Self-Assembled Nanofibrous Membranes by Electrospinning as Efficient Dye Photocatalysts for Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
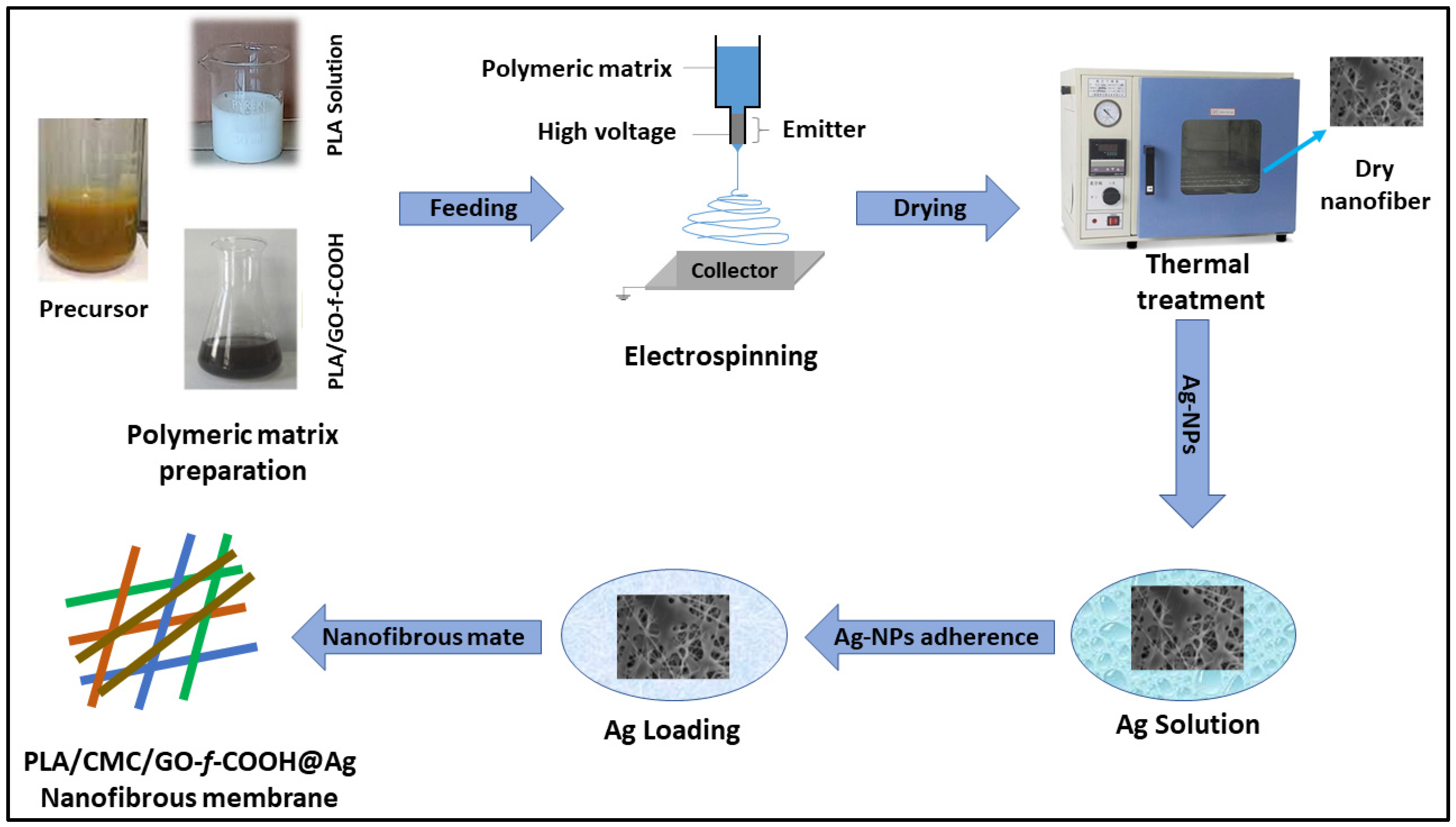
2.2.1. Preparation of Polymeric Matrix Solution
2.2.2. Fabrication of Electrospun Nanofibrous Membrane
2.2.3. Preparation of Nanofibrous Membrane
2.3. Characterizations
2.4. Photocatalytic Tests
3. Results and Discussion
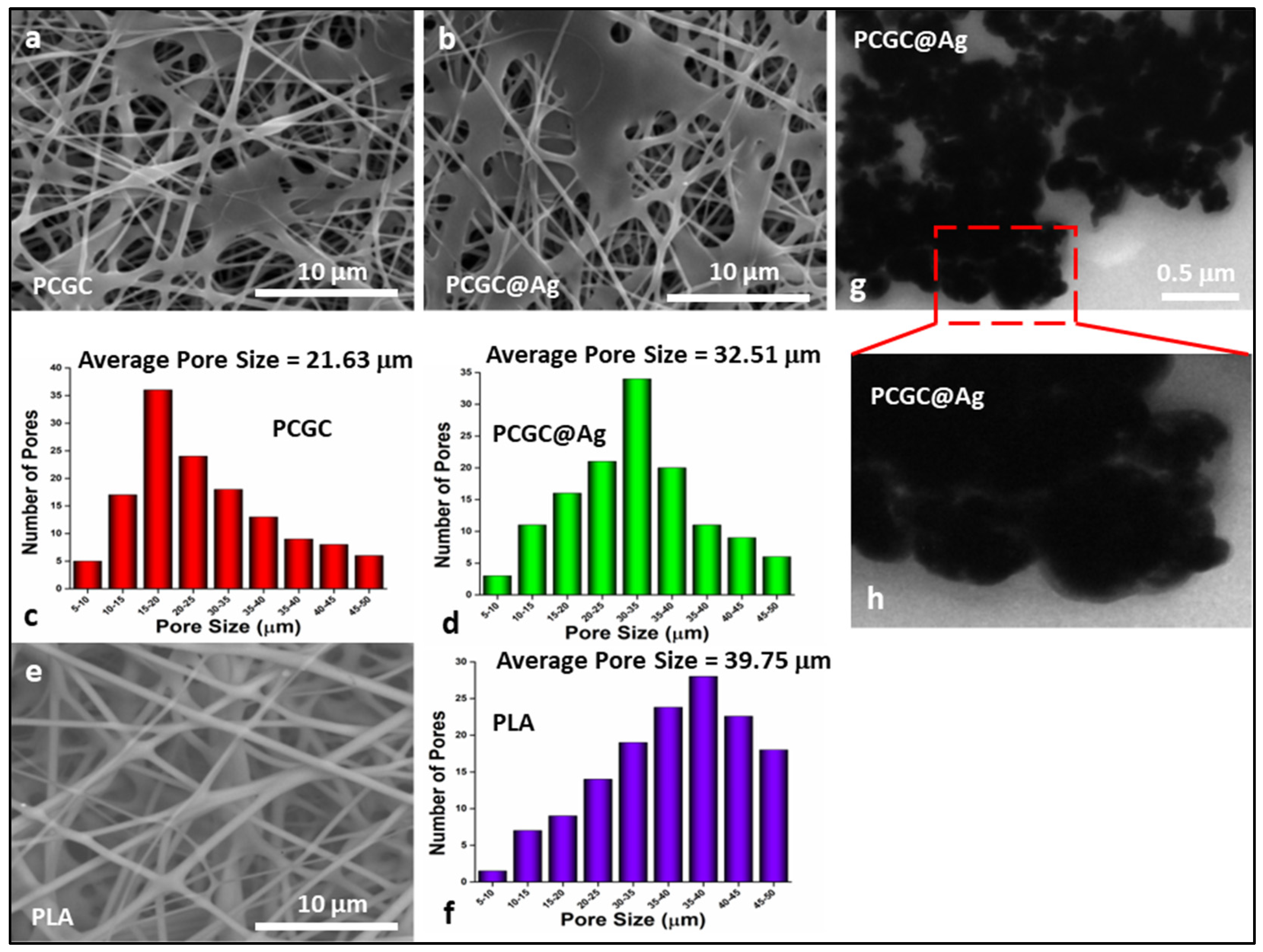
3.1. Surface Morphology
3.2. TEM Analysis
3.3. FTIR Analysis
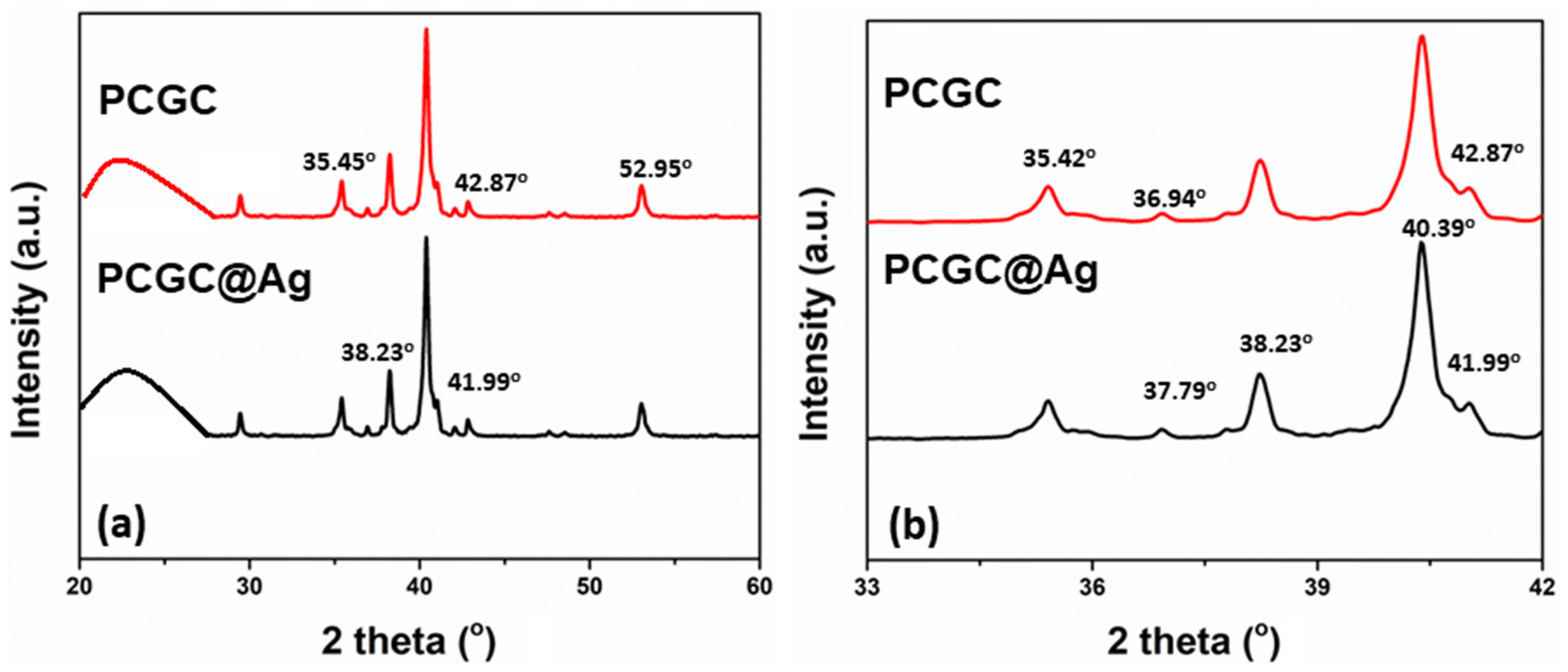
3.4. XRD Analysis
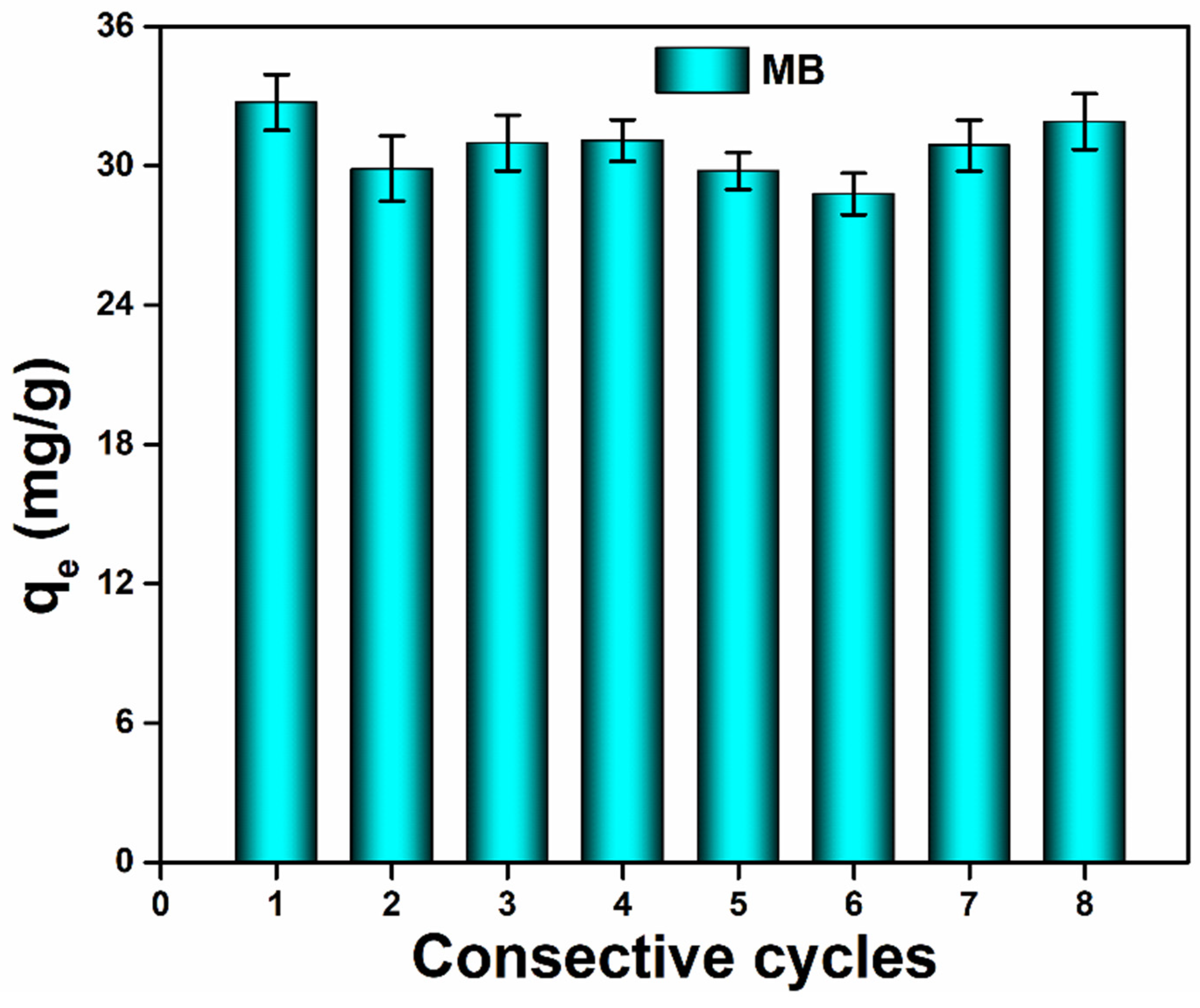
3.5. Photocatalytic Efficiency
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sonone, S.S.; Jadhav, S.; Sankhla, M.S.; Kumar, R. Water contamination by heavy metals and their toxic effect on aquaculture and human health through food Chain. Lett. Appl. NanoBioSci. 2020, 10, 2148–2166. [Google Scholar]
- Chowdhary, P.; Bharagava, R.N.; Mishra, S.; Khan, N. Role of Industries in Water Scarcity and Its Adverse Effects on Environment and Human Health. In Environmental Concerns and Sustainable Development; Springer: Berlin/Heidelberg, Germany, 2020; pp. 235–256. [Google Scholar]
- Mishra, S.; Bharagava, R.N.; More, N.; Yadav, A.; Zainith, S.; Mani, S.; Chowdhary, P. Heavy Metal Contamination: An Alarming Threat to Environment and Human Health. In Environmental Biotechnology: For Sustainable Future; Springer: Berlin/Heidelberg, Germany, 2019; pp. 103–125. [Google Scholar]
- Yap, P.L.; Kabiri, S.; Tran, D.N.; Losic, D. Multifunctional binding chemistry on modified graphene composite for selective and highly efficient adsorption of mercury. ACS Appl. Mater. Interfaces 2018, 11, 6350–6362. [Google Scholar] [CrossRef] [PubMed]
- Abd Rani, U.; Ng, L.Y.; Ng, C.Y.; Mahmoudi, E. A review of carbon quantum dots and their applications in wastewater treatment. Adv. Colloid Interface Sci. 2020, 278, 102124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Meng, Y.; Liu, Z.; Li, D.; Bai, Y.; Yuan, G.; Wang, Y.; Zhang, X.; Li, X. Pyro-catalysis for tooth whitening via oral temperature fluctuation. Nat. Commun. 2022, 13, 1–13. [Google Scholar] [CrossRef]
- Aslam Khan, M.U.; Al-Arjan, W.S.; Binkadem, M.S.; Mehboob, H.; Haider, A.; Raza, M.A.; Abd Razak, S.I.; Hasan, A.; Amin, R. Development of biopolymeric hybrid scaffold-based on AAc/GO/nHAp/TiO2 nanocomposite for bone tissue engineering: In-vitro analysis. Nanomaterials 2021, 11, 1319. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Haider, S.; Shah, S.A.; Abd Razak, S.I.; Hassan, S.A.; Kadir, M.R.A.; Haider, A. Arabinoxylan-co-AA/HAp/TiO2 nanocomposite scaffold a potential material for bone tissue engineering: An in vitro study. Int. J. Biol. Macromol. 2020, 151, 584–594. [Google Scholar] [CrossRef]
- Jafari, A.J.; Moslemzadeh, M.; Esrafili, A.; Kalantary, R.R. Synthesis of new composite based on TiO2 immobilized in glass fibers for photo-catalytic degradation of chlorobenzene in aqueous solutions. Environ. Res. 2022, 204, 112018. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Abd Razak, S.I.; Mehboob, H.; Abdul Kadir, M.R.; Anand, T.J.S.; Inam, F.; Shah, S.A.; Abdel-Haliem, M.E.; Amin, R. Synthesis and characterization of silver-coated polymeric scaffolds for bone tissue engineering: Antibacterial and in vitro evaluation of cytotoxicity and biocompatibility. ACS Omega 2021, 6, 4335–4346. [Google Scholar] [CrossRef]
- Zhou, T.; Prasher, S.; Qi, Z.; George, S.; Mawof, A.; Nzediegwu, C.; Dhiman, J.; Patel, R. Impact of silver nanoparticles in wastewater on heavy metal transport in soil and uptake by radish plants. Water Air Soil Pollut. 2021, 232, 1–13. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Al-Thebaiti, M.A.; Hashmi, M.U.; Aftab, S.; Abd Razak, S.I.; Abu Hassan, S.; Abdul Kadir, M.R.; Amin, R. Synthesis of silver-coated bioactive nanocomposite scaffolds based on grafted beta-glucan/hydroxyapatite via freeze-drying method: Anti-microbial and biocompatibility evaluation for bone tissue engineering. Materials 2020, 13, 971. [Google Scholar] [CrossRef]
- Hassan, G.K.; Mahmoud, W.H.; Al-sayed, A.; Ismail, S.H.; El-Sherif, A.A.; Abd El Wahab, S. Multi-functional of TiO2@ Ag core–shell nanostructure to prevent hydrogen sulfide formation during anaerobic digestion of sewage sludge with boosting of bio-CH4 production. Fuel 2023, 333, 126608. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Abd Razak, S.I.; Haider, S.; Mannan, H.A.; Hussain, J.; Hasan, A. Sodium alginate-f-GO composite hydrogels for tissue regeneration and antitumor applications. Int. J. Biol. Macromol. 2022, 208, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Farjadian, F.; Abbaspour, S.; Sadatlu, M.A.A.; Mirkiani, S.; Ghasemi, A.; Hoseini-Ghahfarokhi, M.; Mozaffari, N.; Karimi, M.; Hamblin, M.R. Recent developments in graphene and graphene oxide: Properties, synthesis, and modifications: A review. ChemistrySelect 2020, 5, 10200–10219. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Koduru, J.R.; Karri, R.R. A comprehensive review of applications of magnetic graphene oxide based nanocomposites for sustainable water purification. J. Environ. Manag. 2019, 231, 622–634. [Google Scholar] [CrossRef]
- Karkooti, A.; Yazdi, A.Z.; Chen, P.; McGregor, M.; Nazemifard, N.; Sadrzadeh, M. Development of advanced nanocomposite membranes using graphene nanoribbons and nanosheets for water treatment. J. Membr. Sci. 2018, 560, 97–107. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, L.; Kang, J.; Kim, K.H. Facile synthesis of Manganese selenide anchored in Three-Dimensional carbon nanosheet matrix with enhanced Lithium storage properties. Chem. Eng. J. 2021, 423, 130243. [Google Scholar] [CrossRef]
- Aslam Khan, M.U.; Haider, A.; Abd Razak, S.I.; Abdul Kadir, M.R.; Haider, S.; Shah, S.A.; Hasan, A.; Khan, R.; Khan, S.-U.D.; Shakir, I. Arabinoxylan/graphene-oxide/nHAp-NPs/PVA bionano composite scaffolds for fractured bone healing. J. Tissue Eng. Regen. Med. 2021, 15, 322–335. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Yaqoob, Z.; Ansari, M.N.M.; Razak, S.I.A.; Raza, M.A.; Sajjad, A.; Haider, S.; Busra, F.M. Chitosan/poly vinyl alcohol/graphene oxide based pH-responsive composite hydrogel films: Drug release, anti-microbial and cell viability studies. Polymers 2021, 13, 3124. [Google Scholar] [CrossRef]
- Al-Arjan, W.S.; Aslam Khan, M.U.; Nazir, S.; Abd Razak, S.I.; Abdul Kadir, M.R. Development of arabinoxylan-reinforced apple pectin/graphene oxide/nano-hydroxyapatite based nanocomposite scaffolds with controlled release of drug for bone tissue engineering: In-vitro evaluation of biocompatibility and cytotoxicity against MC3T3-E1. Coatings 2020, 10, 1120. [Google Scholar] [CrossRef]
- Naeem, H.; Ajmal, M.; Qureshi, R.B.; Muntha, S.T.; Farooq, M.; Siddiq, M. Facile synthesis of graphene oxide–silver nanocomposite for decontamination of water from multiple pollutants by adsorption, catalysis and antibacterial activity. J. Environ. Manag. 2019, 230, 199–211. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Haider, S.; Raza, M.A.; Shah, S.A.; Abd Razak, S.I.; Kadir, M.R.A.; Subhan, F.; Haider, A. Smart and pH-sensitive rGO/Arabinoxylan/chitosan composite for wound dressing: In-vitro drug delivery, antibacterial activity, and biological activities. Int. J. Biol. Macromol. 2021, 192, 820–831. [Google Scholar] [CrossRef] [PubMed]
- El-Aswar, E.I.; Ramadan, H.; Elkik, H.; Taha, A.G. A comprehensive review on preparation, functionalization and recent applications of nanofiber membranes in wastewater treatment. J. Environ. Manag. 2022, 301, 113908. [Google Scholar] [CrossRef] [PubMed]
- Orth, E.S.; Fonsaca, J.E.; Domingues, S.H.; Mehl, H.; Oliveira, M.M.; Zarbin, A.J. Targeted thiolation of graphene oxide and its utilization as precursor for graphene/silver nanoparticles composites. Carbon 2013, 61, 543–550. [Google Scholar] [CrossRef]
- Benslimane, A.; Bahlouli, I.M.; Bekkour, K.; Hammiche, D. Thermal gelation properties of carboxymethyl cellulose and bentonite-carboxymethyl cellulose dispersions: Rheological considerations. Appl. Clay Sci. 2016, 132, 702–710. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Akhtar, M.J.; Cleymand, F.; Desobry, S. Structural, mechanical and barrier properties of active PLA–antioxidant films. J. Food Eng. 2012, 110, 380–389. [Google Scholar] [CrossRef]
- Narayan, R.; Kim, J.E.; Kim, J.Y.; Lee, K.E.; Kim, S.O. Graphene oxide liquid crystals: Discovery, evolution and applications. Adv. Mater. 2016, 28, 3045–3068. [Google Scholar] [CrossRef]
- Zeng, H.; Wang, L.; Zhang, D.; Yan, P.; Nie, J.; Sharma, V.K.; Wang, C. Highly efficient and selective removal of mercury ions using hyperbranched polyethylenimine functionalized carboxymethyl chitosan composite adsorbent. Chem. Eng. J. 2019, 358, 253–263. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Z.; He, W.; Chen, M.; Tu, W.; Zhu, M.; Gan, D.; Liu, S. Multifunctional stable PDA/RGO/MOFs&SiO2-COOH membrane with excellent flux and anti-fouling performance for the separation of organic dye and oil/water. Surf. Interfaces 2022, 33, 102183. [Google Scholar]
- Liu, F.; Guo, R.; Shen, M.; Wang, S.; Shi, X. Effect of processing variables on the morphology of electrospun poly [(lactic acid)-co-(glycolic acid)] nanofibers. Macromol. Mater. Eng. 2009, 294, 666–672. [Google Scholar] [CrossRef]
- Hashemzaei, Z.; Saravani, H.; Sharifitabar, M.; Shahbakhsh, M. Copper nanowires/poly (naphtoquinone chromium (III)) for simultaneous voltammetric detection of para-aminophenol, phenol and para-nitrophenol. Microchem. J. 2022, 175, 107210. [Google Scholar] [CrossRef]
- Khan, Z.; Al-Zahrani, S.A.; AlSulami, Q.A.; Al-Thabaiti, S.A.; Al-Arjan, W.S. Effects of shape-controlling cationic and anionic surfactants on the morphology and surface resonance plasmon intensity of silver@ copper bimetallic nanoparticles. J. Mol. Liq. 2019, 275, 354–363. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Razak, S.I.A.; Ansari, M.N.M.; Zulkifli, R.M.; Ahmad Zawawi, N.; Arshad, M. Development of biodegradable bio-based composite for bone tissue engineering: Synthesis, characterization and in vitro biocompatible evaluation. Polymers 2021, 13, 3611. [Google Scholar] [CrossRef] [PubMed]
- Abou-Zeid, R.E.; Dacrory, S.; Ali, K.A.; Kamel, S. Novel method of preparation of tricarboxylic cellulose nanofiber for efficient removal of heavy metal ions from aqueous solution. Int. J. Biol. Macromol. 2018, 119, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Wang, W.; Jiao, T.; Ma, K.; Zhang, Q.; Hong, W.; Qiu, H.; Zhou, J.; Zhang, L.; Peng, Q. Bioinspired polydopamine sheathed nanofibers containing carboxylate graphene oxide nanosheet for high-efficient dyes scavenger. ACS Sustain. Chem. Eng. 2017, 5, 4948–4956. [Google Scholar] [CrossRef]
- Sa’adon, S.; Ansari, M.N.M.; Razak, S.I.A.; Anand, J.S.; Nayan, N.H.M.; Ismail, A.E.; Khan, M.U.A.; Haider, A. Preparation and physicochemical characterization of a diclofenac sodium-dual layer polyvinyl alcohol patch. Polymers 2021, 13, 2459. [Google Scholar] [CrossRef] [PubMed]
- Sertbakan, T.R.; Al-Shakarchi, E.K.; Mala, S.S. The Preparation of Nano Silver by Chemical Reduction Method. J. Mod. Phys. 2022, 13, 81–88. [Google Scholar] [CrossRef]
- Mohd, S.; Wani, A.A.; Khan, A.M. ZnO/POA functionalized metal-organic framework ZIF-8 nanomaterial for dye removal. Clean. Chem. Eng. 2022, 3, 100047. [Google Scholar] [CrossRef]
- Li, E.; Liao, L.; Lv, G.; Li, Z.; Yang, C.; Lu, Y. The interactions between three typical PPCPs and LDH. Front. Chem. 2018, 6, 16. [Google Scholar] [CrossRef]
- Song, J.; Xing, R.; Jiao, T.; Peng, Q.; Yuan, C.; Möhwald, H.; Yan, X. Crystalline dipeptide nanobelts based on solid–solid phase transformation self-assembly and their polarization imaging of cells. ACS Appl. Mater. Interfaces 2018, 10, 2368–2376. [Google Scholar] [CrossRef]
- El-Ghobashy, M.A.; Salem, I.A.; El-Dahrawy, W.M.; Salem, M.A. Fabrication of α-MnO2/Fe-Mn binary oxide nanocomposite as an efficient adsorbent for the removal of methylene blue from wastewater. J. Mol. Struct. 2023, 1272, 134118. [Google Scholar] [CrossRef]

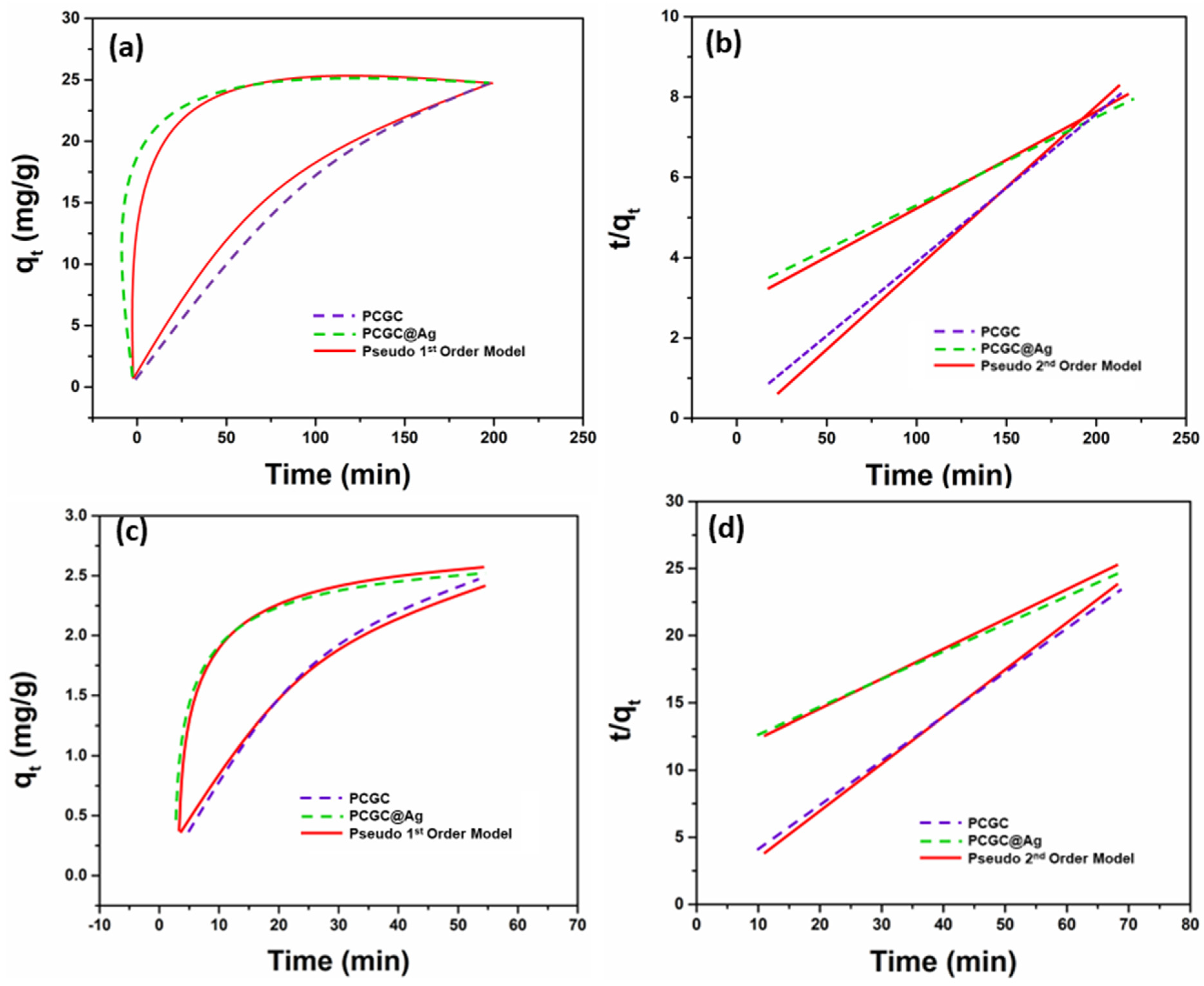
| Dye Name | Sample Name | Pseudo First Order | Pseudo Second Order | ||||
|---|---|---|---|---|---|---|---|
| qe (mg/g) | R2 | K1 (min−1) | qe (mg/g) | R2 | K1 (g/mg min) | ||
| MB | PCGC | 3.19 | 0.983581 | 0.0442 | 2.85 | 0.978329 | 0.0125 |
| PCGC@Ag | 3.37 | 0.992917 | 0.1445 | 2.49 | 0.989839 | 0.1072 | |
| Rh-B | PCGC | 23.93 | 0.973594 | 0.01149 | 33.62 | 0.994392 | 2.07 × 10−4 |
| PCGC@Ag | 22.78 | 0.989832 | 0.07376 | 21.49 | 0.998738 | 1.20 × 10−2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Arjan, W.S. Self-Assembled Nanofibrous Membranes by Electrospinning as Efficient Dye Photocatalysts for Wastewater Treatment. Polymers 2023, 15, 340. https://doi.org/10.3390/polym15020340
Al-Arjan WS. Self-Assembled Nanofibrous Membranes by Electrospinning as Efficient Dye Photocatalysts for Wastewater Treatment. Polymers. 2023; 15(2):340. https://doi.org/10.3390/polym15020340
Chicago/Turabian StyleAl-Arjan, Wafa Shamsan. 2023. "Self-Assembled Nanofibrous Membranes by Electrospinning as Efficient Dye Photocatalysts for Wastewater Treatment" Polymers 15, no. 2: 340. https://doi.org/10.3390/polym15020340
APA StyleAl-Arjan, W. S. (2023). Self-Assembled Nanofibrous Membranes by Electrospinning as Efficient Dye Photocatalysts for Wastewater Treatment. Polymers, 15(2), 340. https://doi.org/10.3390/polym15020340





