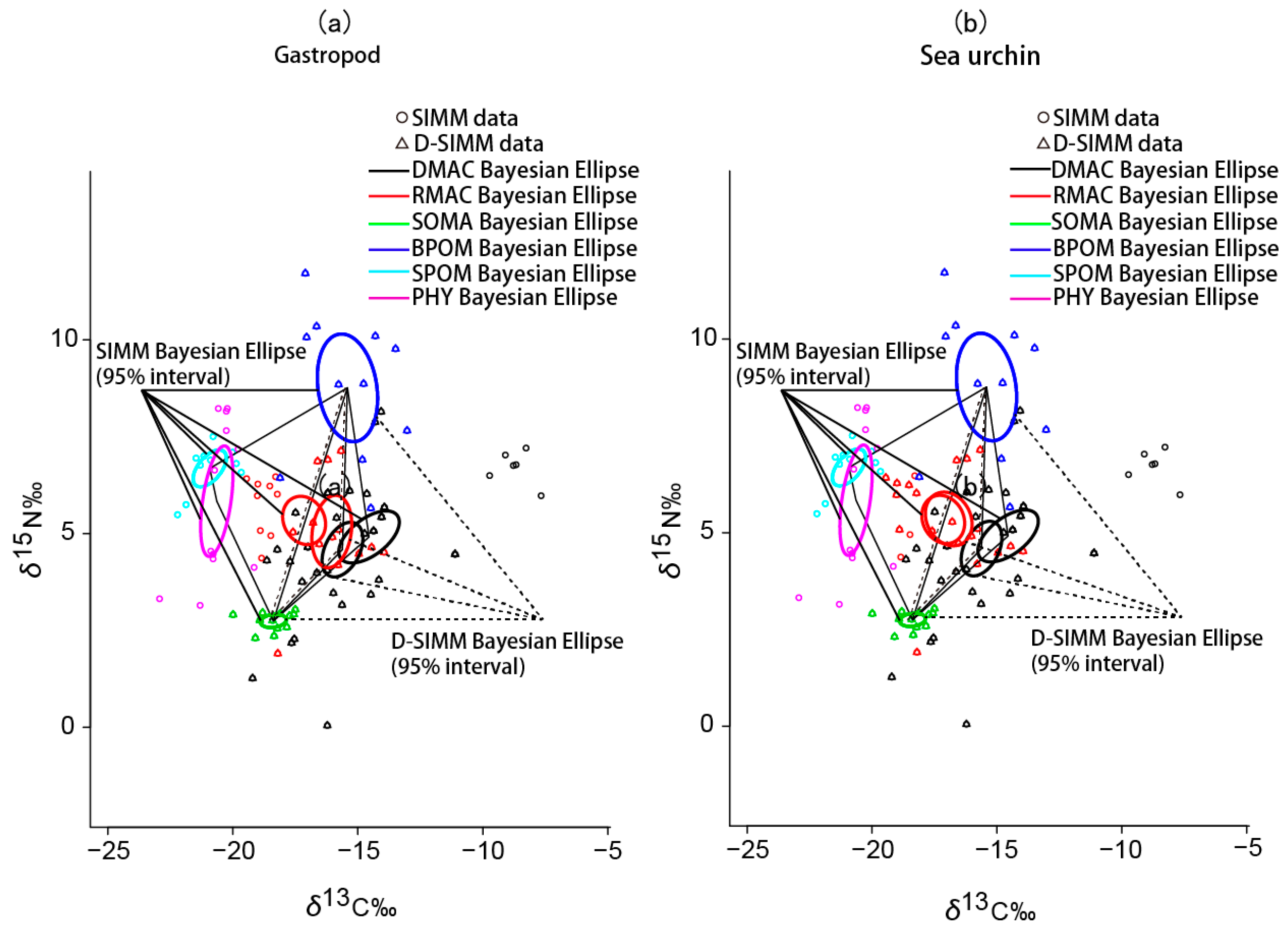
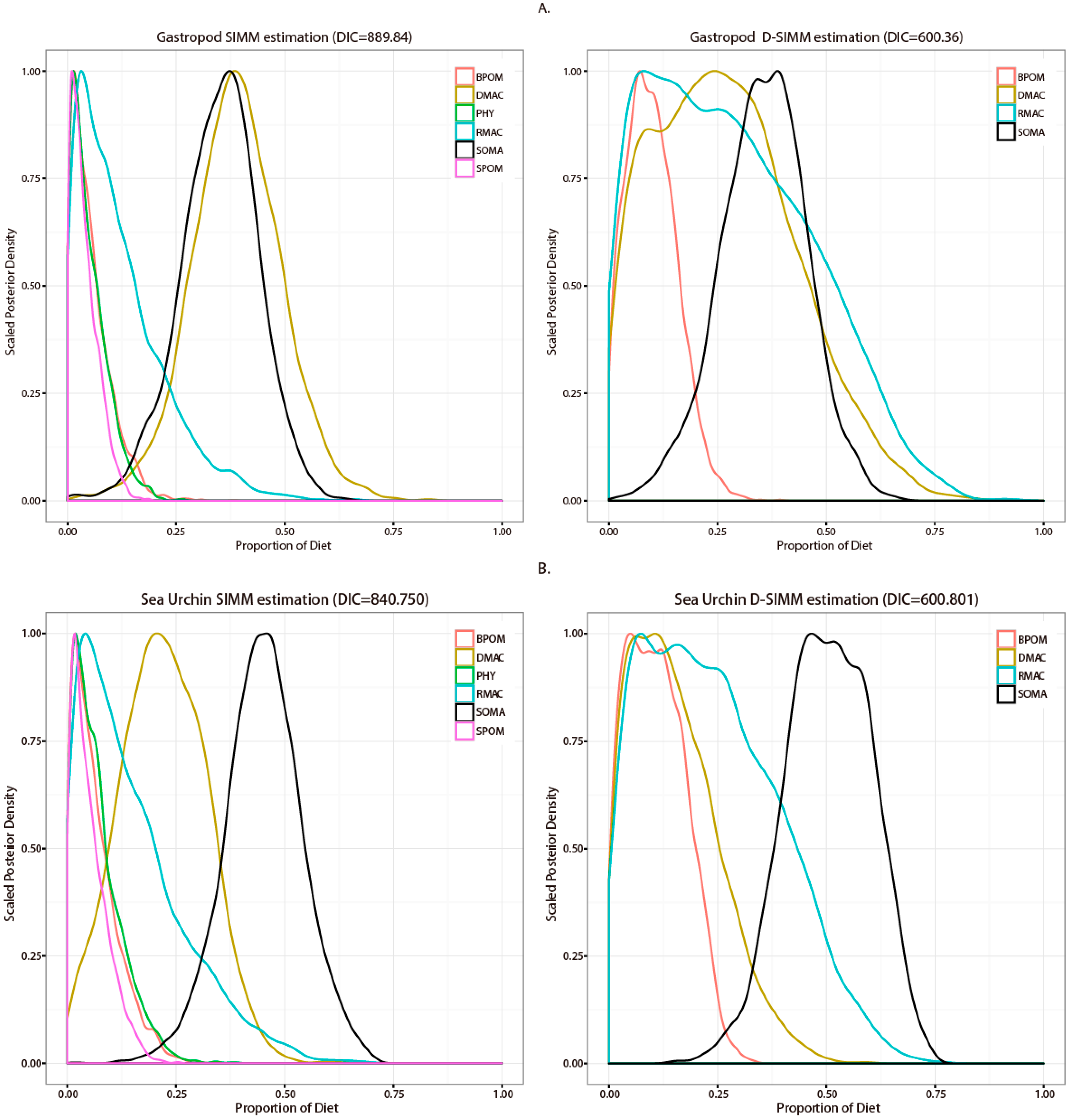
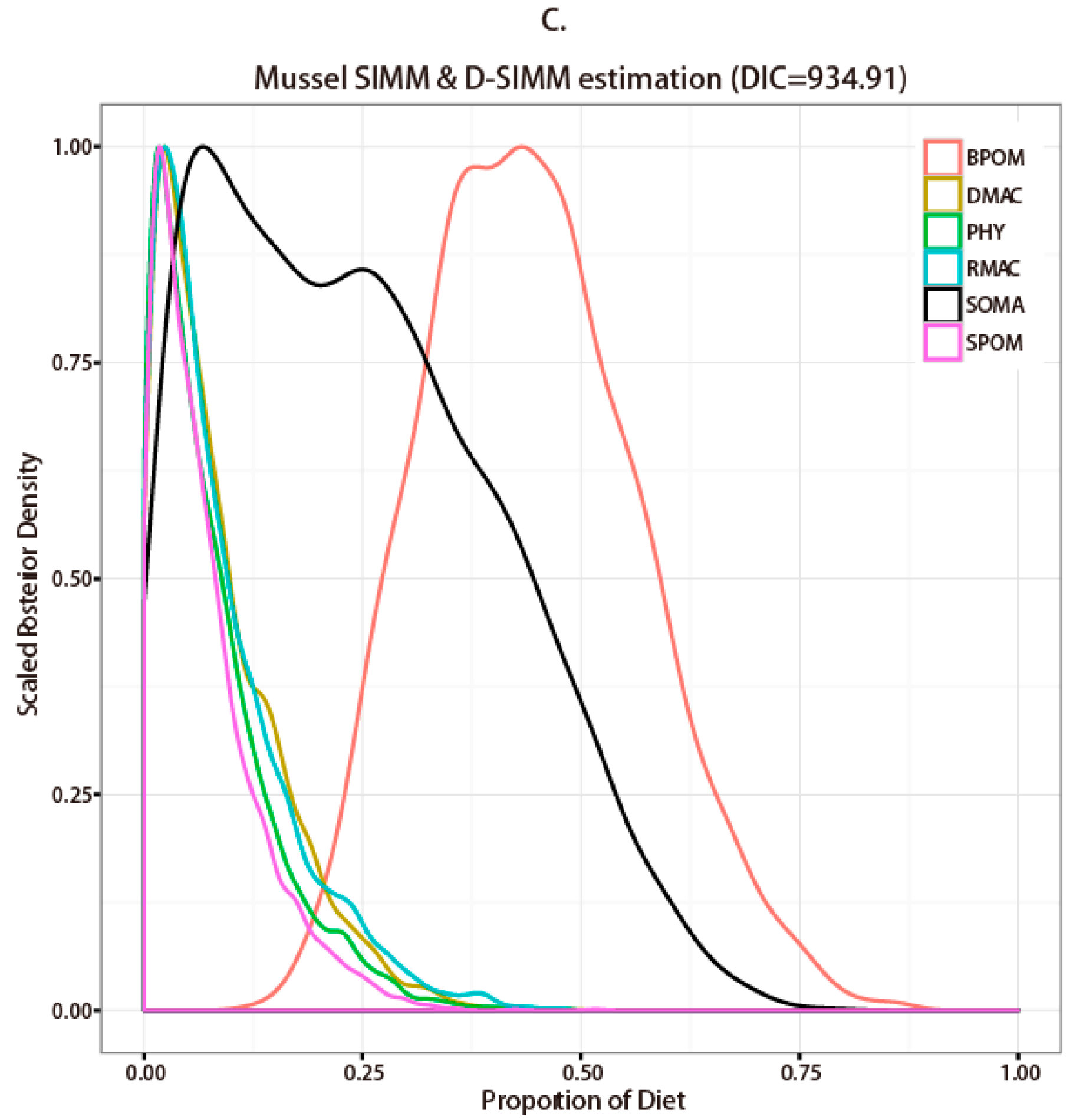
3.2. Stable Isotope Analysis and Source Contribution Evaluation
4. Discussion
4.1. Comparison between SIMM and D-SIMM
4.2. Diet Estimation of Representative Macroinvertebrate Species in Seaweed Beds
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paine, R.T. Road Maps of Interactions or Grist for Theoretical Development? Ecology 1988, 69, 1648–1654. [Google Scholar] [CrossRef]
- Bertness, M.D.; Leonard, G.H. The role of positive interactions in communities: Lessons from intertidal habitats. Ecology 1997, 78, 1976–1989. [Google Scholar] [CrossRef]
- Bertness, M.D.; Leonard, G.H.; Levine, J.M.; Schmidt, P.R.; Ingraham, A.O. Testing the relative contribution of positive and negative interactions in rocky intertidal communities. Ecology 1999, 80, 2711–2726. [Google Scholar] [CrossRef]
- Whittaker, R.H.; Niering, W.A. Vegetation of the Santa Catalina Mountains, Arizona. V. Biomass, Production, and Diversity along the Elevation Gradient. Ecology 1975, 56, 771–790. [Google Scholar] [CrossRef]
- Reviews, B.; Iv, V.; Assemblages, O.; Ecology, M.; Geography, E.A.; Mann, K.H. Ecology of Coastal Waters—A Systems Approach. Studies in Ecology; Blackwell Scientific Publications: Oxford, UK, 1982; Volume 8, pp. 340–341. [Google Scholar]
- Zabala, S.; Bigatti, G.; Botto, F.; Iribarne, O.O.; Galván, D.E. Trophic relationships between a Patagonian gastropod and its epibiotic anemone revealed by using stable isotopes and direct observations. Mar. Biol. 2013, 160, 909–919. [Google Scholar] [CrossRef]
- Harrold, C.; Lisin, S. Radio-tracking fafts of giant-kelp—Local production and regional transport. J. Exp. Mar. Biol. Ecol. 1989, 130, 237–251. [Google Scholar] [CrossRef]
- Pollock, F.J.; Lamb, J.B.; Field, S.N.; Heron, S.F.; Schaffelke, B.; Shedrawi, G.; Bourne, D.G.; Willis, B.L. Sediment and turbidity associated with offshore dredging increase coral disease prevalence on nearby reefs. PLoS ONE 2014, 9, e102498. [Google Scholar] [CrossRef] [PubMed]
- Colombo, F.; Costa, V.; Dubois, S.F.; Gianguzza, P.; Mazzola, A.; Vizzini, S. Trophic structure of vermetid reef community: High trophic diversity at small spatial scales. J. Sea Res. 2013, 77, 93–99. [Google Scholar] [CrossRef]
- Lunt, J.; Smee, D.L. Turbidity influences trophic interactions in estuaries. Limnol. Oceanogr. 2014, 59, 2002–2012. [Google Scholar] [CrossRef]
- Duggins, D.O.; Eckman, J.E. The role of kelp detritus in the growth of benthic suspension feeders in an understory kelp forest. J. Exp. Mar. Biol. Ecol. 1994, 176, 53–68. [Google Scholar] [CrossRef]
- Abrantes, K.G.; Sheaves, M. Importance of freshwater flow in terrestrial-aquatic energetic connectivity in intermittently connected estuaries of tropical Australia. Mar. Biol. 2010, 157, 2071–2086. [Google Scholar] [CrossRef]
- Cardoso, P.G.; Raffaelli, D.; Lillebø, A.I.; Verdelhos, T.; Pardal, M.A. The impact of extreme flooding events and anthropogenic stressors on the macrobenthic communities’ dynamics. Estuar. Coast. Shelf Sci. 2008, 76, 553–565. [Google Scholar] [CrossRef]
- Duffy, J.E.; Cardinale, B.J.; France, K.E.; McIntyre, P.B.; Thébault, E.; Loreau, M. The functional role of biodiversity in ecosystems: Incorporating trophic complexity. Ecol. Lett. 2007, 10, 522–538. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.F.; Shin, P.K.S.; Lin, G.H.; Chen, S.P.; Siu, G.C. Stable isotope and fatty acid evidence for uptake of organic waste by green-lipped mussels Perna viridis in a polyculture fish farm system. Mar. Ecol. Prog. Ser. 2006, 317, 273–283. [Google Scholar] [CrossRef]
- Pauly, D.; Watson, R. Background and interpretation of the “Marine Trophic Index” as a measure of biodiversity. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Leitão, R.; Martinho, F.; Neto, J.M.; Cabral, H.; Marques, J.C.; Pardal, M.A. Feeding ecology, population structure and distribution of Pomatoschistus microps (Krøyer, 1838) and Pomatoschistus minutus (Pallas, 1770) in a temperate estuary, Portugal. Estuar. Coast. Shelf Sci. 2006, 66, 231–239. [Google Scholar] [CrossRef]
- Hopkins, J.B.; Ferguson, J.M. Estimating the diets of animals using stable isotopes and a comprehensive Bayesian mixing model. PLoS ONE 2012, 7, e28478. [Google Scholar] [CrossRef]
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER—Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Ogle, K.; Tucker, C.; Cable, J.M. Beyond simple linear mixing models: Process-based isotope partitioning of ecological processes. Ecol. Appl. 2014, 24, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Winemiller, K.O.; Akin, S.; Zeug, S.C. Production sources and food web structure of a temperate tidal estuary: Integration of dietary and stable isotope data. Mar. Ecol. Prog. Ser. 2007, 343, 63–76. [Google Scholar] [CrossRef]
- Savini, D.; Occhipinti-Ambrogi, A. Consumption rates and prey preference of the invasive gastropod Rapana venosa in the Northern Adriatic Sea. Helgol. Mar. Res. 2006, 60, 153–159. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, S.; Wang, X.; Rijin, J.; Zhao, J. The feeding behaviour and ecological function during summer of one herbivore on seaweed bed in Gouqi Island: The gastropod, Turbo cornutus Solander. J. Fish. China 2015, 39, 511–519. [Google Scholar]
- Pearson, T.H.; Rosenberg, R. Feast and Famine: Structuring Factors in Marine Benthic Communities; Symposium of the British Ecological Society: Sussex, UK, 1987. [Google Scholar]
- Fauchald, K.; Jumars, P.A. The diet of worms: A study of polychaete feeding guilds. Oceanogr. Mar. Biol. Annu. Rev. 1979, 17, 193–284. [Google Scholar]
- McCutchan, J.H.; Lewis, W.M.; Kendall, C.; McGrath, C.C. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 2003, 102, 378–390. [Google Scholar] [CrossRef]
- Vander Zanden, M.J.; Rasmussen, J.B. Primary consumer delta C-13 and delta N-15 and the trophic position of aquatic consumers. Ecology 1999, 80, 1395–1404. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: http://www.R-project.org/ (accessed on 16 March 2016).
- Semmens, B.X.; Stock, B.C.; Ward, E.; Moore, J.W.; Parnell, A.; Jackson, A.L.; Phillips, D.L.; Bearhop, S.; Inger, R. MixSIAR: A Bayesian Stable Isotope Mixing Model for Characterizing Intrapopulation Niche Variation; ESA Convention: Minneapolis, MN, USA, 2013. [Google Scholar]
- Jackson, M.C.; Donohue, I.; Jackson, A.L.; Britton, J.R.; Harper, D.M.; Grey, J. Population-level metrics of trophic structure based on stable isotopes and their application to invasion ecology. PLoS ONE 2012, 7, e31757. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.W.; Semmens, B.X. Incorporating uncertainty and prior information into stable isotope mixing models. Ecol. Lett. 2010, 11, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.A. On the Interpretation of χ2 from Contingency Tables, and the Calculation of P. J. R. Stat. Soc. 1922, 85, 87–94. [Google Scholar] [CrossRef]
- Gelman, A.A.; Carlin, J.B.; Stern, H.S.; Donald, B. Bayesian Data Analysis; Chapman & Hall/CRC, cop.: Boca Raton, FL, USA, 1995. [Google Scholar]
- Saito, L.; Redd, C.; Chandra, S.; Atwell, L.; Fritsen, C.H.; Rosen, M.R. Quantifying foodweb interactions with simultaneous linear equations: Stable isotope models of the Truckee River, USA. J. N. Am. Benthol. Soc. 2007, 26, 642–662. [Google Scholar] [CrossRef]
- Yokoyama, H.; Ishihi, Y. Variation in δ 13C and δ 15N among different tissues of three estuarine bivalves: Implications for dietary. Plankton Benthos Res. 2006, 1, 178–182. [Google Scholar] [CrossRef]
- Kasai, A.; Ishizaki, D.; Isoda, T. Isotopic trophic-step fractionation of the freshwater clam Corbicula sandai. Fish. Sci. 2016, 82, 491–498. [Google Scholar] [CrossRef]
- Dubois, S.; Jean-Louis, B.; Bertrand, B.; Lefebvre, S. Isotope trophic-step fractionation of suspension-feeding species: Implications for food partitioning in coastal ecosystems. J. Exp. Mar. Biol. Ecol. 2007, 351, 121–128. [Google Scholar] [CrossRef]
- Fry, B. Food Web Structure on Georges Bank from Stable C, N, and S Isotopic Compositions. Limnol. Oceanogr. 1988, 33, 1182–1190. [Google Scholar] [CrossRef]
- Gates, E.N. Determining Carbon and Nitrogen Stable Isotope Discrimination for Marine Consumers. Ph.D. Thesis, Edith Cowan University, Joondalup, WA, Australia, 2006. [Google Scholar]
- Barton, D.R.; Johnson, R.A.; Campbell, L.; Petruniak, J.; Patterson, M. Effects of round gobies (Neogobius melanostomus) on dreissenid mussels and other invertebrates in Eastern Lake Erie, 2002–2004. J. Great Lakes Res. 2005, 31, 252–261. [Google Scholar] [CrossRef]
- Layman, C.A.; Quattrochi, J.P.; Peyer, C.M.; Allgeier, J.E. Niche width collapse in a resilient top predator following ecosystem fragmentation. Ecol. Lett. 2007, 10, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Christensen, V.; Walters, C.J. Ecopath with Ecosim: Methods, capabilities and limitations. Ecol. Model. 2004, 172, 109–139. [Google Scholar] [CrossRef]
- Fulton, E.A.; Smith, A.D.M.; Johnson, C.R. Biogeochemical marine ecosystem models I: IGBEM—A model of marine bay ecosystems. Ecol. Model. 2004, 174, 267–307. [Google Scholar] [CrossRef]
- Plagányi, É.E.; Punt, A.E.; Hillary, R.; Morello, E.B.; Thébaud, O.; Hutton, T.; Pillans, R.D.; Thorson, J.T.; Fulton, E.A.; Smith, A.D.M.; et al. Multispecies fisheries management and conservation: Tactical applications using models of intermediate complexity. Fish Fish. 2014, 15, 1–22. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Bhat, H.; Kumar, N. On the Derivation of the Bayesian Information Criterion; School of Natural Sciences, University of California: Oakland, CA, USA, 2015. [Google Scholar]
- Spiegelhalter, D.J.; Best, N.G.; Carlin, B.P.; Van der Linde, A. The deviance information criterion: 12 years on. J. R. Stat. Soc. Ser. B Stat. Methodol. 2014, 76, 485–493. [Google Scholar] [CrossRef]
- Gurney, L.J.; Froneman, P.W.; Pakhomov, E.A.; McQuaid, C.D. Trophic positions of three euphausiid species from the Prince Edward Islands (Southern Ocean): Implications for the pelagic food web structure. Mar. Ecol. Prog. Ser. 2001, 217, 167–174. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, S.; Chen, Y.; Bi, Y. Analysis of functional feeding groups of macroinvertebrates communities in the macroalgae beds of Gouqi Island, Zhejiang Province. J. Fish. China 2015, 39, 381–391. [Google Scholar]
- Fields, L.; Nixon, S.W.; Oviatt, C.; Fulweiler, R.W. Benthic metabolism and nutrient regeneration in hydrographically different regions on the inner continental shelf of Southern New England. Estuar. Coast. Shelf Sci. 2014, 148, 14–26. [Google Scholar] [CrossRef]
- Jiang, R.; Zhang, S.; Bi, Y.; Wang, Z.; Zhou, X.; Zhao, X.; Chen, L. Food sources of small invertebrates in the macroalgal bed of Gouqi Island. J. Fish. China 2015, 39, 1487–1498. [Google Scholar]
- Jiang, R.; Zhang, S.; Wang, K.; Zhou, X.; Zhao, J. Stable isotope analysis of the offshore food web of Gouqi Island. Chin. J. Ecol. 2014, 33, 930–938. [Google Scholar]

| Sample | Sample Size (n) | δ13C (‰) | δ15N (‰) | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Potential trophic source | |||||
| PHY | 12 | −20.64 | 0.92 | 5.84 | 2.02 |
| SPOM | 10 | −20.92 | 0.86 | 6.69 | 0.62 |
| BPOM | 11 | −15.42 | 1.63 | 8.76 | 1.88 |
| SOMA | 12 | −18.38 | 0.73 | 2.75 | 0.24 |
| SOMB | 18 | −21.95 | 0.51 | 2.53 | 0.51 |
| MADS | 39 | −14.55 | 3.08 | 4.99 | 1.55 |
| Corallina officinalis | 3 | −8.48 | 0.73 | 6.6 | 0.55 |
| Jania decussato-dichotoma | 3 | −8.93 | 0.74 | 6.82 | 0.36 |
| Ulva pertuca | 11 | −14.3 | 0.41 | 5.76 | 1.26 |
| Dictyopteris dichotoma | 3 | −14.77 | 3.19 | 5.06 | 1.26 |
| Sargassum vachellianum | 5 | −15.72 | 2.81 | 4.53 | 1.03 |
| Sargassum horueri | 10 | −16.83 | 1.54 | 3.8 | 1.59 |
| Sargassum fusiforme | 4 | −16.7 | 0.7 | 3.84 | 0.54 |
| MARS | 22 | −17.04 | 1.6 | 5.29 | 1.2 |
| Garteloupia livida | 3 | −14.45 | 0.51 | 4.55 | 0.09 |
| Chondria crassicaulis | 3 | −16.83 | 1.28 | 5.31 | 2.94 |
| Undaria pinnatifida | 7 | −16.39 | 0.65 | 5.17 | 0.84 |
| Codium fragile | 3 | −18.54 | 0.29 | 5.26 | 1.08 |
| Ceramium japonicum | 3 | −19.16 | 0.25 | 6.23 | 0.22 |
| Hypnea cervicornis | 3 | −18.55 | 0.34 | 5.78 | 0.61 |
| Consumers | |||||
| Gastropod (T. cornutus) | 26 | −16.3 | 0.85 | 5.86 | 0.54 |
| Sea urchin (A. crassispina) | 17 | −15.44 | 0.65 | 5.08 | 0.5 |
| Mussel (S. virgatus) | 41 | −15.97 | 0.64 | 6.15 | 0.62 |
| Description | Species | Functional Group | Potential Trophic Source | |||
|---|---|---|---|---|---|---|
| MAC | PHY | SOM | POM | |||
| Sea urchin | A. crassispina | Semi-mobile jawed surface omnivore (herbivore and detritus feeder) | ●a | ×c | ○b | ○b |
| Gastropod | T. cornutus | Semi-mobile jawed surface omnivore (herbivore and detritus feeder) | ●a | ×c | ●a | ○b |
| Mussel | S. virgatus | Sessile filter-feeder (MAC and PHY and POM and SOM) | ●a | ●a | ●a | ●a |
| Potential Trophic Source | Replicate Number of Experiments (n) | Macroinvertebrate—MAC Dietary Preference | |
|---|---|---|---|
| Sea Urchin | Gastropod | ||
| Chlorophyta | |||
| Ulva pertuca | 6 | ●a | ●a |
| Codium fragile | 6 | ○b | ×c |
| Rhodophyta | |||
| Ceramium japonicum | 6 | ○b | ○b |
| Chondria crassicaulis | 6 | ○b | ×c |
| Corallina officinalis | 6 | ×c | ×c |
| Jania decussato dichotoma | 6 | ×c | ×c |
| Hypnea cervicornis J.Ag. | 6 | ○b | ×c |
| Garteloupia kurogii | 6 | ●a | ●a |
| Phaeophyta | |||
| Sargassum fusiforme | 6 | ●a | ●a |
| Sargassum horueri | 6 | ●a | ●a |
| Dictyopteris dichotoma | 6 | ●a | ○b |
| Ishige okamurai | 6 | ●a | ○b |
| Hizikia fusifarme | 6 | ●a | ●a |
| Group | Tissue | Diet | Lab. or Field | Δ13C (‰) | Δ15N (‰) | SDΔ13C (‰) | SDΔ15N (‰) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Bivalves (M. veneriformis) | Muscle | POM | Lab. | 0.9 | 3.6 | [35] | ||
| Bivalves (R. philippinarum) | Muscle | POM | Lab. | 0.6 | 3.4 | [35] | ||
| Bivalves (C. sandai) | Soft tissue | PHY | Lab. | 0.7 | 2.1 | [36] | ||
| Bivalves (M. edulis) | Muscle | PHY | Lab. | 2.17 | 3.78 | 0.324 | 0.292 | [37] |
| Bivalves (C. gigas) | Muscle | PHY | Lab. | 1.85 | 3.79 | 0.194 | 0.194 | [37] |
| Bivalves (C. sandai) | Soft tissue | MAC | Lab. | 0.6 | 3.6 | [36] | ||
| Bivalves (C. sandai) | Soft tissue | MAC | Lab. | 0.1 | 3.3 | [36] | ||
| Bivalves (Scallops) | Soft tissue | POM | Field | 3.8 | 0.9 | [38] | ||
| Bivalves (Mussel) | Soft tissue | POM | Field | 3.4 | 1.8 | [38] | ||
| Sea urchin (H. erythrogramma) | Muscle | MAC (Phaeophytes) | Lab. | 1.85 | 2.44 | [39] | ||
| Sea urchin (H. erythrogramma) | Muscle | MAC (Fleshy Rhodophytes) | Lab. | 3.23 | 3.96 | [39] | ||
| Sea urchin (H. erythrogramma) | Muscle | MAC (Calcareous Rhodophytes) | Lab. | 4.39 | 3.02 | [39] | ||
| Sea urchin (H. erythrogramma) | Muscle | MAC (Chlorophytes) | Lab. | 1.2 | 3.15 | [39] | ||
| Gastropod (T. torquatus) | Foot tissue | MAC (Fleshy Rhodophytes) | Lab. | −0.17 | 1.56 | [39] | ||
| Gastropod (T. torquatus) | Foot tissue | MAC (Calcareous Rhodophytes) | Lab. | 0.14 | 1.06 | [39] | ||
| Gastropod (T. cornutus) | Foot tissue | MAC and SOM | Lab. | 0.43 | 1.43 | 0.13 | 0.8 | Present study |
| Sea urchin (A. crassispina) | Gonad | MAC and SOM | Lab. | 1.93 | 0.8 | 1.1 | 0.2 | Present study |
| Source | TA | SEA | SEAC |
|---|---|---|---|
| SIMM (gastropod and sea urchin) | |||
| DMAC | 51.41 | 13.66 | 14.03 |
| RMAC | 16.79 | 6.09 | 6.39 |
| SOMA | 1.21 | 0.54 | 0.6 |
| BPOM | 20.35 | 9.48 | 10.54 |
| SPOM | 2.21 | 1.37 | 1.54 |
| PHY | 10.36 | 5.12 | 5.64 |
| Gastropod D-SIMM | |||
| DMAC | 35.82 | 9.13 | 9.43 |
| RMAC | 10.9 | 4.98 | 5.44 |
| SOMA | 1.12 | 0.54 | 0.6 |
| BPOM | 20.35 | 9.48 | 10.54 |
| Sea Urchin D-SIMM | |||
| DMAC | 35.82 | 9.13 | 9.43 |
| RMAC | 16.79 | 6.34 | 6.71 |
| SOMA | 1.12 | 0.54 | 0.6 |
| BPOM | 20.35 | 9.48 | 10.54 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Liu, Y.; Wang, K.; Zhao, J.; Zhao, X.; Zhang, S. Re-Evaluation of the Impacts of Dietary Preferences on Macroinvertebrate Trophic Sources: An Analysis of Seaweed Bed Habitats Using the Integration of Stable Isotope and Observational Data. Sustainability 2018, 10, 2010. https://doi.org/10.3390/su10062010
Zhou X, Liu Y, Wang K, Zhao J, Zhao X, Zhang S. Re-Evaluation of the Impacts of Dietary Preferences on Macroinvertebrate Trophic Sources: An Analysis of Seaweed Bed Habitats Using the Integration of Stable Isotope and Observational Data. Sustainability. 2018; 10(6):2010. https://doi.org/10.3390/su10062010
Chicago/Turabian StyleZhou, Xijie, Yumeng Liu, Kai Wang, Jing Zhao, Xu Zhao, and Shouyu Zhang. 2018. "Re-Evaluation of the Impacts of Dietary Preferences on Macroinvertebrate Trophic Sources: An Analysis of Seaweed Bed Habitats Using the Integration of Stable Isotope and Observational Data" Sustainability 10, no. 6: 2010. https://doi.org/10.3390/su10062010
APA StyleZhou, X., Liu, Y., Wang, K., Zhao, J., Zhao, X., & Zhang, S. (2018). Re-Evaluation of the Impacts of Dietary Preferences on Macroinvertebrate Trophic Sources: An Analysis of Seaweed Bed Habitats Using the Integration of Stable Isotope and Observational Data. Sustainability, 10(6), 2010. https://doi.org/10.3390/su10062010




