An Astrobiological View on Sustainable Life
Abstract
:1. Introduction
2. Vortex of Life
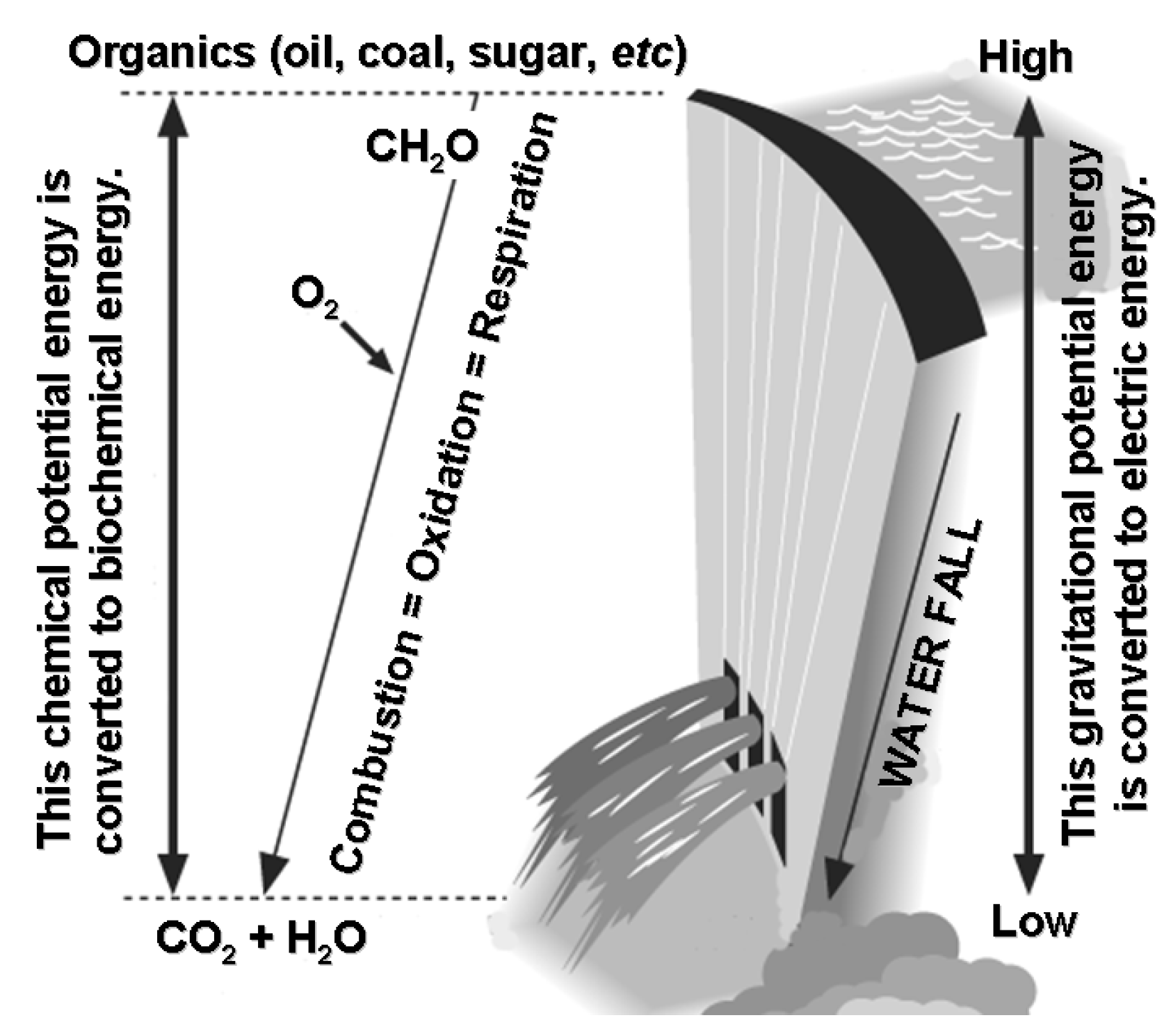
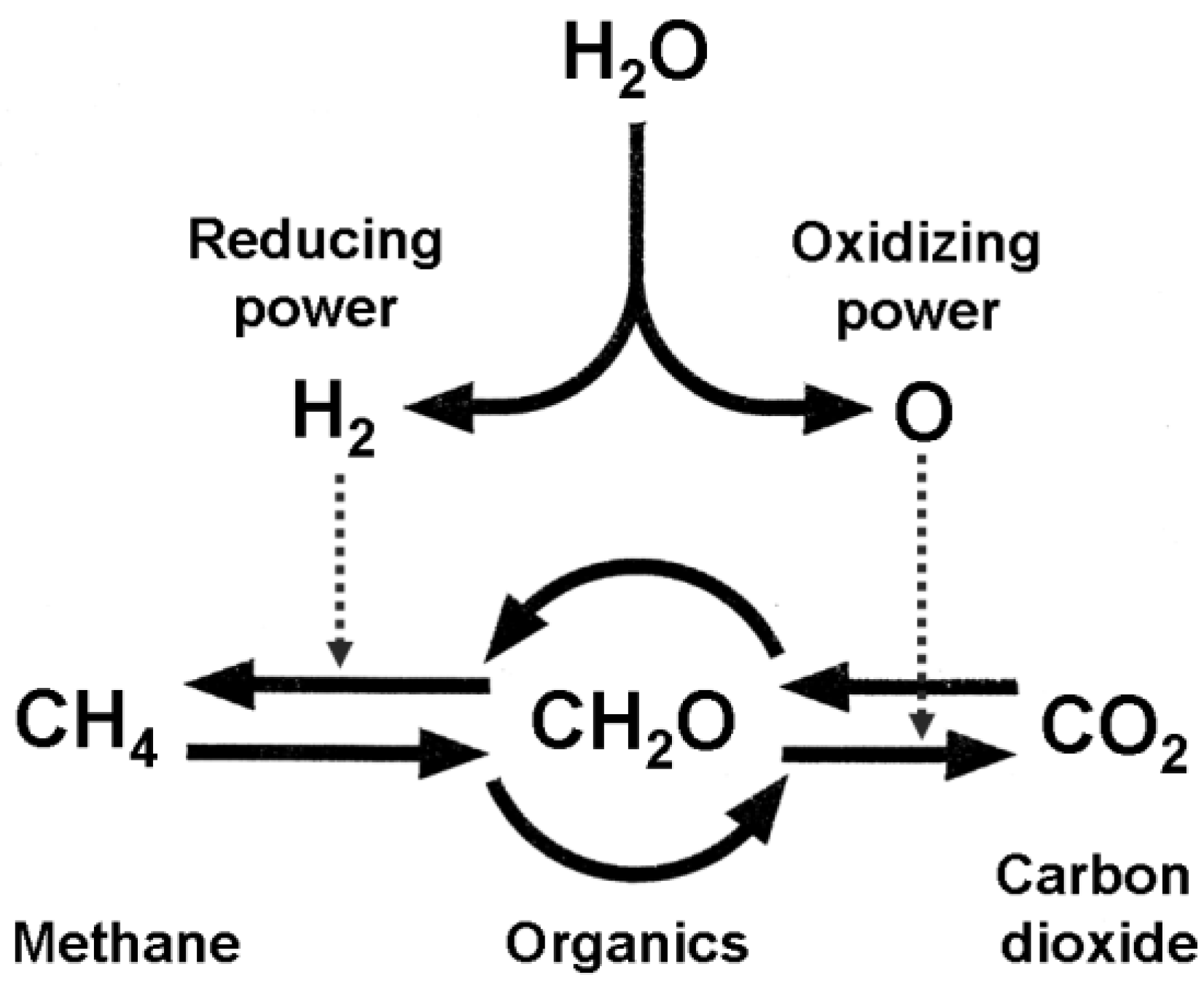
3. Life Vortex as Intermediate between CH4 and CO2


4. Splitting of Water
- (1)
- Water molecules are held together by sharing electrons among two hydrogen and one oxygen atoms via two covalent bonds;
- (2)
- Water is a polar molecule. Although the net electrical charge on the molecule is zero, its structure causes the molecule to become polarized;
- (3)
- Electrostatic bonds (hydrogen bonds) form between the negatively charged oxygen side of one water molecule and the positively charged hydrogen of another molecule;
- (4)
- The existence of these hydrogen bonds explains many of the unique properties of water;
- (5)
- Ice has an orderly, open structure of water molecules held together by hydrogen bonding;
- (6)
- Water ice’s crystal structure results in lower density (0.92 g cm–3) than that of liquid water, 1.0 g cm–3;
- (7)
- Liquid water structure is intermediate between that of ice and water vapor, and consists of two types of aggregates of water molecules;
- (8)
- Structured water is composed of clusters of hydrogen-bonded water molecules that form and reform very quickly but slow enough to influence the physical behavior of water;
- (9)
- Unstructured water is composed of closely packed free water molecules, denser than structured water;
- (10)
- If hydrogen bonding did not exist, water would only occur as a gas at the Earth's surface;
- (11)
- Water is the only naturally occurring substance on the Earth to exist at the surface in all three states: liquid, solid and gas;
- (12)
- Water dissolves more substances in greater quantity than any other common liquid;
- (13)
- Water has the highest surface tension of all liquids;
- (14)
- Water has the highest heat capacity of all common solids and liquids, which prevents extreme range in aquatic temperature;
- (15)
- Boiling and melting points of water are higher than those of other hydrogen compounds of similar size or oxygen-group (8O, 16S, 34Se, 52Te and so on). For example, CH4, NH3, H2S, H2Se and H2Te occur as gases at room temperature; and
- (16)
- Water has the highest heat conductivity of any common liquid.
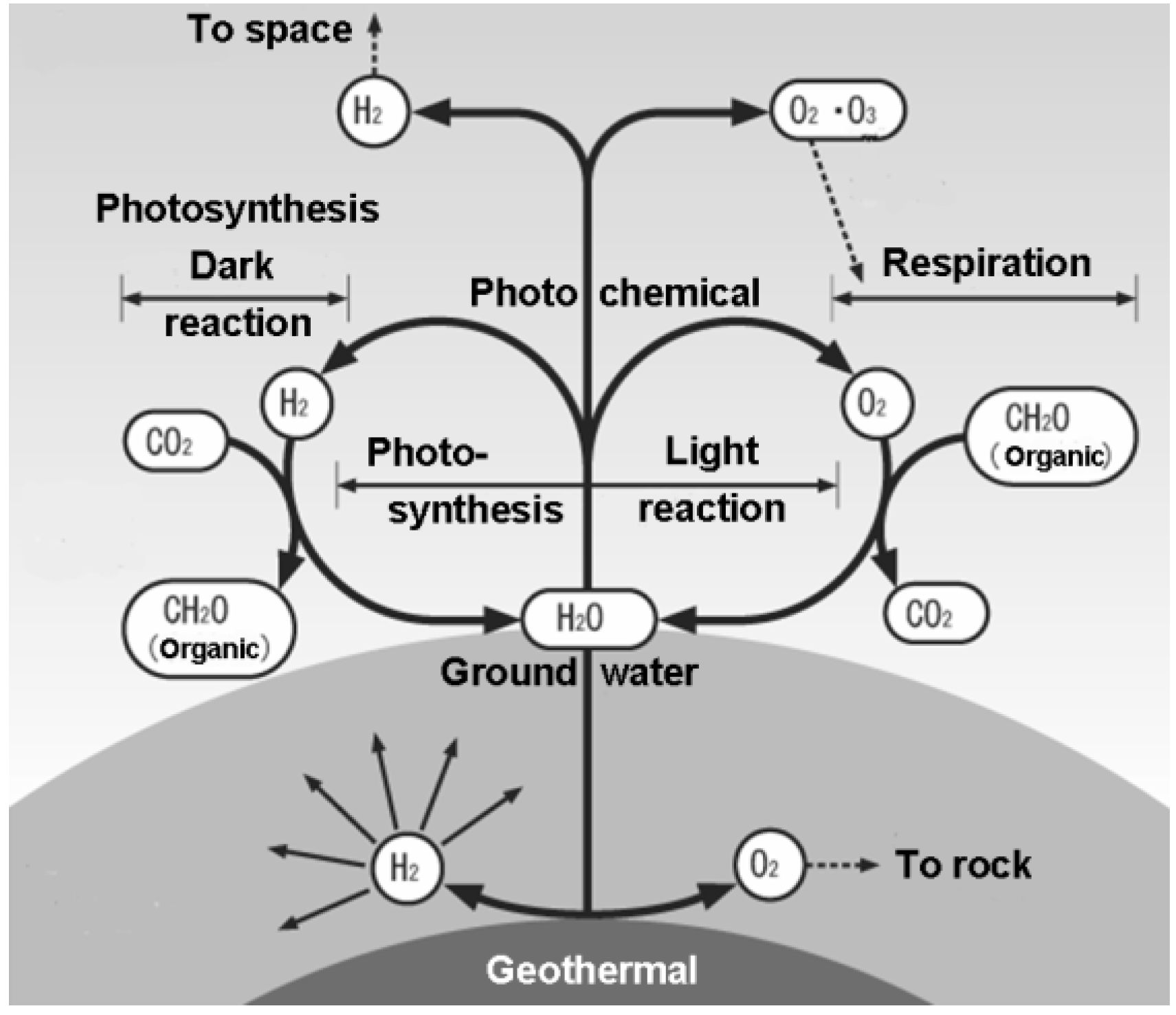
5. Testing Planetary Biospheres
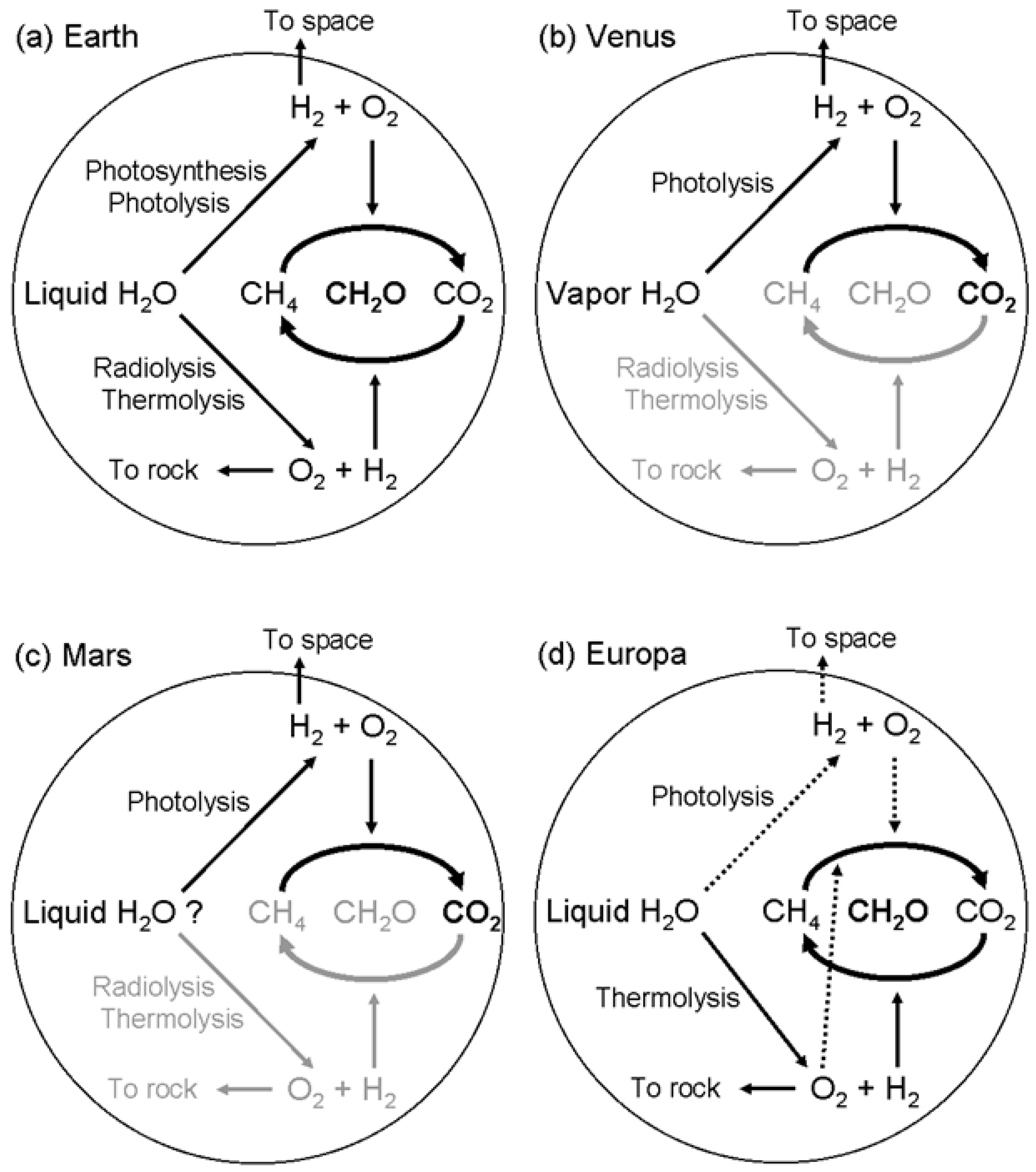
6. Astrobiological Conclusion
References
- Schrödinger, E. What is Life? The Physical Aspect of the Living Cell; Cambridge University Press: Cambridge, UK, 1944. [Google Scholar]
- Prigogine, I. Order out of Chaos: Man's New Dialogue with Nature; Bantam Books: New York, NY, USA, 1984. [Google Scholar]
- Getling, A.V. Rayleigh-Benard Convection; World Scientific Publishing: Singapore, 1988. [Google Scholar]
- Humphris, S.E.; Zierenberg, R.A.; Mullineaux, L.S.; Thomson, R.E. (Eds.) Seafloor Hydrothermal Systems; American Geophysical Union: Washington, DC, USA, 1995.
- Fredrickson, J.K.; Fletcher, M. (Eds.) Sursurface Microbiology and Biogeochemistry; Wiley-Liss: New York, NY, USA, 2001.
- Moriguchi, Y.; Jenkins, D. Hojoki (text by Kamo-no-Chomei). Stone Bridge Press: Berkeley, CA, USA, 1996. [Google Scholar]
- Sabtier, P. Catalysis in Organic Chemistry (translation of La Catalyse en Chimie Organique). Van Nostrand Co: New York, NY, USA, 1923. [Google Scholar]
- Bock, G.R.; Goode, J.A. (Eds.) Evolution of Hydrothermal Ecosystems on Earth (and Mars?); John Wiley & Sons Ltd: Chichester, UK, 1996.
- Bullock, M.A. The flow and ebb of water. Nature 2005, 438, 1087–1088. [Google Scholar] [CrossRef] [PubMed]
- Kransnopolsky, V.A.; Feldman, P.D. Detection of molecular hydrogen in the atmosphere of Mars. Science 2001, 294, 1914–1917. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.S.; Gibson, E.K., Jr.; Thomas-Keprta, K.L.; Vali, H.; Romanek, C.S.; Clemett, S.J.; Chillier, X.D.F.; Maechling, C.R.; Zare, R.N. Search for past life on Mars: possible relic biogenic activity in Martian meteorite ALH84001. Science 1996, 273, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Golden, D.C.; Ming, D.W.; Schwandt, C.S.; Lauer, H.V.; Socki, R.A.; Morris, R.V.; Lofgren, G.E.; McKay, G.A. A simple inorganic process for formation of carbonates, magnetite, and sulfides in Martian meteorite ALH84001. Am. Mineral. 2001, 83, 370–375. [Google Scholar]
- Thomas-Keprta, K.L.; Clemett, S.J.; Bazylinski, D.A.; Kirschvink, J.L.; McKay, D.S.; Wentworth, S.J.; Vali, H.; Gibson, E.K.J.; McKay, M.F.; Romanek, C.S. Truncated hexa-octahedral magnetite crystals in ALH84001: Presumptive biosignatures. Proc. Natl. Acad. Sci. USA 2001, 98, 2164–2169. [Google Scholar] [CrossRef] [PubMed]
- Byrne, S.; Dundas, C.M.; Kennedy, M.R.; Mellon, M.T.; McEwen, A.S.; Cull, S.C.; Daubar, I.J.; Shean, D.E.; Seelos, K.D.; Murchie, S.L.; Cantor, B.A.; Arvidson, R.E.; Edgett, K.S.; Reufer, A.; Thomas, N.; Harrison, T.N.; Posiolova, L.V.; Seelos, F.P. Distribution of Mid-Latitude Ground Ice on Mars from New Impact Craters. Science 2009, 325, 1674–1676. [Google Scholar] [CrossRef] [PubMed]
- Lucey, P.G. A Lunar Waterworld. Science 2009. [Google Scholar] [CrossRef]
- Formisano, V.; Atreya, S.; Encrenaz, T.; Ignatiev, N.; Giuranna, M. Detection of methane in the atmosphere of Mars. Science 2004, 306, 1758–1751. [Google Scholar] [CrossRef] [PubMed]
- Mumma, M.J.; Villanueva, G.L.; Novak, R.E.; Hewagama, T.; Bonev, B.P.; DiSanti, M.A.; Mandell, A.M.; Smith, M.D. Strong Release of Methane on Mars in Northern Summer 2003. Science 2009, 323, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Neukum, G.; Jaumann, R.; Hoffmann, H.; Hauber, E.; Head, J.W.; Basilevsky, A.T.; Ivanov, B.A.; Werner, S.C.; van Gasselt, S.; Murray, J.B.; McCord, T. The HRSC Co-Investigator Team. Recent and episodic volcanic and glacial activity on Mars revealed by the high resolution stereo camera. Nature 2001, 432, 971–979. [Google Scholar] [CrossRef]
- Fisk, M.R.; Giovannoni, S.J. Sources of nutrients and energy for a deep biosphere on Mars. J. Geophys. Res. 1999, 104, 11805–11815. [Google Scholar] [CrossRef]
- Nna-Mvondo, D.; Martinez-Frias, J. Review komatiites: from Earth’s geological settings to planetary and astrobiological contexts. Earth Moon Planet 2007, 100, 157–179. [Google Scholar] [CrossRef]
- Yoshizaki, T.; Shibuya, T.; Suzuki, K.; Shimizu, K.; Nakamura, K.; Takai, K.; Omori, S.; Maruyama, S. H2 generation by experimental hydrothermal alteration of komatiitic glass at 300 °C and 500 bars: A preliminary result from on-going experiment. Geochem. J. 2009, 43, e17–e22. [Google Scholar] [CrossRef]
- Chyba, C.F.; Phillips, C.B. Possible ecosystems and the search for life on Europa. Proc. Natl. Acad. Sci. USA 2001, 98, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Chyba, C.F.; Hand, K.P. Life without photosynthesis. Science 2001, 292, 2026–2027. [Google Scholar] [CrossRef] [PubMed]
- Gaidos, E.J.; Nimmo, F. Tectonics and water on Europa. Nature 2000, 405, 637. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, R. The evil twin of Agenor; tectonic convergence on Europa. Icarus 2004, 167, 313–319. [Google Scholar] [CrossRef]
- Naganuma, T.; Uematsu, H. Dive Europa: A search-for-life initiative. Biol. Sci. Space 1998, 12, 126–130. [Google Scholar] [CrossRef] [PubMed]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Naganuma, T. An Astrobiological View on Sustainable Life. Sustainability 2009, 1, 827-837. https://doi.org/10.3390/su1040827
Naganuma T. An Astrobiological View on Sustainable Life. Sustainability. 2009; 1(4):827-837. https://doi.org/10.3390/su1040827
Chicago/Turabian StyleNaganuma, Takeshi. 2009. "An Astrobiological View on Sustainable Life" Sustainability 1, no. 4: 827-837. https://doi.org/10.3390/su1040827
APA StyleNaganuma, T. (2009). An Astrobiological View on Sustainable Life. Sustainability, 1(4), 827-837. https://doi.org/10.3390/su1040827



