Palmitic Acid Inhibits the Growth and Metastasis of Gastric Cancer by Blocking the STAT3 Signaling Pathway
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
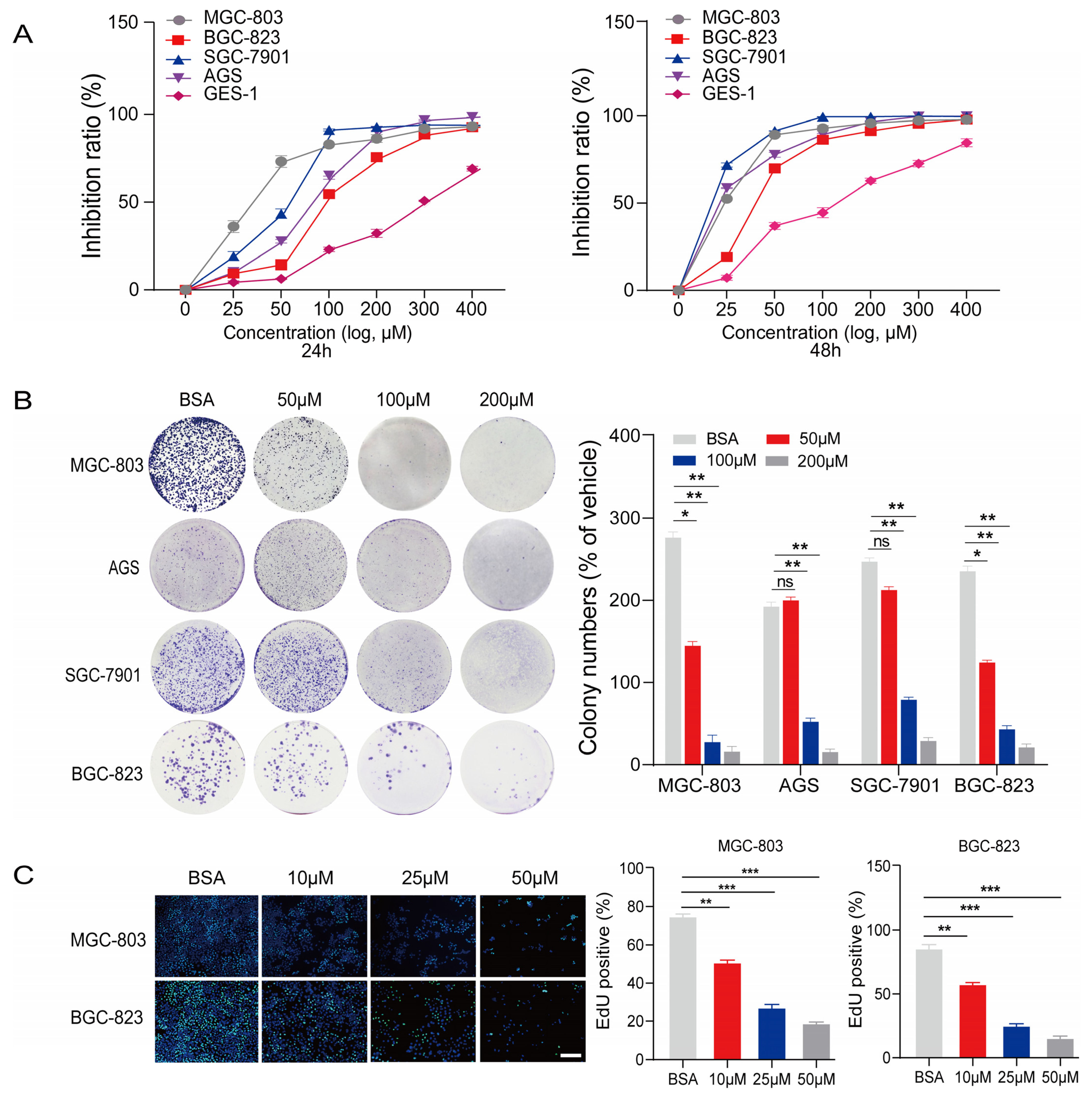
2.1. PA Inhibits the Proliferation and Anchorage-Independent Growth of Gastric Cancer Cells
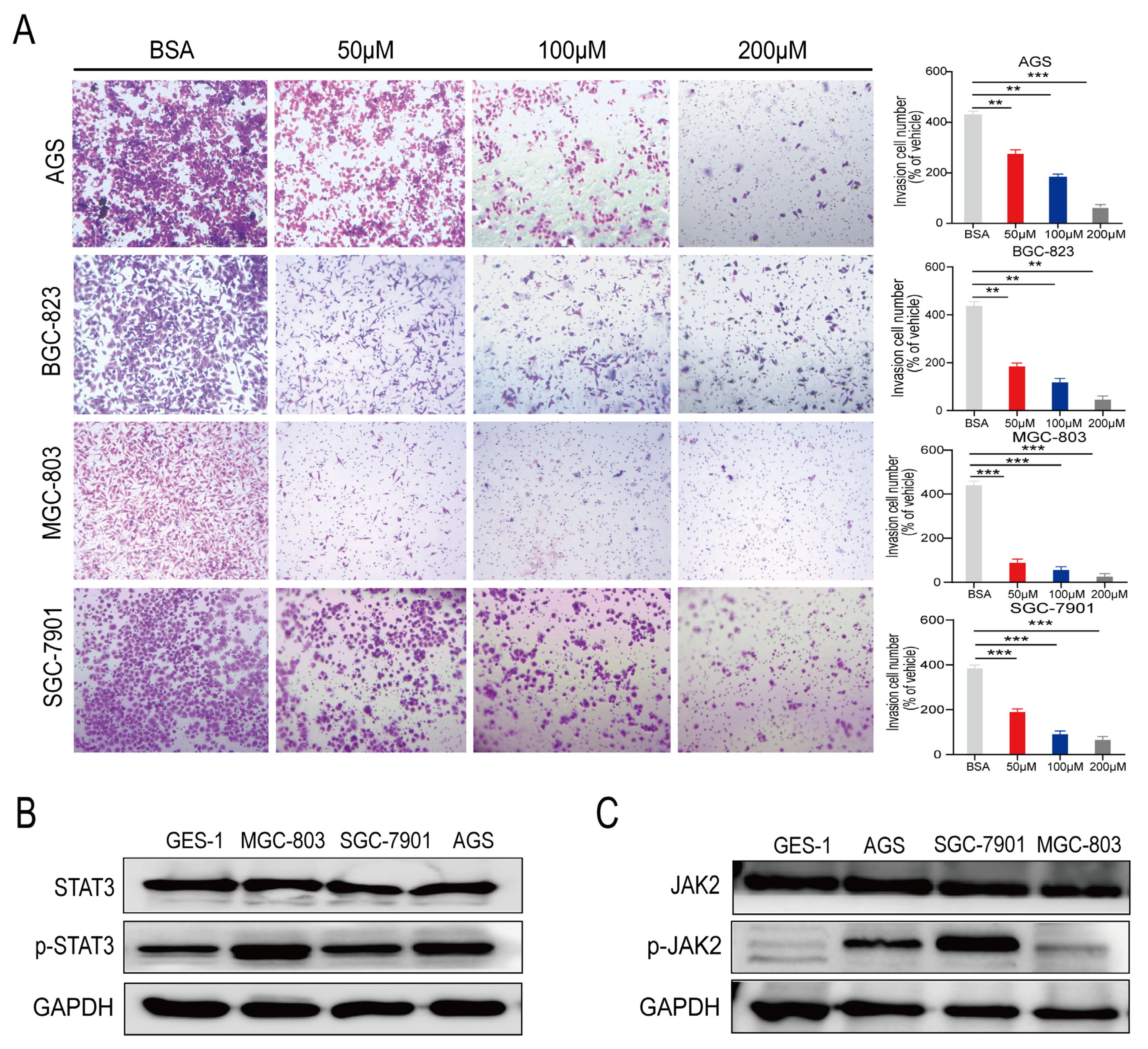
2.2. PA Inhibits the Invasion of Human Gastric Cancer Cells In Vitro
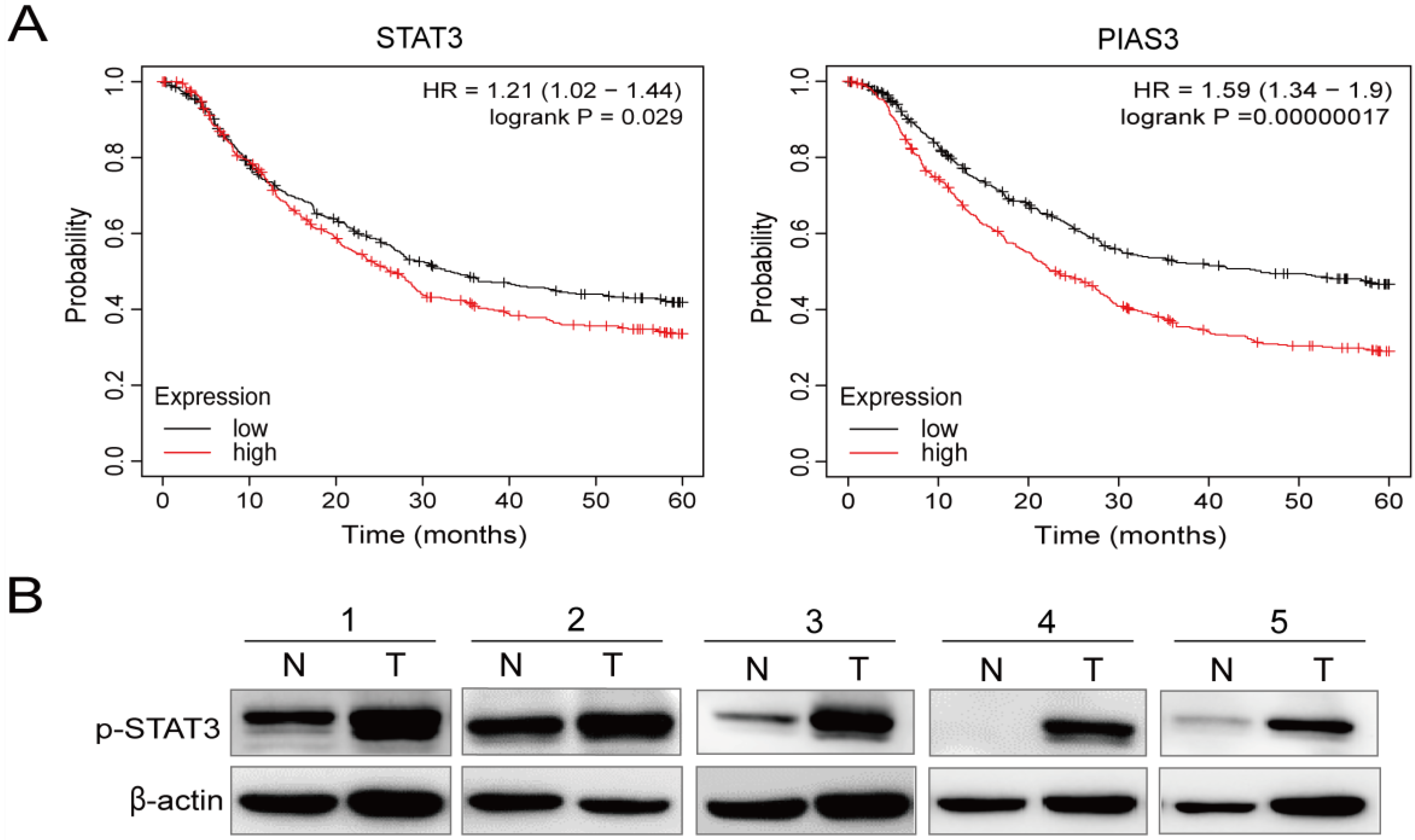
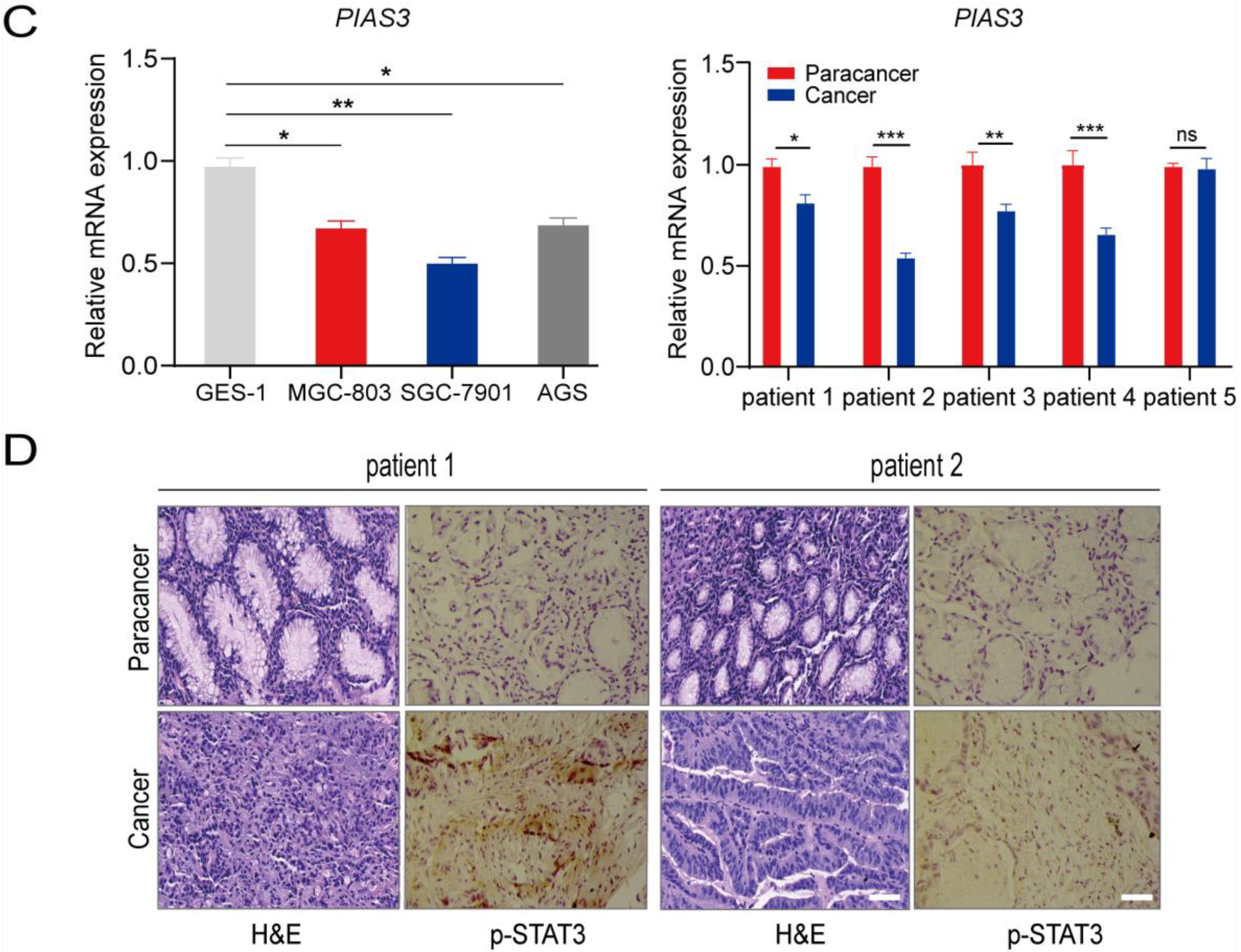
2.3. p-STAT3 Is Highly Expressed in Gastric Cancer Tissues and Cell Lines
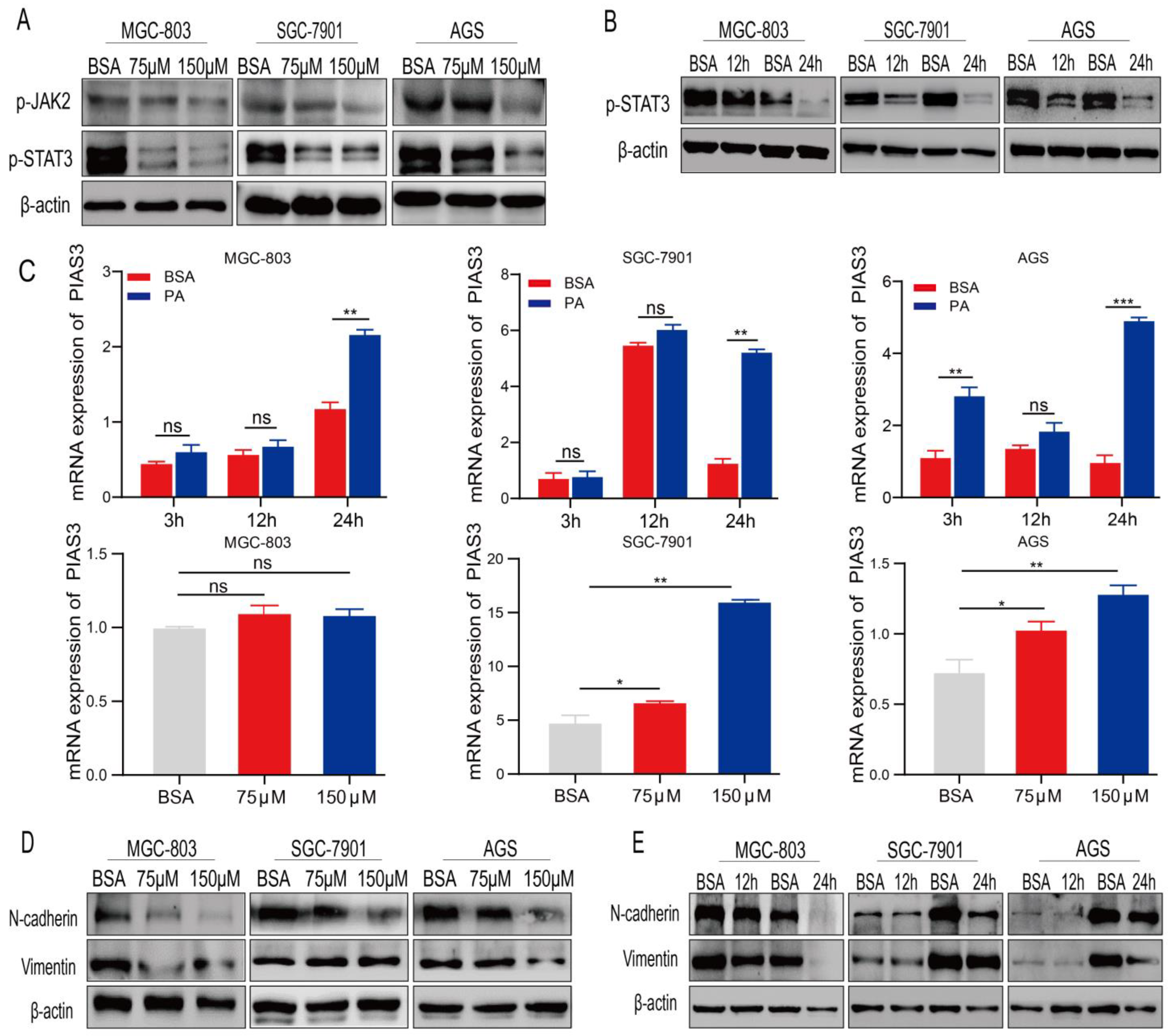
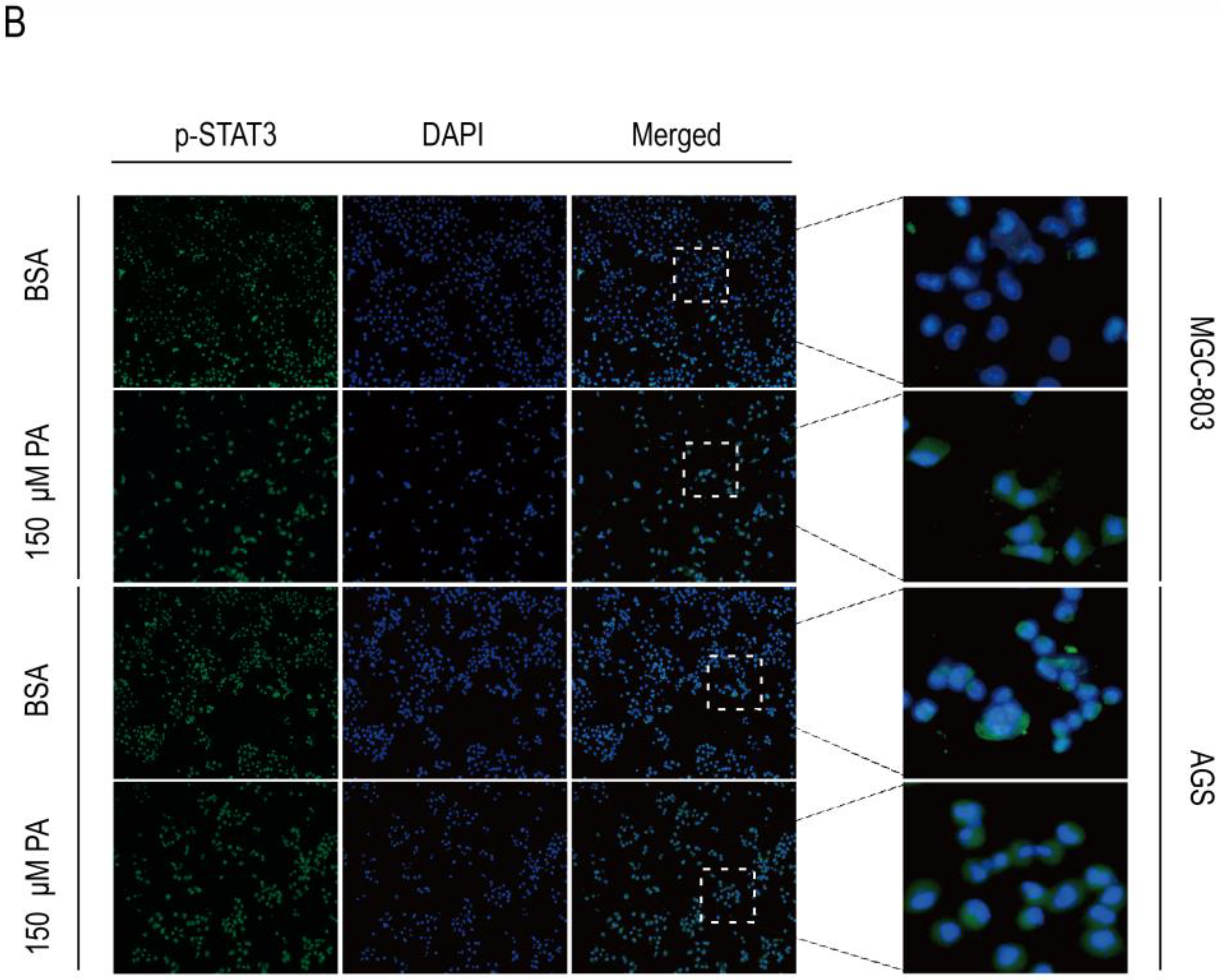
2.4. PA Regulates the Expression of p-STAT3 and EMT-Related Proteins
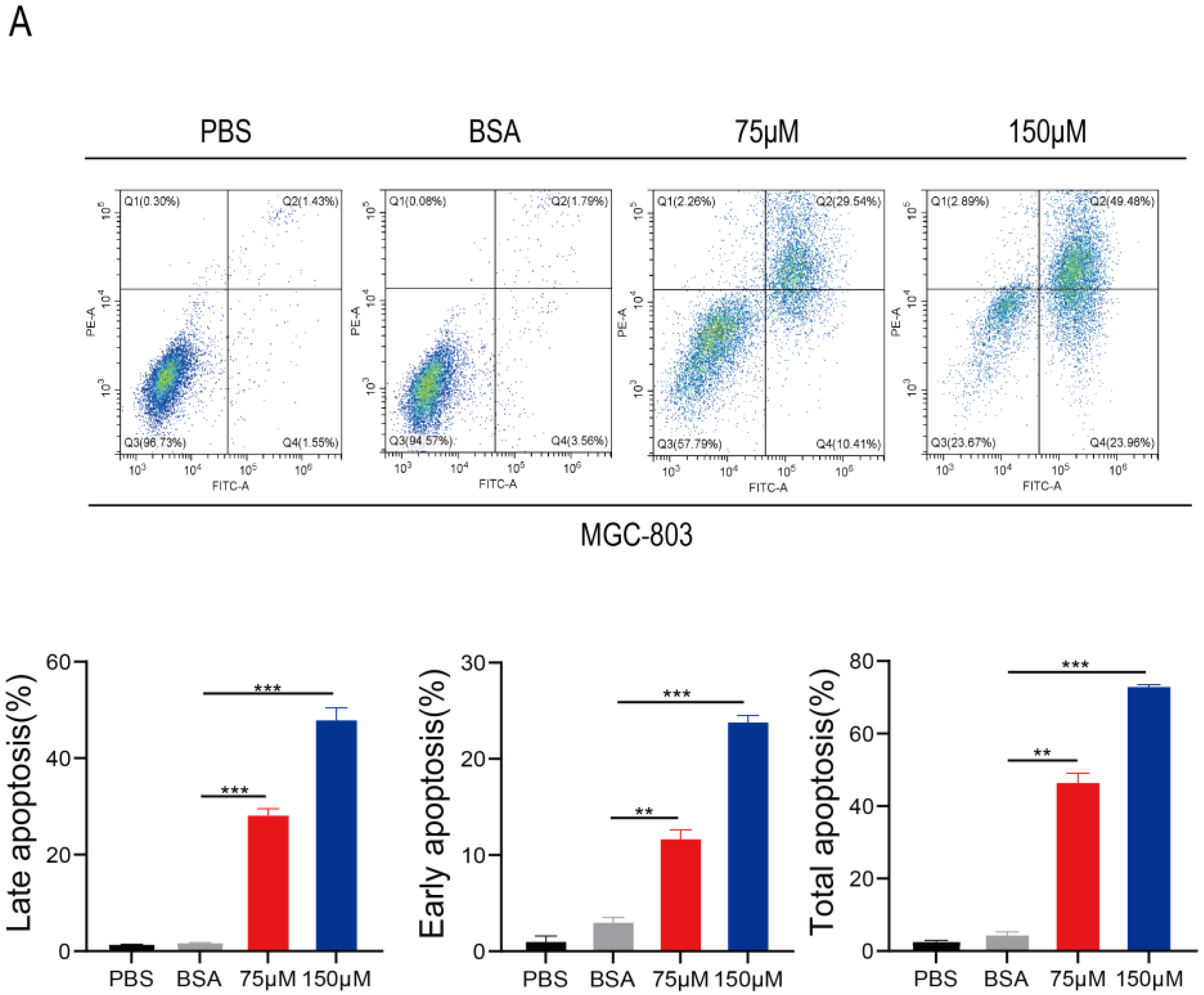
2.5. PA Induces Apoptosis and Inhibits p-STAT3 Expression in Human Gastric Cancer Cells
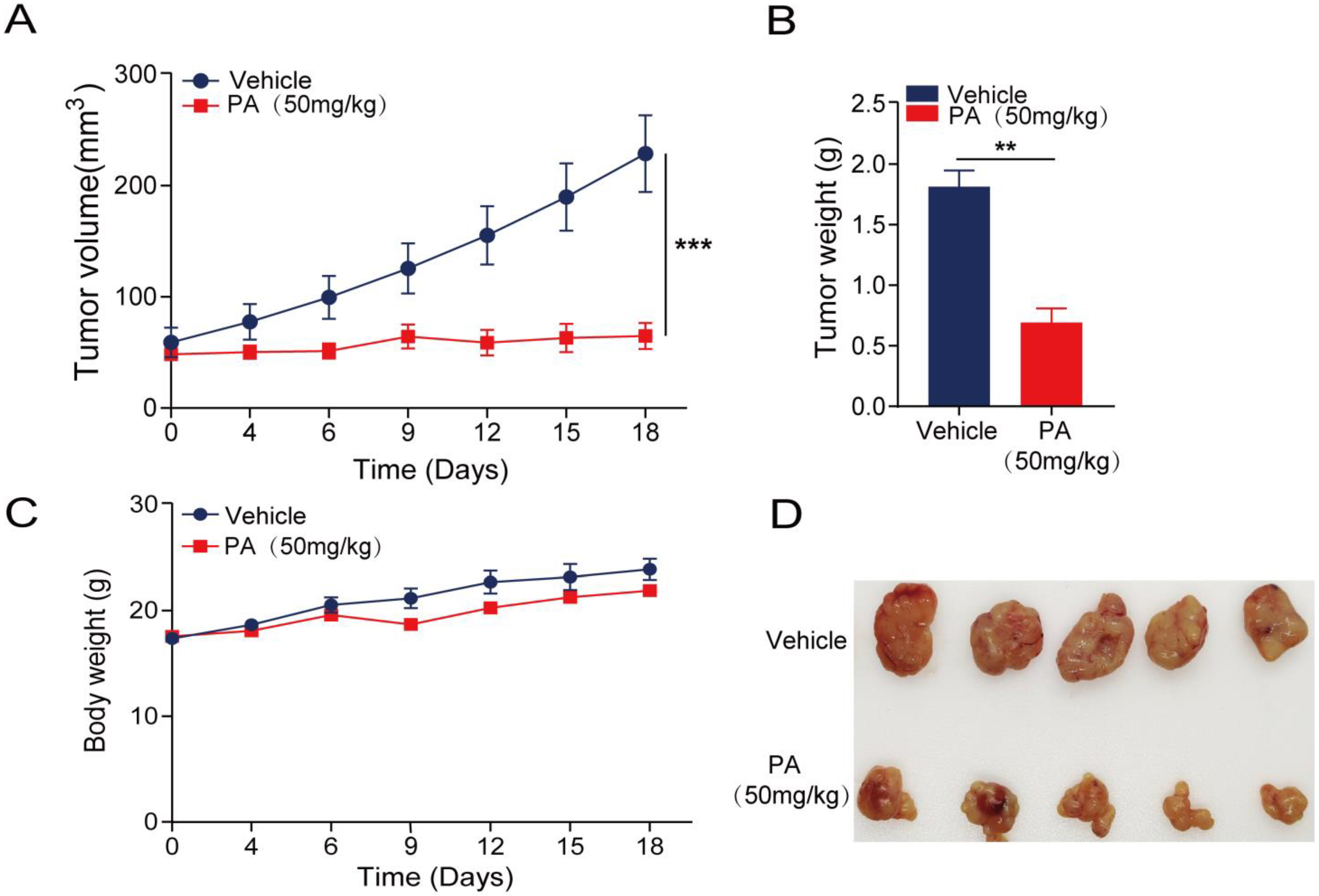
2.6. In Vivo Anticancer Activity of PA
3. Discussion
4. Materials and Methods
4.1. Collection of Clinical Tissue Samples
4.2. Cell Lines and Cell Culture
4.3. Drug Preparation
4.4. Cell Proliferation and Colony Formation Assay
4.5. EdU-DNA Assay
4.6. Transwell Cell Invasion Assay
4.7. WB Analysis
4.8. RNA Isolation and qRT-PCR
4.9. Flow Cytometric Analysis of Cell Apoptosis
4.10. Immunofluorescence Analysis
4.11. In Vivo Human Cancer Xenograft Models
4.12. In Vivo H&E Staining and Immunohistochemical Analysis
4.13. Kaplan–Meier Plotter Analysis
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ajani, J.A.; Lee, J.; Sano, T.; Janjigian, Y.Y.; Fan, D.; Song, S. Gastric adenocarcinoma. Nat. Rev. Dis. Primers 2017, 3, 17036. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yoon, C.; Xu, B.; Xie, J.; Li, P.; Zheng, C.; Huang, C.; Yoon, S.S. Long-Term Survival after Minimally Invasive Versus Open Gastrectomy for Gastric Adenocarcinoma: A Propensity Score-Matched Analysis of Patients in the United States and China. Ann. Surg. Oncol. 2020, 27, 802–811. [Google Scholar] [CrossRef]
- Chang, W.; Wang, H.; Kim, W.; Liu, Y.; Deng, H.; Liu, H.; Jiang, Z.; Niu, Z.; Sheng, W.; Nápoles, O.C.; et al. Hormonal Suppression of Stem Cells Inhibits Symmetric Cell Division and Gastric Tumorigenesis. Cell Stem Cell 2020, 26, 739–754.e738. [Google Scholar] [CrossRef]
- Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2020, 70, 313. [CrossRef]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Geng, F.; Cheng, X.; Guo, D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun. 2018, 38, 27. [Google Scholar] [CrossRef]
- Summons, R.E.; Welander, P.V.; Gold, D.A. Lipid biomarkers: Molecular tools for illuminating the history of microbial life. Nat. Rev. Microbiol 2022, 20, 174–185. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Ma, P.; Chen, J.; Xie, W. Ficus carica leaves extract inhibited pancreatic β-cell apoptosis by inhibiting AMPK/JNK/caspase-3 signaling pathway and antioxidation. Biomed. Pharmacother. 2020, 122, 109689. [Google Scholar] [CrossRef]
- Pascual, G.; Domínguez, D.; Elosúa-Bayes, M.; Beckedorff, F.; Laudanna, C.; Bigas, C.; Douillet, D.; Greco, C.; Symeonidi, A.; Hernández, I.; et al. Dietary palmitic acid promotes a prometastatic memory via Schwann cells. Nature 2021, 599, 485–490. [Google Scholar] [CrossRef]
- Liu, X.Z.; Rulina, A.; Choi, M.H.; Pedersen, L.; Lepland, J.; Takle, S.T.; Madeleine, N.; Peters, S.D.; Wogsland, C.E.; Grøndal, S.M.; et al. C/EBPB-dependent adaptation to palmitic acid promotes tumor formation in hormone receptor negative breast cancer. Nat. Commun. 2022, 13, 69. [Google Scholar] [CrossRef] [PubMed]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Ochoa, E.; Sánchez-Alegría, K.; Gómez-Inclán, C.; Ferrera, P.; Arias, C. Palmitic acid stimulates energy metabolism and inhibits insulin/PI3K/AKT signaling in differentiated human neuroblastoma cells: The role of mTOR activation and mitochondrial ROS production. Neurochem. Int. 2017, 110, 75–83. [Google Scholar] [CrossRef]
- Pascual, G.; Avgustinova, A.; Mejetta, S.; Martín, M.; Castellanos, A.; Attolini, C.S.; Berenguer, A.; Prats, N.; Toll, A.; Hueto, J.A.; et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 2017, 541, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Ding, Y.; Wang, Y.; Wang, Z.; Yin, X.; Yan, G.; Zhang, L.; Yang, P.; Shen, H. Functional lipidomics: Palmitic acid impairs hepatocellular carcinoma development by modulating membrane fluidity and glucose metabolism. Hepatology 2017, 66, 432–448. [Google Scholar] [CrossRef]
- Song, D.; Lan, J.; Chen, Y.; Liu, A.; Wu, Q.; Zhao, C.; Feng, Y.; Wang, J.; Luo, X.; Cao, Z.; et al. NSD2 promotes tumor angiogenesis through methylating and activating STAT3 protein. Oncogene 2021, 40, 2952–2967. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Wang, H.Q.; Man, Q.W.; Huo, F.Y.; Gao, X.; Lin, H.; Li, S.R.; Wang, J.; Su, F.C.; Cai, L.; Shi, Y.; et al. STAT3 pathway in cancers: Past, present, and future. MedComm 2022, 3, e124. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Hong, W.; Zhang, L.; Song, L.; Shi, Q.; Shao, Y.; Hao, G.; Fang, C.; Qiu, Y.; et al. Optineurin modulates the maturation of dendritic cells to regulate autoimmunity through JAK2-STAT3 signaling. Nat. Commun. 2021, 12, 6198. [Google Scholar] [CrossRef]
- Ma, J.; Yang, Y.; Fu, Y.; Guo, F.; Zhang, X.; Xiao, S.; Zhu, W.; Huang, Z.; Zhang, J.; Chen, J. PIAS3-mediated feedback loops promote chronic colitis-associated malignant transformation. Theranostics 2018, 8, 3022–3037. [Google Scholar] [CrossRef]
- Igelmann, S.; Neubauer, H.A.; Ferbeyre, G. STAT3 and STAT5 Activation in Solid Cancers. Cancers 2019, 11, 1428. [Google Scholar] [CrossRef]
- Sen, M.; Kindsfather, A.; Danilova, L.; Zhang, F.; Colombo, R.; LaPorte, M.G.; Kurland, B.F.; Huryn, D.M.; Wipf, P.; Herman, J.G. PTPRT epigenetic silencing defines lung cancer with STAT3 activation and can direct STAT3 targeted therapies. Epigenetics 2020, 15, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Broadfield, L.A.; Pane, A.A.; Talebi, A.; Swinnen, J.V.; Fendt, S.M. Lipid metabolism in cancer: New perspectives and emerging mechanisms. Dev. Cell 2021, 56, 1363–1393. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.D.; Trauner, M. Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 432–450. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yu, X.; Peng, C.; Liu, N.; Chen, W.; Xu, H.; Wei, H.; Fang, K.; Dong, Z.; Fu, C.; et al. Activation of SREBP-1c alters lipogenesis and promotes tumor growth and metastasis in gastric cancer. Biomed. Pharmacother. 2020, 128, 110274. [Google Scholar] [CrossRef]
- Liu, Y.P.; Zheng, C.C.; Huang, Y.N.; He, M.L.; Xu, W.W.; Li, B. Molecular mechanisms of chemo- and radiotherapy resistance and the potential implications for cancer treatment. MedComm 2021, 2, 315–340. [Google Scholar] [CrossRef]
- Urso, C.J.; Zhou, H. Palmitic Acid Lipotoxicity in Microglia Cells Is Ameliorated by Unsaturated Fatty Acids. Int. J. Mol. Sci. 2021, 22, 9093. [Google Scholar] [CrossRef]
- Urso, C.J.; Zhou, H. Role of CD36 in Palmitic Acid Lipotoxicity in Neuro-2a Neuroblastoma Cells. Biomolecules 2021, 11, 1567. [Google Scholar] [CrossRef] [PubMed]
- Seif, F.; Khoshmirsafa, M.; Aazami, H.; Mohsenzadegan, M.; Sedighi, G.; Bahar, M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun. Signal. 2017, 15, 23. [Google Scholar] [CrossRef]
- Iqbal, J.; Walsh, M.T.; Hammad, S.M.; Hussain, M.M. Sphingolipids and Lipoproteins in Health and Metabolic Disorders. Trends Endocrinol. Metab. 2017, 28, 506–518. [Google Scholar] [CrossRef]
- Meikle, P.J.; Summers, S.A. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat. Rev. Endocrinol. 2017, 13, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Sozen, E.; Ozer, N.K. Impact of high cholesterol and endoplasmic reticulum stress on metabolic diseases: An updated mini-review. Redox Biol. 2017, 12, 456–461. [Google Scholar] [CrossRef]
- De Araujo Junior, R.F.; Eich, C.; Jorquera, C.; Schomann, T.; Baldazzi, F.; Chan, A.B.; Cruz, L.J. Ceramide and palmitic acid inhibit macrophage-mediated epithelial-mesenchymal transition in colorectal cancer. Mol. Cell. Biochem. 2020, 468, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Nishimura, Y.; Hatori, Y.; Akagi, R.; Mihara, K.; Yanagihara, K.; Seyama, T. Antitumor effect of palmitic acid-conjugated DsiRNA for colon cancer in a mouse subcutaneous tumor model. Chem. Biol. Drug. Des. 2019, 93, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Snaebjornsson, M.T.; Janaki-Raman, S.; Schulze, A. Greasing the Wheels of the Cancer Machine: The Role of Lipid Metabolism in Cancer. Cell Metab. 2020, 31, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Fabre, A.; Marchal, S.; Barlogis, V.; Mari, B.; Barbry, P.; Rohrlich, P.S.; Forbes, L.R.; Vogel, T.P.; Giovannini-Chami, L. Clinical Aspects of STAT3 Gain-of-Function Germline Mutations: A Systematic Review. J. Allergy Clin. Immunol. Pract. 2019, 7, 1958–1969.e1959. [Google Scholar] [CrossRef]
- Patel, N.J.; Nassal, D.M.; Gratz, D.; Hund, T.J. Emerging therapeutic targets for cardiac arrhythmias: Role of STAT3 in regulating cardiac fibroblast function. Expert Opin. Ther. Targets 2021, 25, 63–73. [Google Scholar] [CrossRef]
- Banerjee, K.; Resat, H. Constitutive activation of STAT3 in breast cancer cells: A review. Int. J. Cancer 2016, 138, 2570–2578. [Google Scholar] [CrossRef]
- Pu, Z.; Xu, M.; Yuan, X.; Xie, H.; Zhao, J. Circular RNA circCUL3 Accelerates the Warburg Effect Progression of Gastric Cancer through Regulating the STAT3/HK2 Axis. Mol. Ther. Nucleic Acids 2020, 22, 310–318. [Google Scholar] [CrossRef]
- Lu, W.J.; Peng, W.; Sun, Q.Q.; Li, Y.H.; Chen, B.; Yu, L.T.; Xu, Y.Z.; Wang, S.Y.; Zhao, Y.L. #2714, a novel active inhibitor with potent G2/M phase arrest and antitumor efficacy in preclinical models. Cell Death Discov. 2018, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.; Liu, Y.; Nie, Y.; Si, B.; Wang, T.; Waqas, A.; Zhao, G.; Wang, M.; Xu, A. Aging-independent and size-dependent genotoxic response induced by titanium dioxide nanoparticles in mammalian cells. J. Environ. Sci. 2019, 85, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Sun, Q.; Xu, H.; Yu, X.; Chen, W.; Wei, H.; Jiang, J.; Xu, Y.; Lu, W. Hyperuricemia induces lipid disturbances mediated by LPCAT3 upregulation in the liver. FASEB J. 2020, 34, 13474–13493. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xu, F.; Xiong, M.; Yang, H.; Lin, W.; Xie, Y.; Xi, H.; Xue, Q.; Ye, T.; Yu, L. Repurposing of antipsychotic trifluoperazine for treating brain metastasis, lung metastasis and bone metastasis of melanoma by disrupting autophagy flux. Pharmacol. Res. 2021, 163, 105295. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Peng, W.; Wang, Y.; Xu, W.; Chen, W.; Huang, L.; Xu, H.; He, X.; Wang, S.; Sun, Q.; et al. Palmitic Acid Inhibits the Growth and Metastasis of Gastric Cancer by Blocking the STAT3 Signaling Pathway. Cancers 2023, 15, 388. https://doi.org/10.3390/cancers15020388
Yu X, Peng W, Wang Y, Xu W, Chen W, Huang L, Xu H, He X, Wang S, Sun Q, et al. Palmitic Acid Inhibits the Growth and Metastasis of Gastric Cancer by Blocking the STAT3 Signaling Pathway. Cancers. 2023; 15(2):388. https://doi.org/10.3390/cancers15020388
Chicago/Turabian StyleYu, Xiaojuan, Wen Peng, Yaoxing Wang, Wenjun Xu, Wentong Chen, Lei Huang, Hu Xu, Xinyu He, Sheng Wang, Qianqian Sun, and et al. 2023. "Palmitic Acid Inhibits the Growth and Metastasis of Gastric Cancer by Blocking the STAT3 Signaling Pathway" Cancers 15, no. 2: 388. https://doi.org/10.3390/cancers15020388
APA StyleYu, X., Peng, W., Wang, Y., Xu, W., Chen, W., Huang, L., Xu, H., He, X., Wang, S., Sun, Q., Lu, W., & Xu, Y. (2023). Palmitic Acid Inhibits the Growth and Metastasis of Gastric Cancer by Blocking the STAT3 Signaling Pathway. Cancers, 15(2), 388. https://doi.org/10.3390/cancers15020388





