Abstract
Green roofs and walls have recently emerged as conservation tools, and they offer promising additional opportunities to enhance biodiversity in cities. However, their ecological conditions remain poorly considered when planning wildlife corridors. To discuss the role of vegetated buildings in landscape connectivity, we reviewed the ecological and technical specificities of green walls and green roofs in light of the key factors concerning urban wildlife (patch size, quality, abundance, and isolation). Green roofs and walls show limited patch sizes, distinct habitat quality at the building scale, and limited redundancy of patch quality within the landscape. We also highlight that the abundance of roof and wall patches is often low. Future research is needed to establish if walls can be vertical corridors for wildlife, thereby reducing the isolation of green roofs. We argue that creating 3D ecological connectivity within the city requires substantial modifications of the design and maintenance of existing green building systems. We suggest that research is needed to integrate the biotic and abiotic characteristics of green buildings to make them more closely resemble those of open green spaces.
1. Introduction
Enhancing biodiversity in cities has now been widely recognized as a concept of improving the sustainability of urban functioning and making the urban environment more resilient to disturbances through the provision of ecosystem services [1,2,3]. In cities, both heavily maintained and transformed patches that contain managed and unmanaged vegetation, along with unmanaged or semi-natural areas, are all habitats for a diversity of both native and non-native species [4]. Many of these habitats include rare species and species that are of concern from a conservation standpoint [5]. These patches support species according to patch size, quality and quantity in the landscape, and according to the heterogeneity both within and among the green spaces [6].
In dense urban areas, biodiversity occurs in green spaces with smaller areas that are more fragmented and isolated [7], and evidence has shown that connectivity enhances biodiversity in such landscapes (e.g., Reference [8]). However, little is known about their ecology and how their functioning conserves the biodiversity of multiple taxa at different spatial scales [9]. Planning ecological corridors is thus a critical way to facilitate the dispersal of species between urban environments and sub-urban reservoirs, and the colonization of novel ecosystems by species coming from the fringes of towns (e.g., Reference [10]).
Corridors are known as useful solutions for enhancing biodiversity in urban landscapes [11]. Continuous greenways should be prioritized for multiple taxa [6], but multiplying stepping-stones (i.e., a succession of disconnected habitat elements) represents a solution in places where opportunities at ground-level remain difficult to find [12]. In such a context, buildings provide additional, large, available surfaces for “greening the grey” [13,14,15,16]. Green roofs and green walls could be important elements of these new urban wildlife corridors [17]. Green walls refer to all systems that enable the greening of a vertical surface with plants [18,19]. Green roofs consist of several layers, including waterproofing, drainage, insulation with soil substrate, and actively growing plants [20]. Depending on their substrate depth, investments in plant care, and irrigation, green roofs are usually categorized as “intensive” or “extensive”. Vegetated buildings may contribute to connectivity. Dispersal is a three-stage process, i.e., emigration, migration, immigration, that results from the interaction between individual species-specific behaviors and the landscape configuration [21]. The connectivity level of an urban greenway depends on the patch specificities (size, quality, redundancy in the matrix, characteristics of the surroundings and species requirements) [22].
Because vegetated buildings are becoming increasingly popular in many countries [23,24], it appears important to discuss their ecological role in biodiversity conservation. We examined the ecological and technical specificities of green walls and green roofs in light of the key factors for urban wildlife (patch size, quality, abundance, and isolation). We compared the habitat values of green walls and roofs to the ground-level green spaces that are mainly found in core city centres (parks, wastelands, pavements). We emphasize that the role of vegetated buildings as components of urban corridors remains questionable. We assume that integrating vegetated buildings in wildlife corridors requires species to disperse within a new 3D green ecological network (Figure 1), but their capabilities of doing so need to be determined with consideration of the species traits and ecological requirements. In addition, through an interdisciplinary approach, we also emphasize that obstacles exist to the wide planning of green roofs and green walls but that methods are being developed to overcome them. Lastly, we argue that recommendations for the design of both green walls and roofs should be similar to enhance the contribution of vegetated buildings to the connectivity of green spaces and wildlife habitats.
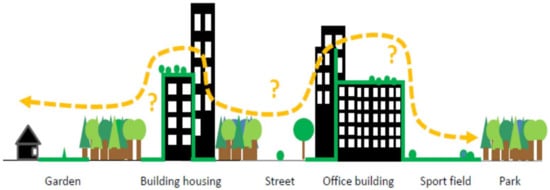
Figure 1.
Species dispersal and vegetated buildings: “do they really contribute to urban connectivity?”.
2. Are Green Walls and Green Roofs Large Enough for Supporting Biodiversity?
Green walls have been demonstrated to be a habitat that supports biodiversity [18,25,26]. Spontaneous flora of stone and masonry walls has been the most commonly examined type (Table 1). Li et al. (2016) [27] recorded 159 species belonging to 77 families on the vertical surfaces of tropical retaining walls. Within European latitudes, rural stone walls [28], historic buildings [29], and both external and retaining walls [30] showed similar values. Researchers have inventoried 207 species of vascular plants and 60 mosses on vertical walls and their tops [28], and published a list of the more common plants and lichens living on historic walls [29], with more than 300 species recorded on vertical walls [30]. Steiner (1994) inventoried 194 taxa of invertebrates, including nematodes, tardigrades, rotifers, and arthropods, among which mites and springtails were the most common together with centipedes, beetles, and other insects [31]. Walls are of particular importance for woodlice and spiders. Indeed, half of the native British woodlice species have been inventoried on walls [18], and over 10% of the British spider species are mural species [15].

Table 1.
Biodiversity of walls in the literature.
It has also been reported in the literature that green roofs can serve as habitats for many spontaneous plants and animals [47,48]. For example, 176 species of plants were inventoried on 115 roofs in a European climate [49]. Studies have also confirmed the presence of arthropods, such as beetles, spiders, true bugs, hymenopterans (ants, bees, wasps), flies [50,51,52], springtails (up to 44 species) [53,54], and mites [55], in addition to fungi [54,55] and bacteria [56]. A recent review conducted in six Swiss cities over the past 20 years showed that 91 out of the 532 species (17%) known in Switzerland are represented on green roofs [57]. Roofs are also habitat for vertebrates. These vertebrates are almost always birds, and few data exists on mammals. 50 bird species have been identified as using roofs for breeding (29 species) or other activities (21 species, e.g., roosting, grooming, foraging for food, drinking, singing, collecting nest material) [48]. Some roofs have been developed specifically for species of concern [58]. By comparing bat activity over four pairs of urban green roofs and conventional urban rooftops for an entire season in New York City, Parkins and Clark (2015) [59] showed higher levels of bat activity and higher bat diversity over green roofs.
The size of habitats is an important factor driving both plant and animal populations in cities (e.g., Reference [60]), but difficulties remain in determining how large urban green spaces should be to support the conservation of biodiversity due to different taxa operating at different spatial scales. Threatened or urban-avoider species would need large areas to be conserved (approximately 53.3 ha on average according to Beninde et al., 2015) [6]. In contrast, 4.4-ha patch sizes may be sufficient to minimize the loss of urban-adapter species. International examples of the implementation of green roofs and recent large-scale sampling research on biodiversity have revealed that the areas of green roofs and green walls are highly variable (Table 2). They range from a few square metres to several hundred square metres for green walls, and to tens of thousands of square metres for green roofs. Based on the patch size criterion, neither green walls nor green roofs would be effective at supporting the conservation of biodiversity of urban-avoider species, and green roofs could satisfy the theoretical threshold of 4.4 ha for supporting urban-adapter species in some cases [6], but not in many others. Green walls areas may remain well below this size.

Table 2.
Variability of patch size for green walls and green roofs (na: not available in the reference).
Like in small urban green spaces (e.g., Reference [64]) and larger ones (e.g., Reference [60]), significant relationships exist between the sizes of green roofs and green walls and species diversity, but those relationships are highly variable among communities, habitats, and time. The relationship between stone wall areas and plant richness has not yet been explored, but it is now known that the size of extensive and semi-extensive roofs (i.e., the more interesting systems for wildlife because of reduced maintenance) acts as a minor determining factor in the species richness of colonizing plants [49,62].
Arthropod species richness is poorly driven by wall size regardless of the system (climbing systems, modular living wall, continuous living wall) [46], but it is impacted by the available area in the case of roofs [50,51]. A weak positive association between green roof size and species abundance or diversity [50,65] has been found, and Kyro et al. (2018) [51] recently added that roof species that are not sensitive to reduced patch size will typically benefit from small roofs, unlike species that require a large area of continuous habitat (e.g., running spiders).
3. Are Green Walls and Green Roofs Identical Habitats?
A comparative analysis of the local microclimatic conditions of green walls to those of green roofs could be very informative to identify if species on the rooftops could also colonize new vertical habitats, extending and building patch sizes.
Walls are not found in natural ecosystems [1]. They are unique habitats where local drivers lead to different species compositions depending on the wall type [25]. In stone walls and masonry walls where flora is only spontaneous, very few microhabitats (cracks, joints, ledges) are available for plants and animals on vertical surfaces because of a poor accumulation of substrates and new building materials. Thus, plants are distributed according to gaps, forming a highly variable and discontinuous plant cover [37]. The abiotic conditions are functions of the physicochemical properties of the building materials, weathering, maintenance intensity and disturbances, pollution, and interactions with animals. The persistence of pioneer species indicates that abiotic conditions tend to remain quite stable over time. This wall flora is mainly native and common [28], and very few species are frequently recorded [28], but urban walls are also described as refuges for some declining species [34], invasive species [26,27,30,66], and ornamental species [36].
The ecological conditions of climbing systems and living walls (modular and continuous systems) are simpler, but different microclimatic conditions exist as a result of different types of façade (Table 2). The microclimatic conditions specifically affect the species richness and community composition in arthropods [46]. Climbing systems are often monospecific and have dry and hot microclimatic conditions. Their beetle communities are associated with floricolous and xerophilous communities that feed mainly on plants and flowers and that are more similar to assemblages observed on green roofs [50]. Beetle communities are characterized by more phytophagous and large assemblages, dominated by Aphthona sp. (Chrysomelidae) or Vibidia duodecimguttata (Coccinellidae). On living walls, the abiotic environment is highly controlled by engineering. Damp conditions are produced by frequent, steady, and widely dispersed drop-by-drop irrigation throughout the entire green wall, while evaporation, runoff, and drainage losses characterize less efficient irrigation systems [67]. Modular herb-shrub systems have been shown to attract a wide range of wildlife, through available substrate and a more structurally complex plant cover (from mosses to small shrubs, with different forms and developments). The communities contain hygrophilous arthropods (many beetles, Latridiidae) and small assemblages of spiders that prefer humid to wet conditions, and are commonly found in moss and litter layers of woodlands and moors (Gongylidiellum vivum), in wetlands (Aphileta misera), or in ground litter (Entelecara omissa) [44,46].
Unlike herb-shrub walls (i.e., living walls), extensive and semi-intensive green roofs generally offer extreme abiotic conditions, similar to those of hard ground surfaces and dry natural habitats on thinner substrates (Table 3), such as brownfields and grasslands [68]. With moisture being the limiting factor controlling plant growth, plants and fauna are mainly heliotropic, thermophilic, xerophilic or mesophilic, acidophilic and oligotrophic, and originate from grasslands and pioneer habitats, showing a wide variety of functional traits for pollination, dispersal, and reproduction [49,50,54,57].

Table 3.
Green walls and green roofs show different microclimates.
4. How Abundant Are Green Roof and Green Wall Patches in Cities?
Although an overall increase of the green space area was measured in European cities from 2000–2006 [69], cities remain often too sparsely vegetated [7]. The role of green spaces in maintaining a high level of biodiversity in cities remains questionable [9] because the amount of green spaces is known to significantly and positively impact urban species richness [6]. Very few data have been collected to show how much the installed green roofs and green walls really contribute to the amount of green spaces in urbanized areas; the city of Paris (France) had only 30 ha of green walls (less than 1% of urban green spaces) in 2016, and 44 ha of green roofs (1.4% of urban green spaces) in 2013, which are mainly on private buildings [70,71]. In 2007, Zurich, Switzerland, had 87 ha of vegetated roofs [72].
The number of opportunities for increasing the amount of green spaces in dense areas by greening buildings appears substantial but uncertain, as many factors act as constraints on the installation, design, and maintenance of green roofs and green walls. Firstly, there are architectural restrictions (e.g., Reference [73]), industry standards, and regulations. Through two types of indexes that assess (i) the building capacity for incorporating green roofs and (ii) the need for additional green spaces in areas [73], it has been noted that many buildings could be suitable for green roof installation in Lisbon (52% of the total city area). In contrast, in Paris (France), only 80 ha out of the 460 ha of empty concrete roofs (17.6%) have been estimated to be potentially valuable sites for vegetation, because flat rooftop areas less than 200 m2 or with a slope greater than 2% are inadequate [71]. Such criteria could be slightly modified to multiply patches in the urban matrix, but those results also indicate that the lack of green spaces in the core city centre cannot always be compensated for by greening roofs, because of the rarity of optimal roofs in the historic centre.
Green walls may be alternatives for increasing the area of green spaces. A first estimate of the best locations for green walls in Melbourne was rather pessimistic, revealing that only approximately 16 ha of wall spaces in the entire city would have potential for vertical greening through climbing systems [74]. However, the modelling approach used had the unique objective of maximizing the thermal effect of climbing species. Thus, the potential for greening walls may have been underestimated in that study.
Other barriers to the adoption of green roofs and green walls in dense areas come from diverse public perceptions; a lack of awareness of the services provided and problems faced; a lack of information about different systems (costs, maintenance, complexity); a lack of examples to give urban designers confidence in the technology; inadequate or absent policy instruments, guidance or incentives [75,76,77]; and lack of scientific data about the effect of particular climate conditions [78]. However, solutions exist, such as education and promotion campaigns aimed at stakeholders, policy makers, and architects. There are also suitable legislation and policies [79] including subsidies for the installation or maintenance of green roofs and green walls [80], or other incentives. Once green walls are installed, their future remains uncertain. Insufficient or inappropriate management can lead to the degradation of the system [81] (Figure 2) and diminish the abundance of wall patches in the matrix. Therefore, knowledge about governance is critical to understand how to maintain green walls as a component of urban greenways.

Figure 2.
Insufficient or inappropriate management lead to the degradation of vegetated walls. (Left) a continuous living wall in in Bordeaux, France (©Flavie. Mayrand). (Right) a direct green façade cut at the base in Paris, France (©Pauline. Watissée).
5. How Redundant Are Green Roofs and Green Walls in Cities?
Habitat redundancy is fundamental to species dispersal because it reduces the distance between two patches and the barrier effect of the urban matrix, particularly for species with low dispersal capabilities. Green walls are often considered analogues of natural vertical habitats for wildlife, such as stone walls for rocky habitats, climbing systems for xerothermophilous vertical habitats like cliffs, and living walls for vegetated waterfalls [30,82]. Unfortunately, no other analogue habitats for walls have been identified in the temperate urban landscape, which raises questions about their role as effective elements of existing greenways. In contrast, arthropod communities on extensive roofs show the same functional diversity as in some urban ground-level habitats (road verges, brownfields, or dry meadows) [83], showing that redundant habitats for extensive green roofs exist in cities. Arthropod communities are mostly composed of generalist species with more flexible habits in terms of resource exploitation (e.g., polyphagous weevils, social bees) or species (e.g., ground-nesting bees) targeting rare resources in cities such as bare soils or sandy areas.
Like roofs, wastelands are unmanaged areas. Wastelands have been demonstrated to be significant original habitat for species in cities [1]. They are characterized by a high diversity of anthropogenic substrates (nutrient-rich soil, rubble, ballast, and brick), restricted public access, restricted uses, and spontaneous vegetation cover with irregular removal [84]. In wastelands, the soils are mostly neutral to alkaline, free-draining, well-aerated, and low in organic matter. Microclimatic conditions are heterogeneous but generally warm and dry in young wastelands. In young wastelands, the pioneer vegetation (in the early stages of succession) attracts open-habitat bird species, ground beetle species associated with open and dry habitats, and, in general, polyphagous species that have reproduction strategies adapted to changing food availability. In the advanced stages of succession, tall grass and meadow vegetation, or pre-forest stages, shelter generalist species that prefer more humid conditions, with perennial species gaining dominance. Unlike green roofs, wastelands change in space and over time according to construction, demolition and succession; they can contribute to species dispersal and biodiversity conservation, but only if they are not too isolated [64]. Extensive and semi-intensive roofs could act as refuges for many wasteland species, thereby reducing the isolation of those habitats in cities.
Unlike extensive roofs, lawns and pavement are often intensively managed (mowing, pesticides, fertilizers) offering limited habitat opportunities for fauna [85,86,87]. However, like roofs, they show very extreme environmental conditions, with flora adapted to a hostile environment that includes limited nutrients, water, and soil accumulation due to highly compacted and thin substrates, extreme light and temperature conditions, a variable soil pH, changing water conditions, eutrophication, and disturbances (trampling, intensive management, pets) [88,89,90,91]. Locally, even in this harsh environment, the heterogeneity of abiotic conditions can enhance species diversity [92]. The most common annual species show very competitive strategies for reproduction, such as non-seasonal reproduction, fast germination, and the absence of seed dormancy. Like the flora on stone walls, plant cover is also highly fragmented (e.g., joints between paving-stones) [90,91].
The conditions of both green walls and green roofs are very distinct from those of the park environment under temperate conditions, and they differ in the following ways: (i) parks offer large areas in which various types of modified habitats can develop [93], such as woody habitats of different ages, remnant vegetation, deadwood, water bodies, open areas, and horticultural borders and (ii) parks contain both managed and unmanaged vegetation areas, leading to diverse ecological niches and community differentiation [85,94,95]. All strata of vegetation can be observed and represented by numerous families, even though often a few groups dominate. Old urban parks show mature successional stages, while no mature successional stages are observed on roofs [52], and roof communities can vary as a function of their age [49,51]. No direct solar radiation on the ground, less trampling and the additions of litter or compost lead to greater amounts of organic matter and higher moisture contents in park soil than in lawns [88]. Therefore, the vegetation is composed of species that prefer wet, shaded, and fresh habitats. A well-developed shrub understory, a higher diversity of native plants (including trees), and ecological management positively impact the presence of native invertebrate and vertebrate species, notably small woodland species [95,96,97]. In contrast, green roofs have been recorded as being nesting sites for ground-nesting species, such as Alauda arvensis and some others [48].
6. In the Connectivity of Green Walls and Roofs to the Ground-Level Green Spaces, Height and Landscapes also Matter
Little is known regarding the source-sink metapopulational dynamics of green roofs, and their connectivity to surrounding green spaces remains poorly assessed [98]. In addition to many local factors and frequent short distance exchanges with ground sites, height strongly influences the diversity of species with low mobility (carabids and spiders) on roofs [50,51], while highly mobile species (bees, weevils) are mainly affected by the exchange of individuals with other green roofs or habitats outside the city [65]. Recent results showed that the abundance of highly mobile species is also impacted by height in the case of tall buildings (>5 building levels) [99]. However, the comparison of the arthropod communities on 40 green roofs with 40 ground-level analogue habitats indicated that whereas green roofs partially replicate the communities of ground-level analogue habitats [83,100], the functional diversity of both communities remained identical [83,100], without relation to mobility. Fewer available resources and harsher local conditions may explain this difference.
No studies have examined the role of green walls in dispersal within fragmented areas, but a few studies have investigated how the surroundings of the wall may influence the wall biodiversity [25]. They showed that isolated stone and masonry walls show a slower development of vegetation cover than those forming a part of networks [27,32], affecting the wall–plant assemblage. The low fauna biodiversity of walls is positively and significantly influenced by landscape factors, but only for species with low dispersal capabilities (beetles), especially those that are frugivorous [46]. Even if some of them are able to fly, the urban matrix can act as a barrier for their dispersal [101], and green walls can act as habitats or stepping stones to enhance their dispersal in the matrix. For fauna with strong dispersal capabilities, the landscape would not act as the main filter, and walls may be additional habitats that enhance species abundance in cities [46]. Research on birds [42] confirmed that green walls could play a critical role in the ecological functioning of very fragmented areas. Based on the surroundings, the spontaneous plants are mostly weeds [33], or show different life/growth forms (mosses, lichens, ferns, herbs, woody species) (e.g., Reference [27]). Few data are available to examine how height could impact wall biodiversity. On stone and masonry walls, the plant cover varies from the wall base to the top because of various local conditions along walls that affect the plant cover [18]. To the best of our knowledge, no study has provided evidence indicating that communities of small animals on walls vary according to the wall height. Such a study would be very helpful in the assessment of whether green walls can act as vertical corridors for wildlife from the ground level to green roofs.
7. Implications for an Ecological Design and Management.
Because arthropods are responsible for numerous ecological functions and ecosystem services (e.g., pollination, decomposition), architecture, design and planning recommendations are needed to increase the contributions of vegetated buildings to greenways. We have concluded that green walls could act as vertical corridors, allowing less mobile species to disperse more easily from the ground to the roofs.
Increasing the patch sizes on buildings can be implemented during building design by simultaneously greening the roof and walls of the same building. Second, changes in designs should focus on increasing vegetation diversity, spatial heterogeneity and resource abundance at the patch scale (e.g., Reference [85]). The ecological conditions of green roofs are close to those of climbing systems, but very different from the conditions of modular systems that are the most efficient systems for hosting biodiversity. We suggest that both green roofs and walls should converge to intermediate conditions for moisture and temperature, reaching more unified ecological conditions at the building scale. The existing modular wall systems should be modified into vertical analogue habitats with drier and warmer conditions. Innovation should result in new support systems (materials, designs) and substrates that enhance the bioreceptibility of the walls, but with a limited environmental footprint [25].
As redundancy of the patch conditions is needed to increase habitat area and the success of species dispersal within cities, changes should consist of tailoring green walls and roofs to the need of the species (or guilds) they are designed to serve, and selecting critical attributes (e.g., exposure, humidity, plants) that can contribute to habitat quality on buildings. Applying the ecological land use complementation approach [102] on walls would be of particular interest to provide supplementary resources for particular species found in surrounding source sites, or to promote different types of responses to environmental disturbance among species. New designs with similar habitat characteristics (kind of vegetation, moisture) and more complex structures (strata of vegetation) could enhance the dispersal of many species from parks and reduce the barrier effect created by streets and buildings.
8. Conclusions
We highlight that the role of green walls and roofs in urban wildlife corridors remains questionable because of limited patch size, distinct habitat quality at the building scale, and limited redundancy of the patch quality within the landscape. Through an interdisciplinary approach, we also emphasized that the abundance of roofs and walls is often low and that barriers exist to the wide implementation of green roofs and green walls, but that methods are being developed to overcome them. We also noted that green roof biodiversity is influenced by the surroundings and height, but more knowledge is needed to establish if walls can be vertical corridors for plants and wildlife, thereby reducing the isolation of the green roofs. We find that the suggestion made by Braaker et al. [65] of using green walls to reduce the isolation of green roofs seems to not be supported. New designs for walls and roofs could enhance the dispersal of many park species beyond the park areas, and reduce the barrier effects created by streets and buildings. At the building scale, we suggest that vegetated buildings should have more unified ecological conditions, without homogenizing the conditions of green spaces.
Acknowledgments
This work was supported by the French National Research Agency (ANR-14-CE22-0021).
Author Contributions
Philippe Clergeau designed the research; Flavie Mayrand performed the research; Flavie Mayrand and Philippe Clergeau wrote the paper. Flavie Mayrand and Philippe Clergeau reviewed and edited the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kowarik, I. Novel Urban Ecosystems, Biodiversity, and Conservation. Environ. Pollut. 2011, 159, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Pickett, S.T.A.; Boone, C.G.; McGrath, B.P.; Cadenasso, M.L.; Childers, D.L.; Ogden, L.A.; McHale, M.; Grove, J.M. Ecological Science and Transformation to the Sustainable City. Cities 2013, 32, S10–S20. [Google Scholar] [CrossRef]
- Elmqvist, T.; Setälä, H.; Handel, S.; van der Ploeg, S.; Aronson, J.; Blignaut, J.; Gómez-Baggethun, E.; Nowak, D.; Kronenberg, J.; de Groot, R. Benefits of Restoring Ecosystem Services in Urban Areas. Curr. Opin. Environ. Sustain. 2015, 14, 101–108. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; La Sorte, F.A.; Aronson, M.F.J.; Goddard, M.A.; MacGregor-Fors, I.; Nilon, C.H.; Warren, P.S. Global Patterns and Drivers of Urban Bird Diversity—Ecology and Conservation of Birds in Urban Environments; Murgui, E., Hedblom, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 13–33. [Google Scholar] [CrossRef]
- Ives, C.D.; Lentini, P.E.; Threlfall, C.G.; Ikin, K.; Shanahan, D.F.; Garrard, G.E.; Bekessy, S.A.; Fuller, R.A.; Mumaw, L.; Rayner, L.; et al. Cities Are Hotspots for Threatened Species. Glob. Ecol. Biogeogr. 2016, 25, 117–126. [Google Scholar] [CrossRef]
- Beninde, J.; Veith, M.; Hochkirch, A. Biodiversity in Cities Needs Space: A Meta-Analysis of Factors Determining Intra-Urban Biodiversity Variation. Ecol. Lett. 2015, 18, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R.A.; Gaston, K.J. The Scaling of Green Space Coverage in European Cities. Biol. Lett. 2009, 5, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, D.F.; Miller, C.; Possingham, H.P.; Fuller, R.A. The Influence of Patch Area and Connectivity on Avian Communities in Urban Revegetation. Biol. Conserv. 2011, 144, 722–729. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; Aronson, M.F.J.; Evans, K.L.; Goddard, M.A.; Lerman, S.B.; Macivor, J.S. Biodiversity in the City: Fundamental Questions for Understanding the Ecology of Urban Green Spaces for Biodiversity Conservation. Bioscience 2017, 67, 799–807. [Google Scholar] [CrossRef]
- Ahern, J. Urban Landscape Sustainability and Resilience: The Promise and Challenges of Integrating Ecology with Urban Planning and Design. Landsc. Ecol. 2013, 28, 1203–1212. [Google Scholar] [CrossRef]
- Vergnes, A.; Kerbiriou, C.; Clergeau, P. Ecological Corridors Also Operate in an Urban Matrix: A Test Case with Garden Shrews. Urban Ecosyst. 2013, 16, 511–525. [Google Scholar] [CrossRef]
- Saura, S.; Bodin, Ö.; Fortin, M.J. Stepping Stones Are Crucial for Species’ Long-Distance Dispersal and Range Expansion through Habitat Networks. J. Appl. Ecol. 2014, 51, 171–182. [Google Scholar] [CrossRef]
- Jim, C. Green-Space Preservation and Allocation for Sustainable Greening of Compact Cities. Cities 2004, 21, 311–320. [Google Scholar] [CrossRef]
- Grimmond, C.S.B.; Cleugh, H.A.; Oke, T.R. An Objective Urban Heat Storage Model and Its Comparison with Other Schemes. Atmos. Environ. Part B 1991, 25, 311–326. [Google Scholar] [CrossRef]
- Darlington, A. Ecology of Walls; Heinemann: London, UK, 1981; ISBN 978-0435602239. [Google Scholar]
- Francis, R.A.; Lorimer, J. Urban Reconciliation Ecology: The Potential of Living Roofs and Walls. J. Environ. Manag. 2011, 92, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Haaland, C.; van den Bosch, C.K. Challenges and Strategies for Urban Green-Space Planning in Cities Undergoing Densification: A Review. Urban For. Urban Green. 2015, 14, 760–771. [Google Scholar] [CrossRef]
- Dover, J.W. Green Walls. In Green Infrastructure. Incorporating Plants and Enhancing Biodiversity in Buildings and Urban Environments; Dover, J.W., Ed.; Routledge: London, UK, 2015; ISBN 9780415521246. [Google Scholar]
- Fernández-Cañero, R.; Pérez Urrestarazu, L. Chapter 2.1—Vertical Greening Systems: Classifications, Plant Species, Substrates. In Nature Based Strategies for Urban and Building Sustainability; Butterworth-Heinemann: Oxford, UK, 2018; pp. 45–54. [Google Scholar]
- Oberndorfer, E.; Lundholm, J.; Bass, B.; Coffman, R.R.; Doshi, H.; Dunnett, N.; Gaffin, S.; Köhler, M.; Liu, K.K.Y.; Rowe, B. Green Roofs as Urban Ecosystems: Ecological Structures, Functions, and Services. Bioscience 2007, 57, 823–833. [Google Scholar] [CrossRef]
- Baguette, M.; Van Dyck, H. Landscape Connectivity and Animal Behavior: Functional Grain as a Key Determinant for Dispersal. Landsc. Ecol. 2007, 22, 1117–1129. [Google Scholar] [CrossRef]
- Douglas, I.; Sadler, J.P. Urban Wildlife Corridors. Conduits for Movement or Linear Habitat? In The Routledge Handbook of Urban Ecology; Douglas, I., Goode, D., Houck, M.C., Wang, R., Eds.; Routledge: New York, NY, USA, 2015; pp. 274–288. ISBN 9780415498135. [Google Scholar]
- Vijayaraghavan, K. Green Roofs: A Critical Review on the Role of Components, Benefits, Limitations and Trends. Renew. Sustain. Energy Rev. 2016, 57, 740–752. [Google Scholar] [CrossRef]
- Jim, C.Y. Green Roof Evolution through Exemplars: Germinal Prototypes to Modern Variants. Sustain. Cities Soc. 2017, 35, 69–82. [Google Scholar] [CrossRef]
- Mayrand, F.; Clergeau, P.; Vergnes, A.; Madre, F. Chapter 3.13—Vertical Greening Systems as Habitat for Biodiversity. In Nature Based Strategies for Urban and Building Sustainability; Butterworth-Heinemann: Oxford, UK, 2018; pp. 227–237. ISBN 978-0-12-812150-4. [Google Scholar]
- Francis, R.A. Wall Ecology: A Frontier for Urban Biodiversity and Ecological Engineering. Prog. Phys. Geogr. 2011, 35, 43–63. [Google Scholar] [CrossRef]
- Li, X.; Yin, X.; Wang, Y. Diversity and Ecology of Vascular Plants Established on the Extant World-Longest Ancient City Wall of Nanjing, China. Urban For. Urban Green. 2016, 18, 41–52. [Google Scholar] [CrossRef]
- Duchoslav, M. Flora and Vegetation of Stony Walls in East Bohemia (Czech Republic). Preslia 2002, 74, 1–25. [Google Scholar]
- Lisci, M.; Monte, M.; Pacini, E. Lichens and Higher Plants on Stone: A Review. Int. Biodeterior. Biodegrad. 2003, 51, 1–17. [Google Scholar] [CrossRef]
- Láníková, D.; Lososová, Z. Rocks and Walls: Natural versus Secondary Habitats. Folia Geobot. 2009, 44, 263–280. [Google Scholar] [CrossRef]
- Steiner, W.A. The Influence of Air Pollution on Moss-Dwelling Animals: 1. Methodology and the Composition of Flora and Fauna. Rev. Suisse Zool. 1994, 101, 533–556. [Google Scholar] [CrossRef]
- Jim, C.Y. Ecology and Conservation of Strangler Figs in Urban Wall Habitats. Urban Ecosyst. 2014, 17, 405–426. [Google Scholar] [CrossRef]
- Dos Reis, V.A.; Lombardi, J.A.; De Figueiredo, R.A. Diversity of Vascular Plants Growing on Walls of a Brazilian City. Urban Ecosyst. 2006, 9, 39–43. [Google Scholar] [CrossRef]
- Shimwell, D.W. Studies in the Floristic Diversity of Durham Walls, 1958–2008. Watsonia 2009, 27, 323–338. [Google Scholar]
- Jim, C.Y.; Chen, W.Y. Habitat Effect on Vegetation Ecology and Occurrence on Urban Masonry Walls. Urban For. Urban Green. 2010, 9, 169–178. [Google Scholar] [CrossRef]
- Payne, R. The Flora of Walls and Buildings in the Isle of Ely. In Nature in Cambridgeshire; Arnold, H.R., Ed.; Cambridge Natural History Society: Cambridge, UK, 2005; pp. 43–58. ISSN 0466-6046. [Google Scholar]
- Qiu, Y.; Chen, B.J.W.; Song, Y.; Huang, Z.Y.X.; Wan, L.; Huang, C.; Liu, M.; Xu, C. Composition, Distribution and Habitat Effects of Vascular Plants on the Vertical Surfaces of an Ancient City Wall. Urban Ecosyst. 2016, 19, 939–948. [Google Scholar] [CrossRef]
- Nedelcheva, A.; Vasileva, A. Vascular Plants from the Old Walls in Kystendil (Southwestern Bulgaria). Biotechnol. Biotechnol. Equip. 2009, 23, 154–157. [Google Scholar] [CrossRef]
- Cervelli, E.W.; Lundholm, J.T.; Du, X. Spontaneous Urban Vegetation and Habitat Heterogeneity in Xi’an, China. Landsc. Urban Plan. 2013, 120, 25–33. [Google Scholar] [CrossRef]
- Jim, C.Y. Assessing Growth Performance and Deficiency of Climber Species on Tropical Greenwalls. Landsc. Urban Plan. 2015, 137, 107–121. [Google Scholar] [CrossRef]
- Mårtensson, L.-M.; Wuolo, A.; Fransson, A.-M.; Emilsson, T. Plant Performance in Living Wall Systems in the Scandinavian Climate. Ecol. Eng. 2014, 71, 610–614. [Google Scholar] [CrossRef]
- Chiquet, C.; Dover, J.W.; Mitchell, P. Birds and the Urban Environment: The Value of Green Walls. Urban Ecosyst. 2013, 16, 453–462. [Google Scholar] [CrossRef]
- Wheater, C.P. Walls and Paved Surfaces: Urban Complexes with Limited Water and Nutrients. In The Routledge Handbook of Urban Ecology; Douglas, I., Goode, D., Houck, M.C., Wang, R., Eds.; Routledge: New York, NY, USA, 2015; ISBN 9780415498135. [Google Scholar]
- Chiquet, C. The Animal Biodiversity of Green Walls in the Urban Environment. Ph.D. Thesis, Staffordshire University, Staffordshire, UK, 2014. [Google Scholar]
- Garbuzov, M.; Ratnieks, F.L.W. Ivy: An Underappreciated Key Resource to Flower-Visiting Insects in Autumn. Insect Conserv. Divers. 2014, 7, 91–102. [Google Scholar] [CrossRef]
- Madre, F.; Clergeau, P.; Machon, N.; Vergnes, A. Building Biodiversity: Vegetated Façades as Habitats for Spider and Beetle Assemblages. Glob. Ecol. Conserv. 2015, 3, 222–233. [Google Scholar] [CrossRef]
- Köhler, M.; Ksiazek-Mikenas, K. Chapter 3.14—Green Roofs as Habitats for Biodiversity. In Nature Based Strategies for Urban and Building Sustainability; Butterworth-Heinemann: Oxford, UK, 2018; pp. 239–249. ISBN 978-0-12-812150-4. [Google Scholar]
- Dover, J.W. Green Roofs. In Green Infrastructure. Incorporating Plants and Enhancing Biodiversity in Buildings and Urban Environments; Dover, J.W., Ed.; Routledge: London, UK, 2015; pp. 163–217. ISBN 9780415521246. [Google Scholar]
- Madre, F.; Vergnes, A.; Machon, N.; Clergeau, P. Green Roofs as Habitats for Wild Plant Species in Urban Landscapes: First Insights from a Large-Scale Sampling. Landsc. Urban Plan. 2014, 122, 100–107. [Google Scholar] [CrossRef]
- Madre, F.; Vergnes, A.; Machon, N.; Clergeau, P. A Comparison of 3 Types of Green Roof as Habitats for Arthropods. Ecol. Eng. 2013, 57, 109–117. [Google Scholar] [CrossRef]
- Kyrö, K.; Brenneisen, S.; Kotze, D.J.; Szallies, A.; Gerner, M.; Lehvävirta, S. Local Habitat Characteristics Have a Stronger Effect than the Surrounding Urban Landscape on Beetle Communities on Green Roofs. Urban For. Urban Green. 2018, 29, 122–130. [Google Scholar] [CrossRef]
- Ksiazek-mikenas, K.; Herrmann, J.; Menke, S.B.; Köhler, M. If You Build It, Will They Come? Plant and Arthropod Diversity on Urban Green Roofs Over Time. Urban Nat. 2018, 1, 52–72. [Google Scholar]
- Joimel, S.; Grard, B.; Auclerc, A.; Hedde, M.; Le Doaré, N.; Salmon, S.; Chenu, C. Are Collembola “flying” onto Green Roofs? Ecol. Eng. 2018, 111, 117–124. [Google Scholar] [CrossRef]
- Rumble, H.; Gange, A.C. Soil Microarthropod Community Dynamics in Extensive Green Roofs. Ecol. Eng. 2013, 57, 197–204. [Google Scholar] [CrossRef]
- John, J.; Lundholm, J.; Kernaghan, G. Colonization of Green Roof Plants by Mycorrhizal and Root Endophytic Fungi. Ecol. Eng. 2014, 71, 651–659. [Google Scholar] [CrossRef]
- Molineux, C.J.; Gange, A.C.; Connop, S.P.; Newport, D.J. Are Microbial Communities in Green Roof Substrates Comparable to Those in Post-Industrial Sites? A Preliminary Study. Urban Ecosyst. 2015, 18, 1245–1260. [Google Scholar] [CrossRef]
- Pétremand, G.; Chittaro, Y.; Braaker, S.; Brenneisen, S.; Gerner, M.; Obrist, M.K.; Rochefort, S.; Szallies, A.; Moretti, M. Ground Beetle (Coleoptera: Carabidae) Communities on Green Roofs in Switzerland: Synthesis and Perspectives. Urban Ecosyst. 2017, 1–14. [Google Scholar] [CrossRef]
- Fernandez-Canero, R.; Gonzalez-Redondo, P. Green Roof as Habitat for Birds: A Review. J. Anim. Vet. Adv. 2010, 9, 2041–2052. [Google Scholar] [CrossRef]
- Parkins, K.L.; Clark, J.A. Green Roofs Provide Habitat for Urban Bats. Glob. Ecol. Conserv. 2015, 4, 349–357. [Google Scholar] [CrossRef]
- Matthies, S.A.; Rüter, S.; Schaarschmidt, F.; Prasse, R. Determinants of Species Richness within and across Taxonomic Groups in Urban Green Spaces. Urban Ecosyst. 2017, 20, 897–909. [Google Scholar] [CrossRef]
- Wong, G.K.L.; Jim, C.Y. Urban-Microclimate Effect on Vector Mosquito Abundance of Tropical Green Roofs. Build. Environ. 2017, 112, 63–76. [Google Scholar] [CrossRef]
- Gabrych, M.; Kotze, D.J.; Lehvävirta, S. Substrate Depth and Roof Age Strongly Affect Plant Abundances on Sedum-Moss and Meadow Green Roofs in Helsinki, Finland. Ecol. Eng. 2016, 86, 95–104. [Google Scholar] [CrossRef]
- MacIvor, J.S.; Lundholm, J. Insect Species Composition and Diversity on Intensive Green Roofs and Adjacent Level-Ground Habitats. Urban Ecosyst. 2011, 14, 225–241. [Google Scholar] [CrossRef]
- Muratet, A.; Machon, N.; Jiguet, F.; Moret, J.; Porcher, E. The Role of Urban Structures in the Distribution of Wasteland Flora in the Greater Paris Area, France. Ecosystems 2007, 10, 661. [Google Scholar] [CrossRef]
- Braaker, S.; Ghazoul, J.; Obrist, M.K.; Moretti, M. Habitat Connectivity Shapes Urban Arthropod Communities: The Key Role of Green Roofs. Ecology 2014, 95, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Gómez, F.; Gil, L.; Jabaloyes, J. Experimental Investigation on the Thermal Comfort in the City: Relationship with the Green Areas, Interaction with the Urban Microclimate. Build. Environ. 2004, 39, 1077–1086. [Google Scholar] [CrossRef]
- Manso, M.; Castro-Gomes, J. Green Wall Systems: A Review of Their Characteristics. Renew. Sustain. Energy Rev. 2015, 41, 863–871. [Google Scholar] [CrossRef]
- Lundholm, J.T.; Richardson, P.J. Habitat Analogues for Reconciliation Ecology in Urban and Industrial Environments. J. Appl. Ecol. 2010, 47, 966–975. [Google Scholar] [CrossRef]
- Kabisch, N.; Haase, D. Green Spaces of European Cities Revisited for 1990–2006. Landsc. Urban Plan. 2013, 110, 113–122. [Google Scholar] [CrossRef]
- Atelier Parisien d’Urbanisme (APUR). Recensement Des Murs Végétaux Parisiens : Cartographie et Typologies État Avancement 2016; APUR: Paris, France, 2016. [Google Scholar]
- Atelier Parisien d’Urbanisme (APUR). Étude Sur Le Potentiel de Végétalisation Des Toitures Terrasses À Paris; APUR: Paris, France, 2013; 39p. [Google Scholar]
- Tschander, B. Flachdachbegruenung in Der Stadt Zurich; Gruen Stadt Zurich, Naturfoerderung, Fachstelle Naturschutz: Zurich, Switzerland, 2007. [Google Scholar]
- Silva, C.M.; Flores-Colen, I.; Antunes, M. Step-by-Step Approach to Ranking Green Roof Retrofit Potential in Urban Areas: A Case Study of Lisbon, Portugal. Urban For. Urban Green. 2017, 25, 120–129. [Google Scholar] [CrossRef]
- Croeser, T. Biological Potential in Cities: An Estimate from Melbourne. Urban For. Urban Green. 2016, 16, 84–94. [Google Scholar] [CrossRef]
- Wong, N.H.; Tan, A.Y.K.; Tan, P.Y.; Sia, A.; Wong, N.C. Perception Studies of Vertical Greenery Systems in Singapore. J. Urban Plan. Dev. 2010, 136, 330–338. [Google Scholar] [CrossRef]
- Magliocco, A.; Perini, K. The Perception of Green Integrated into Architecture: Installation of a Green Facade in Genoa, Italy. AIMS Environ. Sci. 2015, 2, 899–909. [Google Scholar] [CrossRef]
- Brudermann, T.; Sangkakool, T. Green Roofs in Temperate Climate Cities in Europe—An Analysis of Key Decision Factors. Urban For. Urban Green. 2017, 21, 224–234. [Google Scholar] [CrossRef]
- Williams, N.S.G.; Rayner, J.P.; Raynor, K.J. Green Roofs for a Wide Brown Land: Opportunities and Barriers for Rooftop Greening in Australia. Urban For. Urban Green. 2010, 9, 245–251. [Google Scholar] [CrossRef]
- Irga, P.J.; Braun, J.T.; Douglas, A.N.J.; Pettit, T.; Fujiwara, S.; Burchett, M.D.; Torpy, F.R. The Distribution of Green Walls and Green Roofs throughout Australia: Do Policy Instruments Influence the Frequency of Projects? Urban For. Urban Green. 2017, 24, 164–174. [Google Scholar] [CrossRef]
- Claus, K.; Rousseau, S. Public versus Private Incentives to Invest in Green Roofs: A Cost Benefit Analysis for Flanders. Urban For. Urban Green. 2012, 11, 417–425. [Google Scholar] [CrossRef]
- Riley, B. The State of the Art of Living Walls: Lessons Learned. Build. Environ. 2017, 114, 219–232. [Google Scholar] [CrossRef]
- Lundholm, J.T. Urban Cliffs. In The Routledge Handbook of Urban Ecology; Douglas, I., Goode, D., Houck, M.C., Wang, R., Eds.; Routledge: New York, NY, USA, 2015; ISBN 9781136883415. [Google Scholar]
- Braaker, S.; Obrist, M.K.; Ghazoul, J.; Moretti, M. Habitat Connectivity and Local Conditions Shape Taxonomic and Functional Diversity of Arthropods on Green Roofs. J. Anim. Ecol. 2017, 86, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Bonthoux, S.; Brun, M.; Di Pietro, F.; Greulich, S.; Bouché-Pillon, S. How Can Wastelands Promote Biodiversity in Cities? A Review. Landsc. Urban Plan. 2014, 132, 79–88. [Google Scholar] [CrossRef]
- Politi Bertoncini, A.; Machon, N.; Pavoine, S.; Muratet, A. Local Gardening Practices Shape Urban Lawn Floristic Communities. Landsc. Urban Plan. 2012, 105, 53–61. [Google Scholar] [CrossRef]
- Smith, J.; Chapman, A.; Eggleton, P. Baseline Biodiversity Surveys of the Soil Macrofauna of London’s Green Spaces. Urban Ecosyst. 2006, 9, 337. [Google Scholar] [CrossRef]
- Paker, Y.; Yom-Tov, Y.; Alon-Mozes, T.; Barnea, A. The Effect of Plant Richness and Urban Garden Structure on Bird Species Richness, Diversity and Community Structure. Landsc. Urban Plan. 2014, 122, 186–195. [Google Scholar] [CrossRef]
- Sarah, P.; Zhevelev, H.M.; Oz, A. Urban Park Soil and Vegetation: Effects of Natural and Anthropogenic Factors. Pedosphere 2015, 25, 392–404. [Google Scholar] [CrossRef]
- Lundholm, J. Vegetation of Urban Hard Surfaces. In Urban Ecology: Patterns, Processes, and Applications; Niemelä, J., Breuste, J.H., Elmqvist, T., Guntenspergen, G., James, P., McIntyre, N.E., Eds.; Oxford University Press: Oxford, UK, 2011; ISBN 9780199563562. [Google Scholar]
- Melander, B.; Holst, N.; Grundy, A.C.; Kempenaar, C.; Riemens, M.M.; Verschwele, A.; Hansson, D. Weed Occurrence on Pavements in Five North European Towns. Weed Res. 2009, 49, 516–525. [Google Scholar] [CrossRef]
- Fagot, M.; De Cauwer, B.; Beeldens, A.; Boonen, E.; Bulcke, R.; Reheul, D. Weed Flora in Paved Areas in Relation to Environment, Pavement Characteristics and Weed Control. Weed Res. 2011, 51, 650–660. [Google Scholar] [CrossRef]
- Lundholm, J.T.; Marlin, A. Habitat Origins and Microhabitat Preferences of Urban Plant Species. Urban Ecosyst. 2006, 9, 139–159. [Google Scholar] [CrossRef]
- Hermy, M. Landscaped Parks and Open Spaces. In The Routledge Handbook of Urban Ecology; Douglas, I., Goode, D., Houck, M.C., Wang, R., Eds.; Routledge: New York, NY, USA, 2015; pp. 289–300. ISBN 9780415498135. [Google Scholar]
- Li, W.; Ouyang, Z.; Meng, X.; Wang, X. Plant Species Composition in Relation to Green Cover Configuration and Function of Urban Parks in Beijing, China. Ecol. Res. 2006, 21, 221–237. [Google Scholar] [CrossRef]
- Shwartz, A.; Shirley, S.; Kark, S. How Do Habitat Variability and Management Regime Shape the Spatial Heterogeneity of Birds within a Large Mediterranean Urban Park? Landsc. Urban Plan. 2008, 84, 219–229. [Google Scholar] [CrossRef]
- Smith, L.S.; Broyles, M.E.J.; Larzleer, H.K.; Fellowes, M.D.E. Adding Ecological Value to the Urban Lawnscape. Insect Abundance and Diversity in Grass-Free Lawns. Biodivers. Conserv. 2015, 24, 47–62. [Google Scholar] [CrossRef]
- Threlfall, C.G.; Mata, L.; Mackie, J.A.; Hahs, A.K.; Stork, N.E.; Williams, N.S.G.; Livesley, S.J. Increasing Biodiversity in Urban Green Spaces through Simple Vegetation Interventions. J. Appl. Ecol. 2017, 54, 1874–1883. [Google Scholar] [CrossRef]
- Williams, N.S.G.; Lundholm, J.; Scott Macivor, J. Do Green Roofs Help Urban Biodiversity Conservation? J. Appl. Ecol. 2014, 51, 1643–1649. [Google Scholar] [CrossRef]
- MacIvor, J.S. Building Height Matters: Nesting Activity of Bees and Wasps on Vegetated Roofs. Isr. J. Ecol. Evol. 2016, 62, 88–96. [Google Scholar] [CrossRef]
- Tonietto, R.; Fant, J.; Ascher, J.; Ellis, K.; Larkin, D. A Comparison of Bee Communities of Chicago Green Roofs, Parks and Prairies. Landsc. Urban Plan. 2011, 103, 102–108. [Google Scholar] [CrossRef]
- Vergnes, A.; Saux, E.L.; Clergeau, P. Preliminary Data on Low Aerial Plankton in a Large City Center, Paris. Urban For. Urban Green. 2017, 22, 36–40. [Google Scholar] [CrossRef]
- Colding, J. “Ecological Land-Use Complementation” for Building Resilience in Urban Ecosystems. Landsc. Urban Plan. 2007, 81, 46–55. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
