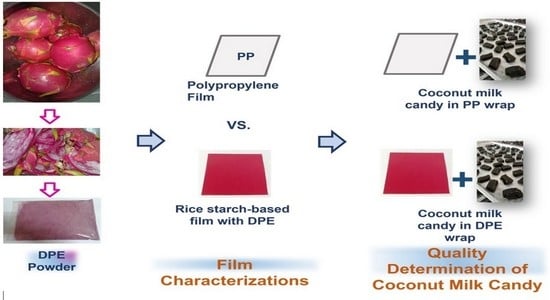
Dragon Fruit Peel Extract Enriched-Biocomposite Wrapping Film: Characterization and Application on Coconut Milk Candy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Dragon Fruit Peel Extract
2.3. Preparation of the DPE-Enriched Biocomposite Film
2.4. Film Property Determination
2.4.1. Film Thickness
2.4.2. Film Appearance and Color
2.4.3. Film Solubility
2.4.4. Mechanical Properties
2.4.5. Water Vapor Permeability
2.4.6. Determination of Total Phenolic Content and Total Betacyanins Content
2.4.7. Determination of DPPH Radical Scavenging Activity
2.4.8. Determination of Ferric Reducing Antioxidant Power (FRAP)
2.4.9. Film Morphology
2.5. Film Application to Coconut Milk Candy
2.6. Determination of Quality Attributes of Coconut Milk Candy
2.6.1. Moisture Content and Water Activity
2.6.2. Texture Profile Analysis
2.6.3. Thiobarbituric Acid Reactive Substances (TBARS)
2.7. Statistical Analysis
3. Results
3.1. Film Characterization
3.1.1. Film Thickness, and Mechanical Properties
3.1.2. Film Solubility, and Barrier Properties
3.1.3. Film Appearance and Color
3.1.4. Determination of Total Phenolic Content and Total Betacyanins Content
3.1.5. Antioxidant Activities
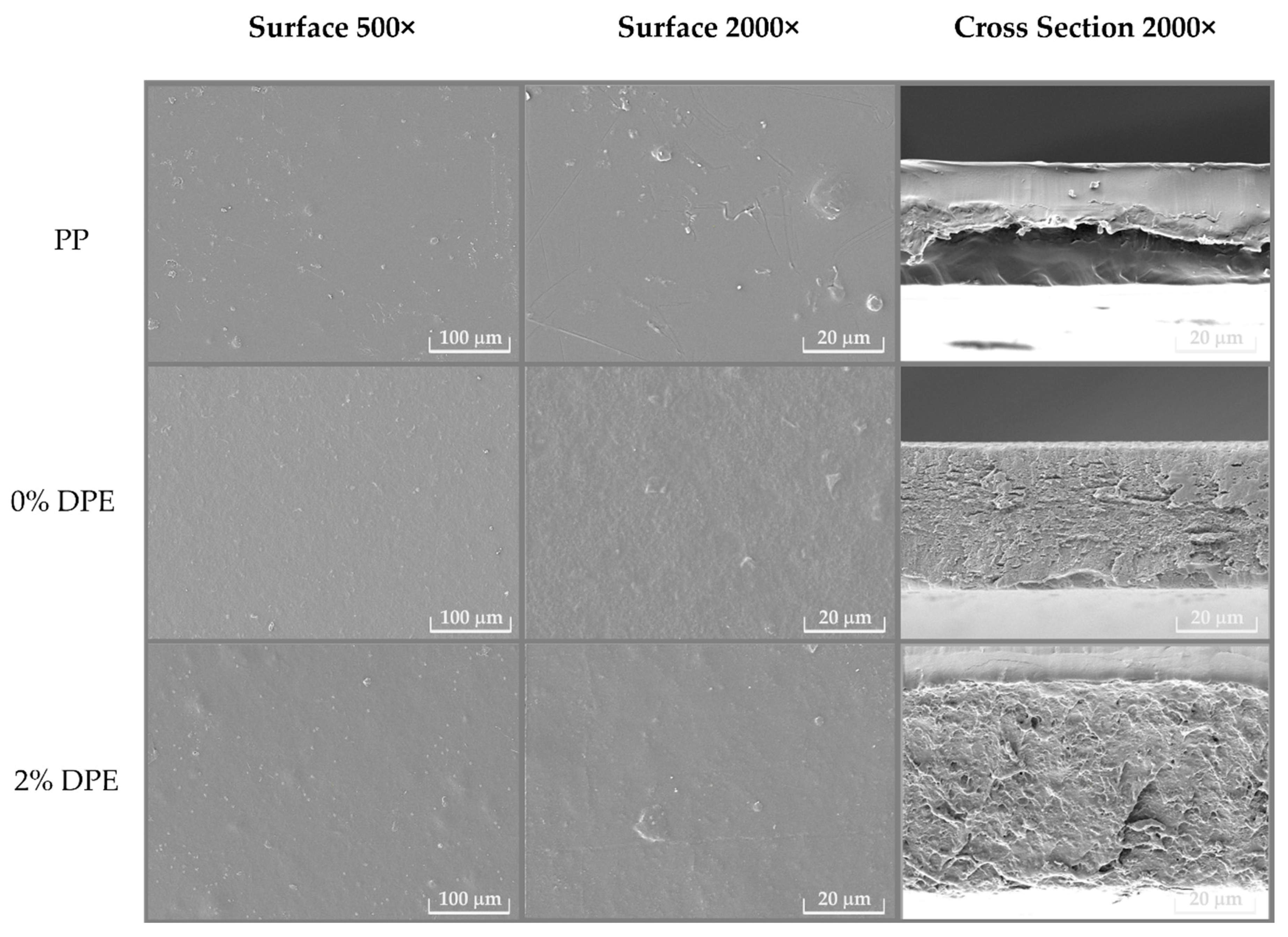
3.1.6. Film Morphology
3.2. Quality Attributes of Coconut Milk Candy
3.2.1. Moisture Content and Water Activity
3.2.2. Thiobarbituric Acid Reactive Substances
3.2.3. Texture Profile Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benito-González, I.; del Ortiz-Gimeno, M.M.; López-Rubio, A.; Martínez-Abad, A.; Garrido-Fernández, A.; Martínez-Sanz, M. Sustainable Starch Biocomposite Films Fully-Based on White Rice (Oryza sativa) Agroindustrial by-Products. Food Bioprod. Process. 2022, 136, 47–58. [Google Scholar] [CrossRef]
- Kumar, L.; Ramakanth, D.; Akhila, K.; Gaikwad, K.K. Edible Films and Coatings for Food Packaging Applications: A Review. Environ. Chem. Lett. 2021, 20, 875–900. [Google Scholar] [CrossRef]
- Amin, U.; Khan, M.U.; Majeed, Y.; Rebezov, M.; Khayrullin, M.; Bobkova, E.; Shariati, M.A.; Chung, I.M.; Thiruvengadam, M. Potentials of Polysaccharides, Lipids and Proteins in Biodegradable Food Packaging Applications. Int. J. Biol. Macromol. 2021, 183, 2184–2198. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Yan, X.; Zhou, J.; Tong, J.; Su, X. Influence of Chitosan Concentration on Mechanical and Barrier Properties of Corn Starch/Chitosan Films. Int. J. Biol. Macromol. 2017, 105, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ji, X.; Cheng, Y.; Tao, Y.; Lu, J.; Du, J.; Wang, H. All-Biodegradable Soy Protein Isolate/Lignin Composite Cross-Linked by Oxidized Sucrose as Agricultural Mulch Films for Green Farming. Int. J. Biol. Macromol. 2022, 223, 120–128. [Google Scholar] [CrossRef]
- Song, G.; Lin, S.; Wu, Y.; Shen, J.; Wu, J.; Zhu, W.; Yu, S.; Li, J.; Wang, S. Emulsifier Free Fish Gelatin Based Films with Excellent Antioxidative and Antibacterial Activity: Preparation, Characterization and Application in Coating Preservation of Fish Fillets. J. Food Eng. 2022, 343, 111362. [Google Scholar] [CrossRef]
- Salim, M.H.; Kassab, Z.; Abdellaoui, Y.; García-Cruz, A.; Soumare, A.; Ablouh, E.; el Achaby, M. Exploration of Multifunctional Properties of Garlic Skin Derived Cellulose Nanocrystals and Extracts Incorporated Chitosan Biocomposite Films for Active Packaging Application. Int. J. Biol. Macromol. 2022, 210, 639–653. [Google Scholar] [CrossRef]
- Gulzar, S.; Tagrida, M.; Nilsuwan, K.; Prodpran, T.; Benjakul, S. Electrospinning of Gelatin/Chitosan Nanofibers Incorporated with Tannic Acid and Chitooligosaccharides on Polylactic Acid Film: Characteristics and Bioactivities. Food Hydrocoll. 2022, 133, 107916. [Google Scholar] [CrossRef]
- de Vargas, V.H.; Marczak, L.D.F.; Flôres, S.H.; Mercali, G.D. Advanced Technologies Applied to Enhance Properties and Structure of Films and Coatings: A Review. Food Bioproc. Tech. 2022, 15, 1224–1247. [Google Scholar] [CrossRef]
- Ji, M.; Wu, J.; Sun, X.; Guo, X.; Zhu, W.; Li, Q.; Shi, X.; Tian, Y.; Wang, S. Physical Properties and Bioactivities of Fish Gelatin Films Incorporated with Cinnamaldehyde-Loaded Nanoemulsions and Vitamin C. LWT 2021, 135, 110103. [Google Scholar] [CrossRef]
- Leguebe, E.; Huart, V.; Krajka, N.; Pellot, J.; le Baillif, M.; Erre, D. Effect of Relaxation Time, Recovery Time and Extension Speed during Characterization of LLDPE Wrap Film Properties. Packag. Technol. Sci. 2022, 35, 913–921. [Google Scholar] [CrossRef]
- Pramanik, N.K.; Katamgari, I.; Dey, A.; Bhardwaj, Y.K.; Alam, T.; Chattopadhyay, S.K.; Saha, N.C. Electron Beam Irradiation on Monolayer Plastic Packaging Films: Studies on Physico-Mechanical and Thermal Properties. Packag. Technol. Sci. 2021, 34, 475–483. [Google Scholar] [CrossRef]
- Baele, M.; Vermeulen, A.; Adons, D.; Peeters, R.; Vandemoortele, A.; Devlieghere, F.; de Meulenaer, B.; Ragaert, P. Selecting Packaging Material for Dry Food Products by Trade-off of Sustainability and Performance: A Case Study on Cookies and Milk Powder. Packag. Technol. Sci. 2021, 34, 303–318. [Google Scholar] [CrossRef]
- Erna, K.H.; Felicia, W.X.L.; Rovina, K.; Vonnie, J.M.; Huda, N. Development of Curcumin/Rice Starch Films for Sensitive Detection of Hypoxanthine in Chicken and Fish Meat. Carbohydr. Polym. Technol. Appl. 2022, 3, 100189. [Google Scholar] [CrossRef]
- Wittaya, T. Rice Starch-Based Biodegradable Films: Properties Enhancement. In Structure and Function of Food Engineering; Eissa, A.A., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 103–104. [Google Scholar]
- Ventura-Cruz, S.; Tecante, A. Nanocellulose and Microcrystalline Cellulose from Agricultural Waste: Review on Isolation and Application as Reinforcement in Polymeric Matrices. Food Hydrocoll. 2021, 118, 106771. [Google Scholar] [CrossRef]
- Hazrati, K.Z.; Sapuan, S.M.; Zuhri, M.Y.M.; Jumaidin, R. Preparation and Characterization of Starch-Based Biocomposite Films Reinforced by Dioscorea Hispida Fibers. J. Mater. Res. Technol. 2021, 15, 1342–1355. [Google Scholar] [CrossRef]
- Tiozon, R.N., Jr.; Bonto, A.P.; Sreenivasulu, N. Enhancing the Functional Properties of Rice Starch through Biopolymer Blending for Industrial Applications: A Review. Int. J. Biol. Macromol. 2021, 192, 100–117. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q. v Starch-Based Films: Major Factors Affecting Their Properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef]
- Said, N.S.; Sarbon, N.M. Response Surface Methodology (RSM) of Chicken Skin Gelatin Based Composite Films with Rice Starch and Curcumin Incorporation. Polym. Test. 2020, 81, 106161. [Google Scholar] [CrossRef]
- Soe, M.T.; Pongjanyakul, T.; Limpongsa, E.; Jaipakdee, N. Modified Glutinous Rice Starch-Chitosan Composite Films for Buccal Delivery of Hydrophilic Drug. Carbohydr. Polym. 2020, 245, 116556. [Google Scholar] [CrossRef]
- Lin, D.; Ma, Y.; Qin, W.; Loy, D.A.; Chen, H.; Zhang, Q. The Structure, Properties and Potential Probiotic Properties of Starch-Pectin Blend: A Review. Food Hydrocoll. 2022, 129, 107644. [Google Scholar] [CrossRef]
- Freitas, P.A.v; González-Martínez, C.; Chiralt, A. Antioxidant Starch Composite Films Containing Rice Straw Extract and Cellulose Fibres. Food Chem. 2023, 400, 134073. [Google Scholar] [CrossRef] [PubMed]
- Salazar Ripoll, C.S.; Hincapié-Llanos, G.A. Evaluation of Sources and Methods of Pectin Extraction from Fruit and Vegetable Wastes: A Systematic Literature Review (SLR). Food Biosci. 2023, 51, 102278. [Google Scholar] [CrossRef]
- Pawar, R.; Jadhav, W.; Bhusare, S.; Borade, R.; Farber, S.; Itzkowitz, D.; Domb, A. Polysaccharides as Carriers of Bioactive Agents for Medical Applications. Nat.-Based Polym. Biomed. Appl. 2008, 3–53. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Osako, K.; Rungraeng, N.; Rawdkuen, S. Characterization of Fish Myofibrillar Protein Film Incorporated with Catechin-Kradon Extract. Int. J. Biol. Macromol. 2018, 107, 1463–1473. [Google Scholar] [CrossRef]
- Adilah, A.N.; Jamilah, B.; Noranizan, M.A.; Hanani, Z.A.N. Utilization of Mango Peel Extracts on the Biodegradable Films for Active Packaging. Food Packag. Shelf Life 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Pramitasari, R.; Gunawicahya, L.N.; Anugrah, D.S.B. Development of an Indicator Film Based on Cassava Starch–Chitosan Incorporated with Red Dragon Fruit Peel Anthocyanin Extract. Polymers 2022, 14, 4142. [Google Scholar] [CrossRef]
- Li, D.; Zhao, X.; Liu, Z.; Liu, H.; Fan, B.; Yang, B.; Zheng, X.; Li, W.; Zou, H. Synergetic Anticorrosion Mechanism of Main Constituents in Chinese Yam Peel for Copper in Artificial Seawater. ACS Omega 2021, 6, 29965–29981. [Google Scholar] [CrossRef]
- Fan, B.; Zhao, X.; Liu, Z.; Xiang, Y.; Zheng, X. Inter-Component Synergetic Corrosion Inhibition Mechanism of Passiflora Edulia Sims Shell Extract for Mild Steel in Pickling Solution: Experimental, DFT and Reactive Dynamics Investigations. Sustain. Chem. Pharm. 2022, 29, 100821. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.-H.; Qiao, J.; Qu, W.; Wang, M.-S.; Gao, X.; Zhang, C.; Brennan, C.S.; Qi, X. Improvement of Betalains Stability Extracted from Red Dragon Fruit Peel by Ultrasound-Assisted Microencapsulation with Maltodextrin. Ultrason. Sonochem 2022, 82, 105897. [Google Scholar] [CrossRef]
- Ruzlan, N.; Kamarudin, K.R.; Idid, S.Z.; Rehan, A.M.; Koya, M.S. Antioxidant Study of Pulps and Peels of Dragon Fruits: A Comparative Study. Int. Food Res. J. 2010, 17, 367–375. [Google Scholar]
- Jiménez-García, S.N.; García-Mier, L.; Ramírez-Gómez, X.S.; Aguirre-Becerra, H.; Escobar-Ortiz, A.; Contreras-Medina, L.M.; García-Trejo, J.F.; Feregrino-Pérez, A.A. Pitahaya Peel: A By-Product with Great Phytochemical Potential, Biological Activity, and Functional Application. Molecules 2022, 27, 5339. [Google Scholar] [CrossRef]
- Bhagya Raj, G.V.S.; Dash, K.K. Microencapsulation of Betacyanin from Dragon Fruit Peel by Complex Coacervation: Physicochemical Characteristics, Thermal Stability, and Release Profile of Microcapsules. Food Biosci. 2022, 49, 101882. [Google Scholar] [CrossRef]
- Manihuruka, F.M.; Suryatib, T.; Ariefb, I.I. Effectiveness of the Red Dragon Fruit (Hylocereus Polyrhizus) Peel Extract as the Colorant, Antioxidant, and Antimicrobial on Beef Sausage. Media Peternak. 2017, 40, 47–54. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Eight. The Sticky Science of Malaysian Dodol. In Reflections on the Science of Food and Cooking; Vega, C., Ubbink, J., van der Linden, E., Eds.; Columbia University Press: New York, NY, USA, 2012; pp. 52–58. ISBN 9780231526920. [Google Scholar]
- Nasaruddin, F.; Chin, N.L.; Yusof, Y.A. Effect of Processing on Instrumental Textural Properties of Traditional Dodol Using Back Extrusion. Int. J. Food Prop. 2012, 15, 495–506. [Google Scholar] [CrossRef]
- Ismail, N.; Ab Karim, M.S.; Che Ishak, F.A.; Arsyad, M.M.; Karnjamapratum, S.; Sirison, J. The Malay’s Traditional Sweet, Dodol: A Review of the Malaysia’s Heritage Delicacy alongside with the Rendition of Neighbouring Countries. J. Ethn. Foods 2021, 8, 19. [Google Scholar] [CrossRef]
- Chuah, T.G.; Hairul Nisah, H.; Thomas Choong, S.Y.; Chin, N.L.; Nazimah Sheikh, A.H. Effects of Temperature on Viscosity of Dodol (Concoction). J. Food Eng. 2007, 80, 423–430. [Google Scholar] [CrossRef]
- Seow, E.K.; Tan, T.C.; Easa, A.M. Role of Honey Diastase on Textural, Thermal, Microstructural, Chemical, and Sensory Properties of Different Dodols. LWT 2021, 148, 111715. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Marzlan, A.A.; Kadum, H.; Arulrajah, B.; Mohamad Asri, N.; Fathallah, S.; Meor Hussin, A.S. Metabolomics Profiling and Antimicrobial Activity of Fermented Date Fruit (Khastawi) Used as Functional Ingredients for Making Asian Confectionary (Dodol). Biotechnol. Biotechnol. Equip. 2021, 35, 478–486. [Google Scholar] [CrossRef]
- Nurhayati, R.; Herawati, E.R.N.; Putri, A.S.; Adyana, G.P. The Addition of Sorbitol and Glycerol to Improve the Physicochemical and Sensory Characteristics of Chocolate Dodol. IOP Conf. Ser. Mater. Sci. Eng. 2019, 633, 012004. [Google Scholar] [CrossRef]
- Seow, E.; Tan, T.; Lee, L.K.; Easa, A.M. Effects of Honey Types and Heating Treatment on the Textural, Thermal, Microstructural, and Chemical Properties of Glutinous Rice Flour Gels. J. Texture Stud. 2020, 51, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Zahid, K.; Wahid, M.A.; Ahamad, N. Enzymatic Dodol. Bul. Teknol. MARDI 2012, 2, 113–117. [Google Scholar]
- Azman Hamzah, M.M.M.; Hussain, A. Algorithms Development in Detection of the Gelatinization Process during Enzymatic ‘Dodol’Processing. ITB J. IC 2007, 1, 99–116. [Google Scholar] [CrossRef]
- Fathordoobady, F.; Mirhosseini, H.; Selamat, J.; Manap, M.Y.A. Effect of Solvent Type and Ratio on Betacyanins and Antioxidant Activity of Extracts from Hylocereus Polyrhizus Flesh and Peel by Supercritical Fluid Extraction and Solvent Extraction. Food Chem. 2016, 202, 70–80. [Google Scholar] [CrossRef] [PubMed]
- ASTM. Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM International: West Conchhocken, PA, USA, 2002. [Google Scholar]
- Kumaran, M.K. Interlaboratory Comparison of the ASTM Standard Test Methods for Water Vapor Transmission of Materials (E 96 95). J. Test. Eval. 1998, 26, 83–88. [Google Scholar]
- Tenore, G.C.; Novellino, E.; Basile, A. Nutraceutical Potential and Antioxidant Benefits of Red Pitaya (Hylocereus Polyrhizus) Extracts. J. Funct. Foods 2012, 4, 129–136. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Properties and Antioxidant Activity of Fish Skin Gelatin Film Incorporated with Citrus Essential Oils. Food Chem. 2012, 134, 1571–1579. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Cunniff, P.; Washington, D. Official Methods of Analysis of Aoac International. J. AOAC Int. 1997, 80, 127A. [Google Scholar]
- Pattarasiriroj, K.; Kaewprachu, P.; Rawdkuen, S. Properties of Rice Flour-Gelatine-Nanoclay Film with Catechin-Lysozyme and Its Use for Pork Belly Wrapping. Food Hydrocoll. 2020, 107, 105951. [Google Scholar] [CrossRef]
- Zhu, F. Interactions between Starch and Phenolic Compound. Trends Food Sci. Technol. 2015, 43, 129–143. [Google Scholar] [CrossRef]
- Giuberti, G.; Rocchetti, G.; Lucini, L. Interactions between Phenolic Compounds, Amylolytic Enzymes and Starch: An Updated Overview. Curr. Opin. Food Sci. 2020, 31, 102–113. [Google Scholar] [CrossRef]
- Mu, J.; Wang, L.; Lv, J.; Chen, Z.; Brennan, M.; Ma, Q.; Wang, W.; Liu, W.; Wang, J.; Brennan, C. Phenolics from Sea Buckthorn (Hippophae Rhamnoides L.) Modulate Starch Digestibility through Physicochemical Modifications Brought about by Starch—Phenolic Molecular Interactions. LWT 2022, 165, 113682. [Google Scholar] [CrossRef]
- Tarique, J.; Zainudin, E.S.; Sapuan, S.M.; Ilyas, R.A.; Khalina, A. Physical, Mechanical, and Morphological Performances of Arrowroot (Maranta Arundinacea) Fiber Reinforced Arrowroot Starch Biopolymer Composites. Polymers 2022, 14, 388. [Google Scholar] [CrossRef]
- Versino, F.; García, M.A. Cassava (Manihot Esculenta) Starch Films Reinforced with Natural Fibrous Filler. Ind. Crops Prod. 2014, 58, 305–314. [Google Scholar] [CrossRef]
- Jumaidin, R.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Thermal, Mechanical, and Physical Properties of Seaweed/Sugar Palm Fibre Reinforced Thermoplastic Sugar Palm Starch/Agar Hybrid Composites. Int. J. Biol. Macromol. 2017, 97, 606–615. [Google Scholar] [CrossRef]
- Shubhra, Q.T.H.; Alam, A.; Quaiyyum, M.A. Mechanical Properties of Polypropylene Composites: A Review. J. Thermoplast. Compos. Mater. 2011, 26, 362–391. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Tessaro, L.; Ramos, A.P.; Tapia-Blácido, D.R. Which Plasticizer Is Suitable for Films Based on Babassu Starch Isolated by Different Methods? Food Hydrocoll. 2019, 89, 143–152. [Google Scholar] [CrossRef]
- Lin, L.; Peng, S.; Shi, C.; Li, C.; Hua, Z.; Cui, H. Preparation and Characterization of Cassava Starch/Sodium Carboxymethyl Cellulose Edible Film Incorporating Apple Polyphenols. Int. J. Biol. Macromol. 2022, 212, 155–164. [Google Scholar] [CrossRef]
- Homthawornchoo, W.; Han, J.; Kaewprachu, P.; Romruen, O.; Rawdkuen, S. Green Tea Extract Enrichment: Mechanical and Physicochemical Properties Improvement of Rice Starch-Pectin Composite Film. Polymers 2022, 14, 2696. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Yang, Y.; Wang, C.; Zhang, T. Formation and Characterization of Noncovalent Ternary Complexes Based on Whey Protein Concentrate, High Methoxyl Pectin, and Phenolic Acid. J. Dairy Sci. 2022, 105, 2963–2977. [Google Scholar] [CrossRef] [PubMed]
- Bertuzzi, M.A.; Castro Vidaurre, E.F.; Armada, M.; Gottifredi, J.C. Water Vapor Permeability of Edible Starch Based Films. J. Food Eng. 2007, 80, 972–978. [Google Scholar] [CrossRef]
- Luo, D.; Xie, Q.; Gu, S.; Xue, W. Potato Starch Films by Incorporating Tea Polyphenol and MgO Nanoparticles with Enhanced Physical, Functional and Preserved Properties. Int. J. Biol. Macromol. 2022, 221, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical Properties and Antioxidant Activity of Gelatin-Sodium Alginate Edible Films with Tea Polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Talón, E.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of the Incorporation of Antioxidants on Physicochemical and Antioxidant Properties of Wheat Starch–Chitosan Films. J. Food Eng. 2013, 118, 271–278. [Google Scholar] [CrossRef]
- Gao, L.; Liu, P.; Liu, L.; Li, S.; Zhao, Y.; Xie, J.; Xu, H. κ-Carrageenan-Based PH-Sensing Films Incorporated with Anthocyanins or/and Betacyanins Extracted from Purple Sweet Potatoes and Peels of Dragon Fruits. Process Biochem. 2022, 121, 463–480. [Google Scholar] [CrossRef]
- Som, A.M.; Ahmat, N.; Abdul Hamid, H.A.; Azizuddin, N.M. A Comparative Study on Foliage and Peels of Hylocereus Undatus (White Dragon Fruit) Regarding Their Antioxidant Activity and Phenolic Content. Heliyon 2019, 5, e01244. [Google Scholar] [CrossRef]
- Nur Hanani, Z.A.; Aelma Husna, A.B.; Nurul Syahida, S.; Nor Khaizura, M.A.B.; Jamilah, B. Effect of Different Fruit Peels on the Functional Properties of Gelatin/Polyethylene Bilayer Films for Active Packaging. Food Packag. Shelf Life 2018, 18, 201–211. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible Cassava Starch Films Carrying Rosemary Antioxidant Extracts for Potential Use as Active Food Packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Azlim, N.A.; Mohammadi Nafchi, A.; Oladzadabbasabadi, N.; Ariffin, F.; Ghalambor, P.; Jafarzadeh, S.; Al-Hassan, A.A. Fabrication and Characterization of a PH-sensitive Intelligent Film Incorporating Dragon Fruit Skin Extract. Food Sci. Nutr. 2022, 10, 597–608. [Google Scholar] [CrossRef]
- Meng, S.; Liu, Z.; Zhao, X.; Fan, B.; Liu, H.; Guo, M.; Hao, H. Efficient Corrosion Inhibition by Sugarcane Purple Rind Extract for Carbon Steel in HCl Solution: Mechanism Analyses by Experimental and in Silico Insights. RSC Adv. 2021, 11, 31693–31711. [Google Scholar] [CrossRef]
- Fan, B.; Liu, Z.; Zhao, X.; Liu, H.; Fan, G.; Hao, H. Fabrication, Characterization and Efficient Surface Protection Mechanism of Poly(Trans-Cinnamaldehyde) Electropolymerized Coatings for EH36 Steel in Simulated Seawater. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127434. [Google Scholar] [CrossRef]
- Kroh, L.W. Caramelisation in Food and Beverages. Food Chem. 1994, 51, 373–379. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Lime Peel Pectin Integrated with Coconut Water and Lime Peel Extract as a New Bioactive Film Sachet to Retard Soybean Oil Oxidation. Food Hydrocoll. 2019, 97, 105173. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Tang, J.; Adhikari, B.; Cao, P. Innovative Technologies for Producing and Preserving Intermediate Moisture Foods: A Review. Food Res. Int. 2019, 116, 90–102. [Google Scholar] [CrossRef]
- Vermeulen, A.; Marvig, C.L.; Daelman, J.; Xhaferi, R.; Nielsen, D.S.; Devlieghere, F. Strategies to Increase the Stability of Intermediate Moisture Foods towards Zygosaccharomyces Rouxii: The Effect of Temperature, Ethanol, PH and Water Activity, with or without the Influence of Organic Acids. Food Microbiol. 2015, 45, 119–125. [Google Scholar] [CrossRef]
- Lu, N.; Zhang, L.; Zhang, X.; Li, J.; Labuza, T.P.; Zhou, P. Molecular Migration in High-Protein Intermediate-Moisture Foods during the Early Stage of Storage: Variations between Dairy and Soy Proteins and Effects on Texture. Food Res. Int. 2016, 82, 34–43. [Google Scholar] [CrossRef]
- Kaur, K.; Singh, N. Amylose-Lipid Complex Formation during Cooking of Rice Flour. Food Chem. 2000, 71, 511–517. [Google Scholar] [CrossRef]
- Faas, N.; Röcker, B.; Smrke, S.; Yeretzian, C.; Yildirim, S. Prevention of Lipid Oxidation in Linseed Oil Using a Palladium-Based Oxygen Scavenging Film. Food Packag. Shelf Life 2020, 24, 100488. [Google Scholar] [CrossRef]
- Denchai, N.; Suwannaporn, P.; Lin, J.; Soontaranon, S.; Kiatponglarp, W.; Huang, T. Retrogradation and Digestibility of Rice Starch Gels: The Joint Effect of Degree of Gelatinization and Storage. J. Food Sci. 2019, 84, 1400–1410. [Google Scholar] [CrossRef]
- Chao, C.; Huang, S.; Yu, J.; Copeland, L.; Wang, S.; Wang, S. Molecular Mechanisms Underlying the Formation of Starch-Lipid Complexes during Simulated Food Processing: A Dynamic Structural Analysis. Carbohydr. Polym. 2020, 244, 116464. [Google Scholar] [CrossRef] [PubMed]


| Film Sample (%, w/v) | Thickness (mm) | TS (MPa) | EAB (%) | FS (%) | WVP |
|---|---|---|---|---|---|
| PP | 0.036 ± 0.003 c | 42.91 ± 4.35 a | 606.08 ± 88.77 a | 39.50 ± 7.48 b | 0.08 ± 0.01 c |
| 0% DPE | 0.049 ± 0.002 b | 5.72 ± 1.42 b | 28.06 ± 1.41 b | 74.31 ± 2.94 a | 9.57 ± 1.13 a |
| 2% DPE | 0.075 ± 0.001 a | 1.23 ± 0.38 c | 25.28 ± 1.19 c | 76.00 ± 3.22 a | 5.22 ± 0.22 b |
| Film Sample (%, w/v) | Appearance | L* | a* | b* | ΔE* |
|---|---|---|---|---|---|
| PP |  | 82.60 ± 0.34 b | 0.62 ± 0.01 b | −6.74 ± 0.06 c | 34.94 ± 0.15 b |
| 0% DPE (Control) |  | 83.09 ± 0.13 a | 0.37 ± 0.01 c | −3.04 ± 0.13 b | 32.53 ± 0.16 c |
| 2% DPE |  | 34.48 ± 0.21 c | 49.87 ± 0.25 a | 8.54 ± 0.22 a | 50.37 ± 0.21 a |
| Film Sample (%, w/v) | TPC (mg GAE/g) | TBC (mg/g) | FRAP (mM Fe (II)/g) | DPPH (mM Trolox/g) |
|---|---|---|---|---|
| PP | ND | ND | ND | ND |
| 0% DPE | ND | ND | ND | ND |
| 2% DPE | 18.18 ± 0.78 a | 0.91 ± 0.01 a | 30.08 ± 2.68 a | 194.50 ± 7.43 a |
| Day | Hardness (N) | Springiness (%) | ||||
|---|---|---|---|---|---|---|
| PP | 0% DPE | 2% DPE | PP | 0% DPE | 2% DPE | |
| 0 | 5.57 ± 0.86 eA | 6.36 ± 1.33 cA | 6.54 ± 1.71 cA | 92.75 ± 4.19 aA | 93.65 ± 4.69 aA | 90.06 ± 3.26 bA |
| 1 | 7.30 ± 0.75 dA | 6.67 ± 1.06 cA | 5.91 ± 1.18 cA | 95.91 ± 3.22 aA | 94.53 ± 2.81 aA | 96.97 ± 4.77 abA |
| 3 | 9.34 ± 0.89 cdA | 6.82 ± 1.32 cB | 6.80 ± 2.22 bcAB | 95.22 ± 4.44 aA | 97.88 ± 2.72 aA | 97.91 ± 3.63 aA |
| 5 | 8.28 ± 1.54 cA | 10.21 ± 1.71 bA | 10.24 ± 1.58 bA | 94.11 ± 3.54 aA | 94.31 ± 0.97 aA | 99.42 ± 5.10 aA |
| 7 | 13.78 ± 2.69 bB | 23.65 ± 2.88 aA | 17.52 ± 2.63 aAB | 96.90 ± 3.86 aA | 97.28 ± 5.24 aA | 98.96 ± 3.53 aA |
| 9 | 19.71 ± 2.53 aB | 27.15 ± 2.98 aA | 16.62 ± 1.14 aB | 95.53 ± 4.16 aA | 95.94 ± 3.53 aA | 99.9 ± 1.34 aA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Homthawornchoo, W.; Hakimi, N.F.S.M.; Romruen, O.; Rawdkuen, S. Dragon Fruit Peel Extract Enriched-Biocomposite Wrapping Film: Characterization and Application on Coconut Milk Candy. Polymers 2023, 15, 404. https://doi.org/10.3390/polym15020404
Homthawornchoo W, Hakimi NFSM, Romruen O, Rawdkuen S. Dragon Fruit Peel Extract Enriched-Biocomposite Wrapping Film: Characterization and Application on Coconut Milk Candy. Polymers. 2023; 15(2):404. https://doi.org/10.3390/polym15020404
Chicago/Turabian StyleHomthawornchoo, Wantida, Nur Fairuza Syahira Mohamad Hakimi, Orapan Romruen, and Saroat Rawdkuen. 2023. "Dragon Fruit Peel Extract Enriched-Biocomposite Wrapping Film: Characterization and Application on Coconut Milk Candy" Polymers 15, no. 2: 404. https://doi.org/10.3390/polym15020404
APA StyleHomthawornchoo, W., Hakimi, N. F. S. M., Romruen, O., & Rawdkuen, S. (2023). Dragon Fruit Peel Extract Enriched-Biocomposite Wrapping Film: Characterization and Application on Coconut Milk Candy. Polymers, 15(2), 404. https://doi.org/10.3390/polym15020404