Adoption of High-Yielding Groundnut Varieties: The Sustainability of a Farmer-Led Multiplication-Dissemination Program in Eastern Uganda
Abstract
1. Introduction
2. Background
2.1. Seed Adoption Literature
2.2. Groundnut in Uganda
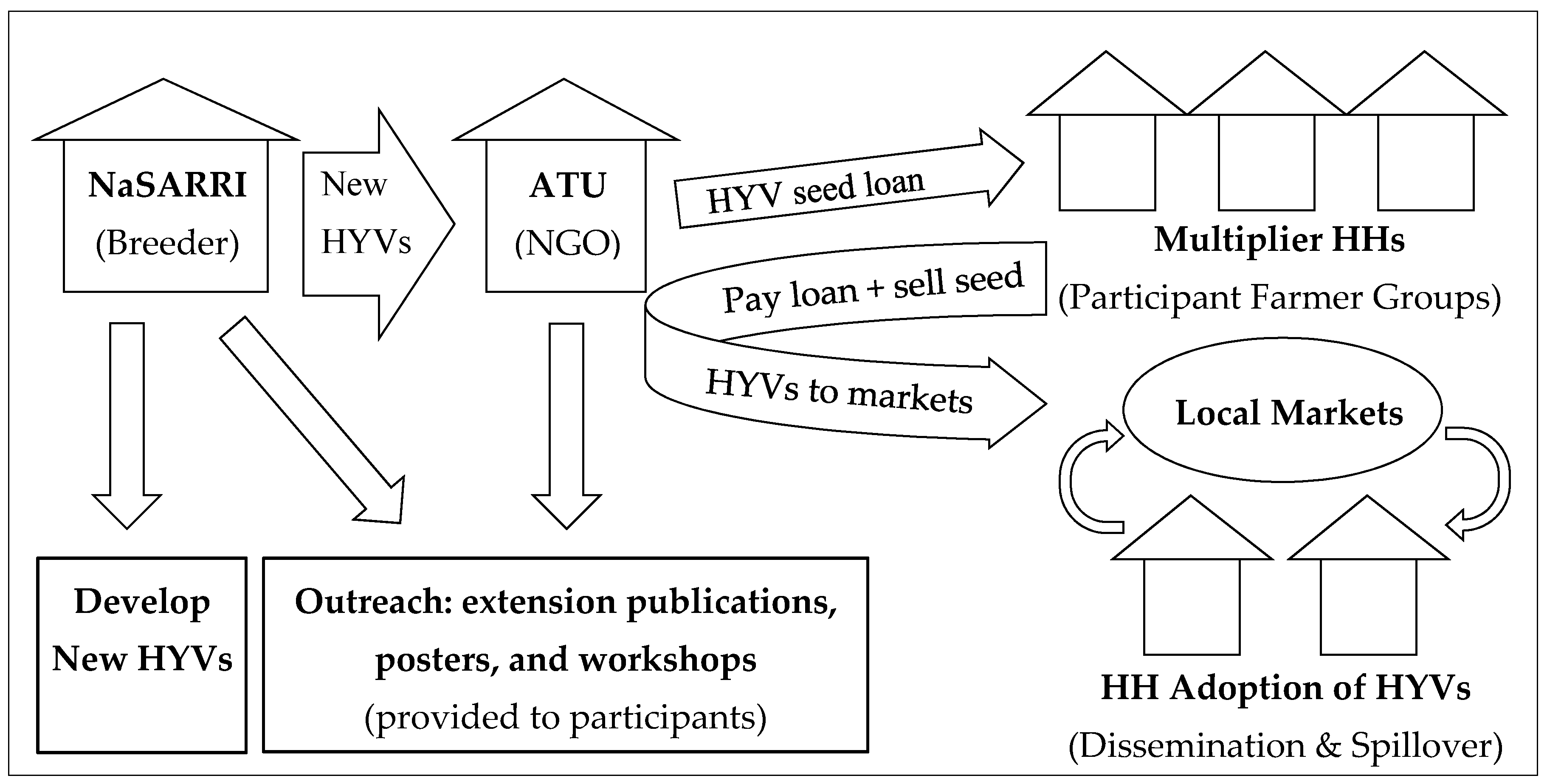
2.3. The ATU Farmer-Led Multiplication and Dissemination Program
3. Materials and Methods
3.1. Data
3.2. Methodological Framework
4. Results
5. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. FAOSTAT Statistics Database; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017. [Google Scholar]
- Okello, D.K.; Biruma, M.; Deom, C.M. Overview of groundnuts research in Uganda: Past, present and future. Afr. J. Biotechnol. 2013, 9, 6448–6459. [Google Scholar]
- Kassie, M.; Shiferaw, B.; Muricho, G. Agricultural Technology, Crop Income, and Poverty Alleviation in Uganda. World Dev. 2011, 39, 1784–1795. [Google Scholar] [CrossRef]
- Moyo, S.; Norton, G.W.; Alwang, J.; Rhinehart, I.; Deom, C.M. Peanut Research and Poverty Reduction: Impacts of Variety Improvement to Control Peanut Viruses in Uganda. Am. J. Agric. Econ. 2007, 89, 448–460. [Google Scholar] [CrossRef]
- Shiferaw, B.; Muricho, G.; Okello, J.; Kebede, T.A.; Okecho, G. Adoption of Improved Groundnut Varieties in Uganda; ICRISAT: Hyderabad, India, 2010. [Google Scholar]
- Tanellari, E.; Kostandini, G.; Bonabana-Wabbi, J.; Murray, A. Gender impacts on adoption of new technologies: The case of improved groundnut varieties in Uganda. Afr. J. Agric. Resour. Econ. Vol. 2014, 9, 300–308. [Google Scholar]
- Thuo, M.; Bell, A.A.; Bravo-Ureta, B.E.; Lachaud, M.A.; Okello, D.K.; Okoko, E.N.; Kidula, N.L.; Deom, C.M.; Puppala, N. Effects of social network factors on information acquisition and adoption of improved groundnut varieties: The case of Uganda and Kenya. Agric. Hum. Values 2014, 31, 339–353. [Google Scholar] [CrossRef]
- Thuo, M.; Bell, A.A.; Bravo-Ureta, B.E.; Okello, D.K.; Okoko, E.N.; Kidula, N.L.; Deom, C.M.; Puppala, N. Social Network Structures among Groundnut Farmers. J. Agric. Educ. Ext. 2013, 19, 339–359. [Google Scholar] [CrossRef]
- Bold, T.; Kaizzi, K.C.; Svensson, J.; Yanagizawa-Drott, D. Lemon Technologies and Adoption: Measurement, Theory and Evidence from Agricultural Markets in Uganda. Q. J. Econ. 2017, 132, 1055–1100. [Google Scholar] [CrossRef]
- Sheahan, M.; Barrett, C.B. Ten striking facts about agricultural input use in Sub-Saharan Africa. Food Policy 2017, 67, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.; Kandiwa, V. Can agricultural input subsidies reduce the gender gap in modern maize adoption? Evidence from Malawi. Food Policy 2014, 45, 101–111. [Google Scholar] [CrossRef]
- Doss, C.R. Analyzing technology adoption using microstudies: Limitations, challenges, and opportunities for improvement. Agric. Econ. 2006, 34, 207–219. [Google Scholar] [CrossRef]
- Tino, G.; Laker-Ojok, R.; Namisi, S. Impact Assessment Report for Farmer Led Groundnut Multiplication in Uganda; AT Uganda Ltd.: Kampala, Uganda, 2004. [Google Scholar]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- World Bank. World Development Report 2008: Agriculture for Development; World Bank: Washington, DC, USA, 2007. [Google Scholar]
- Food and Agriculture Organization (FAO); Organization for Economic Co-Operation and Development (OECD). OECD-FAO Agricultural Outlook 2016-2025; OECD-FAO Agricultural Outlook; OECD Publishing: Paris, France, 2016; ISBN 978-92-64-25322-3. [Google Scholar]
- Field, C.; Van Aalst, M. Climate Change 2014: Impacts, Adaptation, and Vulnerability; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2014; Volume 1. [Google Scholar]
- Waithaka, M.; Nelson, G.C.; Thomas, T.S.; Kyotalimye, M. East African Agriculture and Climate Change: A Comprehensive Analysis; International Food Policy Research Institute: Washington, DC, USA, 2013. [Google Scholar]
- Gladwin, C.H.; Thomson, A.M.; Peterson, J.S.; Anderson, A.S. Addressing food security in Africa via multiple livelihood strategies of women farmers. Food Policy 2001, 26, 177–207. [Google Scholar] [CrossRef]
- Hanjra, M.A.; Qureshi, M.E. Global water crisis and future food security in an era of climate change. Food Policy 2010, 35, 365–377. [Google Scholar] [CrossRef]
- Upton, J.B.; Cissé, J.D.; Barrett, C.B. Food security as resilience: Reconciling definition and measurement. Agric. Econ. 2016, 47, 135–147. [Google Scholar] [CrossRef]
- Barrett, C.B.; Constas, M.A. Toward a theory of resilience for international development applications. Proc. Natl. Acad. Sci. USA 2014, 111, 14625–14630. [Google Scholar] [CrossRef] [PubMed]
- Barrett, C.B. Measuring food insecurity. Science 2010, 327, 825–828. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.C.; El Obeid, A.E.; Jensen, H.H. The geography and causes of food insecurity in developing countries. Agric. Econ. 2000, 22, 199–215. [Google Scholar] [CrossRef]
- Smith, L.C.; Alderman, H.; Aduayom, D. Food Insecurity in Sub-Saharan Africa: New Estimates from Household Expenditure Surveys; International Food Policy Research Institute: Washington, DC, USA, 2006; Volume 146. [Google Scholar]
- Pinstrup-Andersen, P. Food policy research for developing countries: Emerging issues and unfinished business. Food Policy 2000, 25, 125–141. [Google Scholar] [CrossRef]
- Evenson, R.E.; Gollin, D. Assessing the impact of the Green Revolution, 1960 to 2000. Science 2003, 300, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Garnett, T.; Appleby, M.C.; Balmford, A.; Bateman, I.J.; Benton, T.G.; Bloomer, P.; Burlingame, B.; Dawkins, M.; Dolan, L.; Fraser, D.; et al. Sustainable Intensification in Agriculture: Premises and Policies. Science 2013, 341, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Pretty, J.; Toulmin, C.; Williams, S. Sustainable intensification in African agriculture. Int. J. Agric. Sustain. 2011, 9, 5–24. [Google Scholar] [CrossRef]
- Binswanger-Mkhize, H.P.; Savastano, S. Agricultural intensification: The status in six African countries. Food Policy 2016. [Google Scholar] [CrossRef]
- Carter, M.R.; Laajaj, R.; Yang, D. Subsidies and the Persistence of Technology Adoption: Field Experimental Evidence from Mozambique; National Bureau of Economic Research: Cambridge, MA, USA, 2014. [Google Scholar]
- Maredia, M.K.; Byerlee, D.; Pee, P. Impacts of food crop improvement research: Evidence from sub-Saharan Africa. Food Policy 2000, 25, 531–559. [Google Scholar] [CrossRef]
- O’Gorman, M.; Pandey, M. Cross-Country Disparity in Agricultural Productivity: Quantifying the Role of Modern Seed Adoption. J. Dev. Stud. 2010, 46, 1767–1785. [Google Scholar] [CrossRef] [PubMed]
- Griliches, Z. Hybrid corn: An exploration in the economics of technological change. Econom. J. Econom. Soc. 1957, 25, 501–522. [Google Scholar] [CrossRef]
- Feder, G.; Just, R.E.; Zilberman, D. Adoption of agricultural innovations in developing countries: A survey. Econ. Dev. Cult. Chang. 1985, 33, 255–298. [Google Scholar] [CrossRef]
- Besley, T.; Case, A. Modeling technology adoption in developing countries. Am. Econ. Rev. 1993, 83, 396–402. [Google Scholar]
- Foster, A.D.; Rosenzweig, M.R. Learning by doing and learning from others: Human capital and technical change in agriculture. J. Polit. Econ. 1995, 103, 1176–1209. [Google Scholar] [CrossRef]
- Sunding, D.; Zilberman, D. The agricultural innovation process: Research and technology adoption in a changing agricultural sector. Handb. Agric. Econ. 2001, 1, 207–261. [Google Scholar]
- Hoff, K.; Stiglitz, J. Modern economic theory and development. Front. Dev. Econ. Future Perspect. 2001, 389. [Google Scholar]
- Conley, T.G.; Udry, C.R. Learning about a new technology: Pineapple in Ghana. Am. Econ. Rev. 2010, 35–69. [Google Scholar] [CrossRef]
- Cunguara, B.; Darnhofer, I. Assessing the impact of improved agricultural technologies on household income in rural Mozambique. Food Policy 2011, 36, 378–390. [Google Scholar] [CrossRef]
- Smale, M.; Just, R.E.; Leathers, H.D. Land allocation in HYV adoption models: An investigation of alternative explanations. Am. J. Agric. Econ. 1994, 76, 535–546. [Google Scholar] [CrossRef]
- Smale, M.; Heisey, P.W.; Leathers, H.D. Maize of the ancestors and modern varieties: The microeconomics of high-yielding variety adoption in Malawi. Econ. Dev. Cult. Chang. 1995, 43, 351–368. [Google Scholar] [CrossRef]
- Langyintuo, A.S.; Mwangi, W.; Diallo, A.O.; MacRobert, J.; Dixon, J.; Bänziger, M. Challenges of the maize seed industry in eastern and southern Africa: A compelling case for private–public intervention to promote growth. Food Policy 2010, 35, 323–331. [Google Scholar] [CrossRef]
- Maredia, M.K.; Shankar, B.; Kelley, T.G.; Stevenson, J.R. Impact assessment of agricultural research, institutional innovation, and technology adoption: Introduction to the special section. Food Policy 2014, 44, 214–217. [Google Scholar] [CrossRef]
- Miguel, E.; Kremer, M. Worms: Identifying impacts on education and health in the presence of treatment externalities. Econometrica 2004, 72, 159–217. [Google Scholar] [CrossRef]
- Kremer, M.; Miguel, E. The illusion of sustainability. Q. J. Econ. 2007, 122, 1007–1065. [Google Scholar] [CrossRef]
- Evenson, R.E. Economic impacts of agricultural research and extension. Handb. Agric. Econ. 2001, 1, 573–628. [Google Scholar]
- Atlin, G.N.; Cairns, J.E.; Das, B. Rapid breeding and varietal replacement are critical to adaptation of cropping systems in the developing world to climate change. Glob. Food Secur. 2017, 12, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Khandker, S.R.; Koolwal, G.B. How has microcredit supported agriculture? Evidence using panel data from Bangladesh. Agric. Econ. 2016, 47, 157–168. [Google Scholar] [CrossRef]
- Tripp, R. Can biotechnology reach the poor? The adequacy of information and seed delivery. Food Policy 2001, 26, 249–264. [Google Scholar] [CrossRef]
- Tripp, R.; Rohrbach, D. Policies for African seed enterprise development. Food Policy 2001, 26, 147–161. [Google Scholar] [CrossRef]
- Rohrbach, D.; Minde, I.J.; Howard, J. Looking beyond national boundaries: Regional harmonization of seed policies, laws and regulations. Food Policy 2003, 28, 317–333. [Google Scholar] [CrossRef]
- Coomes, O.T.; McGuire, S.J.; Garine, E.; Caillon, S.; McKey, D.; Demeulenaere, E.; Jarvis, D.; Aistara, G.; Barnaud, A.; Clouvel, P.; et al. Farmer seed networks make a limited contribution to agriculture? Four common misconceptions. Food Policy 2015, 56, 41–50. [Google Scholar] [CrossRef]
- Joughin, J. Fake Seeds are Keeping Uganda’s Farmers Poor. 2014. Available online: https://www.theguardian.com/global-development-professionals-network/2014/jul/16/fake-seeds-uganda (accessed on 10 May 2018).
- Mastenbroek, A.; Ntare, B. Uganda Early Generation Seed Study; Integrated Seed Sector Development Uganda: Kampala, Uganda, 2016. [Google Scholar]
- Kansiime, M.K.; Mastenbroek, A. Enhancing resilience of farmer seed system to climate-induced stresses: Insights from a case study in West Nile region, Uganda. J. Rural Stud. 2016, 47, 220–230. [Google Scholar] [CrossRef]
- Mathenge, M.K.; Smale, M.; Olwande, J. The impacts of hybrid maize seed on the welfare of farming households in Kenya. Food Policy 2014, 44, 262–271. [Google Scholar] [CrossRef]
- Mugisa, I.O.; Karungi, J.; Akello, B.; Ochwo-Ssemakula, M.K.N.; Biruma, M.; Okello, D.K.; Otim, G. Assessing the effect of farmers’ practices on the severity of groundnut rosette virus disease in Uganda. Afr. J. Agric. Res. 2015, 10, 998–1003. [Google Scholar] [CrossRef]
- Bonabana-Wabbi, J.; Taylor, D.B.; Kasenge, V. A limited dependent variable analysis of integrated pest management adoption in Uganda. In Proceedings of the American Agricultural Economics Association Annual Meeting, Long Beach, CA, USA, 23 July 2006. [Google Scholar]
- Naidu, R.A.; Kimmins, R.M.; Deom, C.M.; Subrahmanyam, P.; Chiyembekeza, A.J.; Van der Merwe, P.J.A. Groundnut Rosette: A Virus Disease Affecting Groundnut Production in Sub-Saharan Africa. Plant Dis. 1999, 83, 700–709. [Google Scholar] [CrossRef]
- Okello, D.K.; Okori, P.; Bravo-Ureta, B.; Deom, C.M.; Ininda, J.; Anguria, P.; Biruma, M.; Asekenye, C. Groundnuts Seed Production Manual for Uganda; National Agricultural Research Organization: Entebbe, Uganda, 2015; ISBN 978-9970-401-12-3.
- Okello, D.K.; Deom, C.M.; Puppala, N.; Monyo, E.; Bravo-Ureta, B. Registration of ‘Serenut 5R’ Groundnut. J. Plant Regist. 2016. [Google Scholar] [CrossRef]
- Wilber, W.; Pinehas, T.; Sivananda, V.T.; David, K.O.; Carl, M.D.; Boris, E.B.U.; Naveen, P. Genetic variability studies of Valencia groundnut varieties for late leaf spot (Phaeoisariopsis personata) resistance. Afr. J. Plant Sci. 2015, 9, 327–333. [Google Scholar] [CrossRef]
- Deom, C.M.; Kapewa, T.; Busolo-Bulafu, C.M.; Naidu, R.A.; Chiyembekeza, A.J.; Kimmins, F.M.; Subrahmanyam, P.; Van der Merwe, P.J.A. Registration of ICG 12991 peanut germplasm line. Crop Sci. 2006, 46, 481–482. [Google Scholar] [CrossRef]
- Mwebaze, S.M. Country Pasture/Forage Resource Profiles, Uganda; Grassland and Pasture Crops; FAO: Rome, Italy, 2002. [Google Scholar]
- Joughin, J. The Political Economy of Seed Reform in Uganda: Promoting a Regional Seed Trade Market; World Bank Group: Washington, DC, USA, 2014. [Google Scholar]
- Kansiime, M.K. Baseline Study on Farmers’ Access to Seed and Other Planting Materials in Uganda; Integrated Seed Sector Development Uganda: Kampala, Uganda, 2014. [Google Scholar]
- Benin, S.; Nkonya, E.; Okecho, G.; Randriamamonjy, J.; Kato, E.; Lubade, G.; Kyotalimye, M. Returns to spending on agricultural extension: The case of the National Agricultural Advisory Services (NAADS) program of Uganda. Agric. Econ. 2011, 42, 249–267. [Google Scholar] [CrossRef]
- Benin, S.; Nkonya, E.; Okecho, G.; Pender, J.; Nahdy, S.; Mugarura, S. Assessing the Impact of the National Agricultural Advisory Services (NAADS) in the Uganda Rural Livelihoods; International Food Policy Research Institute: Washington, DC, USA, 2007. [Google Scholar]
- Anderson, J.R.; Feder, G. Agricultural extension: Good intentions and hard realities. World Bank Res. Obs. 2004, 19, 41–60. [Google Scholar] [CrossRef]
- Lamb, J.N.; Moore, K.M.; Norton, J.; Omondi, E.C.; Laker-Ojok, R.; Sikuku, D.N.; Ashilenje, D.S.; Odera, J. A social networks approach for strengthening participation in technology innovation: Lessons learnt from the Mount Elgon region of Kenya and Uganda. Int. J. Agric. Sustain. 2016, 14, 65–81. [Google Scholar] [CrossRef]
- Rwabwogo, M.O. Uganda Districts Information Handbook; Fountain Publishers: Kampala, Uganda, 2007. [Google Scholar]
- Ramalho, E.A.; Ramalho, J.J.; Murteira, J.M. Alternative estimating and testing empirical strategies for fractional regression models. J. Econ. Surv. 2011, 25, 19–68. [Google Scholar] [CrossRef]
- Papke, L.E.; Wooldridge, J.M. Econometric methods for fractional response variables with an application to 401 (k) plan participation rates. J. Appl. Econom. 1996, 11, 619–632. [Google Scholar] [CrossRef]
- Papke, L.E.; Wooldridge, J.M. Panel data methods for fractional response variables with an application to test pass rates. J. Econom. 2008, 145, 121–133. [Google Scholar] [CrossRef]
- Imbens, G.W.; Wooldridge, J.M. Recent Developments in the Econometrics of Program Evaluation. J. Econ. Lit. 2009, 47, 5–86. [Google Scholar] [CrossRef]
- Ravallion, M. Chapter 59 Evaluating Anti-Poverty Programs. In Handbook of Development Economics; Elsevier: New York, NY, USA, 2007; Volume 4, pp. 3787–3846. ISBN 978-0-444-53100-1. [Google Scholar]
- Deaton, A. Instruments, Randomization, and Learning about Development. J. Econ. Lit. 2010, 48, 424–455. [Google Scholar] [CrossRef]
- Duflo, E.; Glennerster, R.; Kremer, M. Chapter 61 Using randomization in development economics research: A Toolkit. In Handbook of Development Economics; Elsevier: New York, NY, USA, 2008; Volume 4, pp. 3895–3962. ISBN 978-0-444-53100-1. [Google Scholar]
- Khandker, S.R.; Koolwal, G.B.; Samad, H.A. Handbook on Impact Evaluation: Quantitative Methods and Practices; The World Bank: Washington, DC, USA, 2009; ISBN 978-0-8213-8028-4. [Google Scholar]
- Rosenbaum, P.R.; Rubin, D.B. The central role of the propensity score in observational studies for causal effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]
- Winters, P.; Salazar, L.; Maffioli, A. Designing impact evaluations for agricultural projects. Inter Am. Dev. Bank 2010, 14, 2012. [Google Scholar]
- Hirano, K.; Imbens, G.W.; Ridder, G. Efficient estimation of average treatment effects using the estimated propensity score. Econometrica 2003, 71, 1161–1189. [Google Scholar] [CrossRef]
- Guo, S.; Fraser, M.W. Propensity Score Analysis: Statistical Methods and Applications; Sage Publications: Thousand Oaks, CA, USA, 2014; Volume 11. [Google Scholar]
- Bravo-Ureta, B.E.; Almeida, A.N.; Solís, D.; Inestroza, A. The Economic Impact of Marena’s Investments on Sustainable Agricultural Systems in Honduras: Impact of Investments on Sustainable Agriculture. J. Agric. Econ. 2011, 62, 429–448. [Google Scholar] [CrossRef]
- Sanglestsawai, S.; Rejesus, R.M.; Yorobe, J.M. Economic impacts of integrated pest management (IPM) farmer field schools (FFS): Evidence from onion farmers in the Philippines. Agric. Econ. 2015, 46, 149–162. [Google Scholar] [CrossRef]
- Abadie, A.; Imbens, G.W. Matching on the estimated propensity score. Econometrica 2016, 84, 781–807. [Google Scholar] [CrossRef]
- Abadie, A.; Imbens, G.W. Large sample properties of matching estimators for average treatment effects. Econometrica 2006, 74, 235–267. [Google Scholar] [CrossRef]
- DiPrete, T.A.; Gangl, M. Assessing bias in the estimation of causal effects: Rosenbaum bounds on matching estimators and instrumental variables estimation with imperfect instruments. Sociol. Methodol. 2004, 34, 271–310. [Google Scholar] [CrossRef]
- Angrist, J.D.; Imbens, G.W.; Rubin, D.B. Identification of causal effects using instrumental variables. J. Am. Stat. Assoc. 1996, 91, 444–455. [Google Scholar] [CrossRef]
- Caliendo, M.; Kopeinig, S. Some practical guidance for the implementation of propensity score matching. J. Econ. Surv. 2008, 22, 31–72. [Google Scholar] [CrossRef]
- Cavatassi, R.; González-flores, M.; Winters, P.; Andrade-Piedra, J.; Espinosa, P.; Thiele, G. Linking Smallholders to the New Agricultural Economy: The Case of the Plataformas de Concertación in Ecuador. J. Dev. Stud. 2011, 47, 1545–1573. [Google Scholar] [CrossRef]
- De los Santos Montero, L.A.; Bravo-Ureta, B.E. Natural Resource Management and Household Well-being: The Case of POSAF-II in Nicaragua. World Dev. 2017, 99, 42–59. [Google Scholar] [CrossRef]
- Angrist, J.D. Estimation of limited dependent variable models with dummy endogenous regressors: Simple strategies for empirical practice. J. Bus. Econ. Stat. 2001, 19, 2–28. [Google Scholar] [CrossRef]
- Barrett, C.B.; Carter, M.R.; Timmer, C.P. A century-long perspective on agricultural development. Am. J. Agric. Econ. 2010, 92, 447–468. [Google Scholar] [CrossRef]
| Variable | Definition |
|---|---|
| Outcome Indicator | |
| ADOPT | Groundnut land allocation to HYVs (%) |
| Participation | |
| BEN | Participant (1 = yes, 0 = no) |
| C_IN | Control neighbor (1 = yes, 0 = no) |
| C_OUT | Control non-neighbor (1 = yes, 0 = no) |
| PV | Project village (1 = yes, 0 = no) |
| Demographic Characteristics | |
| GRESP | Gender of respondent (1 = male, 0 = female) |
| HH_SIZE | Total HH members (#) |
| LOC | Location: Sub-county, District (1 = Nyero, Kumi; 2 = Kidongole, Bukedea; 3 = Kasodo, Pallisa; 4 = Lyama, Budaka; 5 = Kachonga, Butaleja; 6 = Nagongera, Tororo; 7 = Butiru, Manafwa; and 8 = Bukhalu, Sironko) |
| AGE | Age household head (HHH) (years) |
| GHHH | Gender HHH (1 = male, 0 = female) |
| EDU | Schooling HHH (1 = none, 2 = primary, 3 = secondary, 4 = tertiary) |
| M_STAT | Married HHH (1 = yes, 0 = no) |
| Agricultural Production | |
| AREA | Total area cultivated (ha) |
| MEM | Member of a farm group (1 = yes, 0 = no) |
| GROW | Grew groundnuts 2013 Season A (1 = yes, 0 = no) |
| SAVE | Groundnuts grown from home saved seed (1 = yes, 0 = no) |
| G_CASH | Groundnuts grown as cash crop (1 = yes, 0 = no) |
| G_AREA | Area allocated to groundnuts (ha) |
| G_PROP | Proportion farmland in groundnut production (%) |
| COST | Cost of groundnut seed (per kg) 2013 season A (USD *) |
| SEED | Quantity of groundnut seed planted 2013 season A (kg) |
| HARV | Quantity groundnut (unshelled) harvested 2013 season A (kg) |
| YIELD | Yield groundnuts (unshelled) 2013 season A (kg/ha) |
| Groundnut Varieties | |
| LRV | Land race varieties (1 = Red Beauty ^; 2 = Igola 1 ^; 3 = Erudurudu Red; 4 = Etesot; 5 = Magwere; 6 = Kitambi) |
| HYV | High yielding varieties (1 = Serenut1; 2 = Serenut2; 3 = Serenut3; 4 = Serenut4; 5 = Serenut5; 6 = Serenut6) |
| Pooled | BEN | C_ALL | C_IN | C_OUT | |
|---|---|---|---|---|---|
| GRESP | 0.48 | 0.39 | 0.57 | 0.50 | 0.62 |
| (0.501) | (0.488) | (0.495) | (0.501) | (0.486) | |
| HH_SIZE | 8.47 | 8.24 | 8.70 | 8.35 | 9.05 |
| (3.99) | (3.98) | (4.00) | (4.18) | (3.79) | |
| AGE | 51.4 | 53.2 | 49.6 | 49.9 | 49.3 |
| (12.9) | (12.9) | (12.7) | (14.0) | (11.3) | |
| GHHH | 0.79 | 0.79 | 0.79 | 0.81 | 0.78 |
| (0.407) | (0.407) | (0.407) | (0.395) | (0.419) | |
| EDU_1 | 0.14 | 0.15 | 0.12 | 0.12 | 0.12 |
| (0.343) | (0.358) | (0.327) | (0.332) | (0.322) | |
| EDU_2 | 0.56 | 0.53 | 0.59 | 0.64 | 0.54 |
| (0.497) | (0.500) | (0.493) | (0.481) | (0.500) | |
| EDU_3 | 0.21 | 0.20 | 0.22 | 0.17 | 0.27 |
| (0.410) | (0.404) | (0.416) | (0.382) | (0.444) | |
| EDU_4 | 0.09 | 0.11 | 0.07 | 0.06 | 0.07 |
| (0.286) | (0.317) | (0.250) | (0.235) | (0.264) | |
| M_STAT | 0.82 | 0.80 | 0.85 | 0.83 | 0.87 |
| (0.382) | (0.404) | (0.358) | (0.374) | (0.341) | |
| AREA | 1.64 | 1.60 | 1.68 | 1.48 | 1.89 |
| (1.05) | (1.01) | (1.09) | (0.88) | (1.24) | |
| MEM | 0.61 | 0.97 | 0.25 | 0.19 | 0.30 |
| (0.489) | (0.180) | (0.431) | (0.395) | (0.460) | |
| GROW | 0.78 | 0.80 | 0.76 | 0.71 | 0.82 |
| (0.415) | (0.404) | (0.426) | (0.456) | (0.389) | |
| n | 480 | 240 | 240 | 120 | 120 |
| Pooled | BEN | C_ALL | C_IN | C_OUT | ||
|---|---|---|---|---|---|---|
| ADOPT | 0.52 | 0.56 | 0.48 | 0.56 | 0.40 | |
| (0.452) | (0.439) | (0.463) | (0.463) | (0.452) | ||
| AREA | 1.77 | 1.71 | 1.84 | 1.62 | 2.02 | |
| (1.07) | (1.04) | (1.11) | (0.85) | (1.27) | ||
| MEM | 0.64 | 0.97 | 0.28 | 0.22 | 0.34 | |
| (0.482) | (0.160) | (0.452) | (0.419) | (0.475) | ||
| GROW_HYV | 0.63 | 0.70 | 0.57 | 0.64 | 0.50 | |
| (0.483) | (0.461) | (0.497) | (0.482) | (0.503) | ||
| SAVE | 0.49 | 0.55 | 0.42 | 0.40 | 0.44 | |
| (0.501) | (0.498) | (0.495) | (0.493) | (0.499) | ||
| G_CASH | 0.91 | 0.91 | 0.92 | 0.95 | 0.89 | |
| (0.284) | (0.293) | (0.275) | (0.213) | (0.317) | ||
| G_AREA | 0.32 | 0.34 | 0.30 | 0.28 | 0.32 | |
| (0.378) | (0.457) | (0.271) | (0.242) | (0.294) | ||
| G_PROP | 0.21 | 0.24 | 0.18 | 0.17 | 0.18 | |
| (0.304) | (0.405) | (0.128) | (0.123) | (0.132) | ||
| COST | 1.002 | 1.001 | 1.003 | 0.946 | 1.052 | |
| (1.060) | (1.081) | (1.041) | (1.023) | (1.060) | ||
| SEED | 24.8 | 26.0 | 23.5 | 22.0 | 24.7 | |
| (35.0) | (44.4) | (21.3) | (19.3) | (22.8) | ||
| HARV | 166 | 170 | 162 | 166 | 160 | |
| (226) | (240) | (210) | (238) | (183) | ||
| YIELD | 97.3 | 102.4 | 92.0 | 92.6 | 91.4 | |
| (90.3) | (97.7) | (82.0) | (78.9) | (85.0) | ||
| n | 374 | 191 | 183 | 85 | 98 | |
| YIELD_LRV | 114.0 | 116.2 | 111.9 | 119.2 | 107.0 | |
| (94.2) | (98.2) | (90.5) | (87.5) | (92.7) | ||
| n | 218 | 108 | 108 | 44 | 110 | |
| YIELD_LRV_1 | 133.7 | 135.5 | 131.9 | 139.9 | 124.1 | |
| (100.7) | (98.9) | (103.2) | (95.4) | (111.3) | ||
| n | 125 | 62 | 62 | 31 | 63 | |
| YIELD_HYV | 86.2 | 95.4 | 74.5 | 77.6 | 71.1 | |
| (87.9) | (97.6) | (72.4) | (77.7) | (66.7) | ||
| n | 236 | 133 | 133 | 54 | 103 | |
| YIELD_HYV_2 | 77.8 | 84.5 | 69.5 | 66.5 | 72.6 | |
| (75.9) | (85.3) | (61.6) | (56.1) | (67.4) | ||
| n | 219 | 121 | 98 | 51 | 47 |
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | ||
|---|---|---|---|---|---|---|---|---|---|
| OLSR | OLSU | OLSR | OLSU | FRR+ | FRU+ | FRR+ | FRU+ | ||
| BEN | 0.157 *** | 0.141 *** | 0.156 *** | 0.138 *** | |||||
| (0.056) | (0.045) | (0.054) | (0.044) | ||||||
| C_IN | 0.158 ** | 0.137 *** | 0.157 ** | 0.139 *** | |||||
| (0.066) | (0.052) | (0.066) | (0.051) | ||||||
| PV | 0.158 *** | 0.140*** | 0.156 *** | 0.138 *** | |||||
| (0.053) | (0.042) | (0.052) | (0.042) | ||||||
| GRESP | 0.0441 | 0.043 | 0.0510 | 0.0511 | |||||
| (0.043) | (0.043) | (0.041) | (0.041) | ||||||
| HH_SIZE | −0.005 | −0.005 | −0.006 | −0.006 | |||||
| (0.005) | (0.005) | (0.005) | (0.005) | ||||||
| LOC_1 | 0.728 *** | 0.728 *** | 0.648 *** | 0.648 *** | |||||
| (0.077) | (0.077) | (0.067) | (0.067) | ||||||
| LOC_2 | 0.430 *** | 0.431 *** | 0.380 *** | 0.380 *** | |||||
| (0.080) | (0.080) | (0.072) | (0.072) | ||||||
| LOC_3 | 0.718 *** | 0.718 *** | 0.632 *** | 0.632 *** | |||||
| (0.078) | (0.078) | (0.069) | (0.069) | ||||||
| LOC_4 | 0.230 ** | 0.230 *** | 0.237 *** | 0.237 *** | |||||
| (0.084) | (0.084) | (0.080) | (0.080) | ||||||
| LOC_5 | −0.0611 | −0.0611 | −0.0516 | −0.0516 | |||||
| (0.080) | (0.080) | (0.081) | (0.081) | ||||||
| LOC_6 | 0.491 *** | 0.491 *** | 0.428 *** | 0.428 *** | |||||
| (0.098) | (0.098) | (0.091) | (0.091) | ||||||
| LOC_7 | 0.321 *** | 0.322 *** | 0.297 *** | 0.297 *** | |||||
| (0.079) | (0.079) | (0.072) | (0.072) | ||||||
| AGE | 0.0008 | 0.0008 | 0.0007 | 0.0007 | |||||
| (0.001) | (0.001) | (0.001) | (0.001) | ||||||
| GHHH | 0.0631 | 0.0634 | 0.0645 | 0.0644 | |||||
| (0.068) | (0.068) | (0.070) | (0.070) | ||||||
| EDU_1 | −0.141 * | −0.141 * | −0.126 | −0.126 | |||||
| (0.083) | (0.083) | (0.088) | (0.088) | ||||||
| EDU_2 | −0.071 | −0.071 | −0.060 | −0.060 | |||||
| (0.066) | (0.065) | (0.071) | (0.071) | ||||||
| EDU_3 | −0.128 * | −0.128* | −0.118 | −0.118 | |||||
| (0.071) | (0.071) | (0.075) | (0.075) | ||||||
| M_STAT | 0.022 | 0.0221 | 0.0085 | 0.0085 | |||||
| (0.068) | (0.068) | (0.072) | (0.072) | ||||||
| AREA | −0.004 | −0.004 | −0.002 | −0.002 | |||||
| (0.019) | (0.019) | (0.018) | (0.018) | ||||||
| CONST | 0.405 *** | 0.052 | 0.405 *** | 0.0516 | |||||
| (0.045) | (0.126) | (0.045) | (0.126) | ||||||
| R2 | 0.024 | 0.445 | 0.024 | 0.445 | |||||
| F | 4.475 | 15.82 | 8.974 | 16.80 | |||||
| n | 374 | 374 | 374 | 374 | 374 | 374 | 374 | 374 |
| PSM (1) | PSM (2) | PSM (3) | PSM (4) | PSM (5) | |
|---|---|---|---|---|---|
| BEN/C_ALL | PV/C_OUT | BEN/C_OUT | C_IN/C_OUT | BEN/C_IN | |
| HH_SIZE | −0.014 | −0.004 | −0.001 | 0.005 | −0.018 |
| (0.019) | (0.021) | (0.024) | (0.028) | (0.022) | |
| LOC_1 | 0.187 | 0.157 | 0.291 | 0.057 | 0.068 |
| (0.286) | (0.304) | (0.342) | (0.404) | (0.343) | |
| LOC_2 | 0.387 | 0.287 | 0.477 | 0.057 | 0.264 |
| (0.296) | (0.317) | (0.353) | (0.423) | (0.356) | |
| LOC_3 | 0.147 | 0.036 | 0.176 | −0.101 | 0.117 |
| (0.288) | (0.306) | (0.341) | (0.415) | (0.348) | |
| LOC_4 | 0.131 | −0.205 | 0.020 | −0.514 | 0.380 |
| (0.313) | (0.326) | (0.358) | (0.463) | (0.406) | |
| LOC_5 | 0.216 | 0.245 | 0.367 | 0.213 | 0.028 |
| (0.295) | (0.315) | (0.353) | (0.424) | (0.351) | |
| LOC_6 | 0.264 | 0.165 | 0.353 | 0.005 | 0.190 |
| (0.360) | (0.395) | (0.436) | (0.542) | (0.428) | |
| LOC_7 | 0.309 | 0.206 | 0.437 | −0.014 | 0.203 |
| (0.290) | (0.312) | (0.351) | (0.420) | (0.348) | |
| AGE | 0.015 *** | 0.009 | 0.016 ** | −0.002 | 0.014 ** |
| (0.005) | (0.006) | (0.007) | (0.008) | (0.006) | |
| GHHH | 0.0851 | 0.219 | 0.175 | 0.187 | 0.006 |
| (0.235) | (0.254) | (0.291) | (0.331) | (0.285) | |
| EDU_1 | −0.316 | −0.439 | −0.508 | −0.451 | 0.014 |
| (0.306) | (0.343) | (0.374) | (0.478) | (0.379) | |
| EDU_2 | −0.335 | −0.295 | −0.402 | −0.207 | −0.209 |
| (0.242) | (0.275) | (0.293) | (0.386) | (0.293) | |
| EDU_3 | −0.321 | −0.465 | −0.531 * | −0.531 | −0.059 |
| (0.262) | (0.292) | (0.311) | (0.417) | (0.321) | |
| M_STAT | −0.238 | −0.346 | −0.401 | −0.241 | −0.113 |
| (0.253) | (0.286) | (0.314) | (0.383) | (0.304) | |
| AREA | −0.063 | −0.171 ** | −0.159 ** | −0.240 ** | 0.091 |
| (0.028) | (0.029) | (0.032) | (0.046) | (0.039) | |
| CONSTANT | −0.266 | 0.847 * | 0.221 | 0.759 | −0.130 |
| (0.447) | (0.497) | (0.555) | (0.672) | (0.531) | |
| n | 374 | 374 | 289 | 183 | 276 |
| PSM (1) | PSM (2) | PSM (3) | PSM (4) | PSM (5) | |
|---|---|---|---|---|---|
| BEN/C_ALL | PV/C_OUT | BEN/C_OUT | C_IN/C_OUT | BEN/C_IN | |
| ATET | 0.051 | 0.037 | 0.213 *** | 0.145 ** | −0.025 |
| (0.056) | (0.061) | (0.072) | (0.065) | (0.063) | |
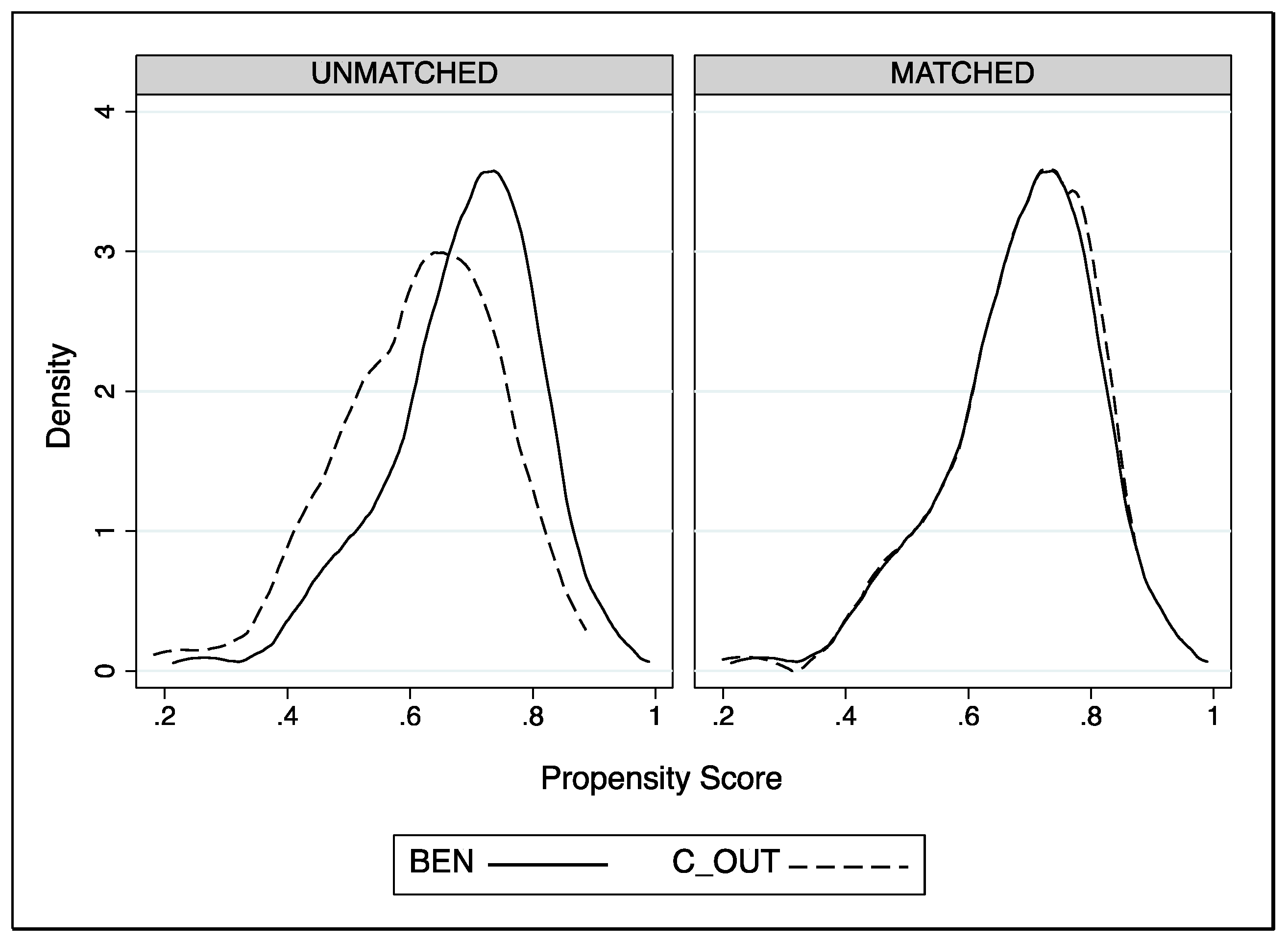
| Rosenbaum Bounds Delta (Г) | 1.90 + | 1.85 + | 3.65 + | 3.55 + | 1.75 − |
| IV(1) | IV(2a) | IV(2b) | |
|---|---|---|---|
| (iii) 1st Stage | (iv) 2nd Stage: OLS | (iv) 2nd Stage: FR + | |
| PV | 0.656 *** | ||
| (0.0489) | |||
| BEN | 0.213 *** | 0.211 *** | |
| (0.066) | (0.063) | ||
| GRESP | −0.138 ** | 0.073 | 0.080 * |
| (0.049) | (0.047) | (0.045) | |
| HH_SIZE | −0.004 | −0.005 | −0.005 |
| (0.006) | (0.005) | (0.005) | |
| LOC_1 | 0.013 | 0.725 *** | 0.645 *** |
| (0.089) | (0.080) | (0.067) | |
| LOC_2 | 0.098 | 0.410 *** | 0.359 *** |
| (0.092) | (0.083) | (0.073) | |
| LOC_3 | 0.026 | 0.713 *** | 0.626 *** |
| (0.090) | (0.081) | (0.069) | |
| LOC_4 | 0.079 | 0.213 ** | 0.220 *** |
| (0.097) | (0.087) | (0.079) | |
| LOC_5 | 0.010 | −0.063 | −0.054 |
| (0.091) | (0.082) | (0.081) | |
| LOC_6 | 0.038 | 0.483 *** | 0.420 *** |
| (0.112) | (0.101) | (0.091) | |
| LOC_7 | 0.080 | 0.305 *** | 0.280 *** |
| (0.090) | (0.081) | (0.072) | |
| AGE | 0.004 ** | −2.0e-5 | −1.0e-4 |
| (0.002) | (0.002) | (0.001) | |
| GHHH | 0.065 | 0.049 | 0.051 |
| (0.078) | (0.070) | (0.071) | |
| EDU_1 | −0.049 | −0.131 | −0.116 |
| (0.095) | (0.086) | (0.089) | |
| EDU_2 | −0.069 | −0.056 | −0.045 |
| (0.075) | (0.068) | (0.072) | |
| EDU_3 | −0.030 | −0.122 * | −0.112 |
| (0.082) | (0.073) | (0.075) | |
| M_STAT | −0.032 | 0.029 | 0.015 |
| (0.078) | (0.070) | (0.072) | |
| AREA | 0.019 | −0.008 | −0.006 |
| (0.022) | (0.019) | (0.018) | |
| CONSTANT | −0.124 | 0.078 | |
| (0.145) | (0.127) | ||
| R2 | 0.402 | 0.413 | |
| F | 14.06 | 15.89 | |
| n | 374 | 374 | 374 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jelliffe, J.L.; Bravo-Ureta, B.E.; Deom, C.M.; Okello, D.K. Adoption of High-Yielding Groundnut Varieties: The Sustainability of a Farmer-Led Multiplication-Dissemination Program in Eastern Uganda. Sustainability 2018, 10, 1597. https://doi.org/10.3390/su10051597
Jelliffe JL, Bravo-Ureta BE, Deom CM, Okello DK. Adoption of High-Yielding Groundnut Varieties: The Sustainability of a Farmer-Led Multiplication-Dissemination Program in Eastern Uganda. Sustainability. 2018; 10(5):1597. https://doi.org/10.3390/su10051597
Chicago/Turabian StyleJelliffe, Jeremy L., Boris E. Bravo-Ureta, C. Michael Deom, and David K. Okello. 2018. "Adoption of High-Yielding Groundnut Varieties: The Sustainability of a Farmer-Led Multiplication-Dissemination Program in Eastern Uganda" Sustainability 10, no. 5: 1597. https://doi.org/10.3390/su10051597
APA StyleJelliffe, J. L., Bravo-Ureta, B. E., Deom, C. M., & Okello, D. K. (2018). Adoption of High-Yielding Groundnut Varieties: The Sustainability of a Farmer-Led Multiplication-Dissemination Program in Eastern Uganda. Sustainability, 10(5), 1597. https://doi.org/10.3390/su10051597





