Modification and Expression of mRNA m6A in the Lateral Habenular of Rats after Long-Term Exposure to Blue Light during the Sleep Period
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Blue Light Treatment
2.3. RNA Preparation
2.4. High-Throughput m6A
2.5. Sequencing Data Analysis
2.6. Western Blotting Assay
2.7. Enzyme-Linked Immunosorbent Assays for Serum Determinations
2.8. Molecular Docking
2.9. Statistical Analysis
3. Results
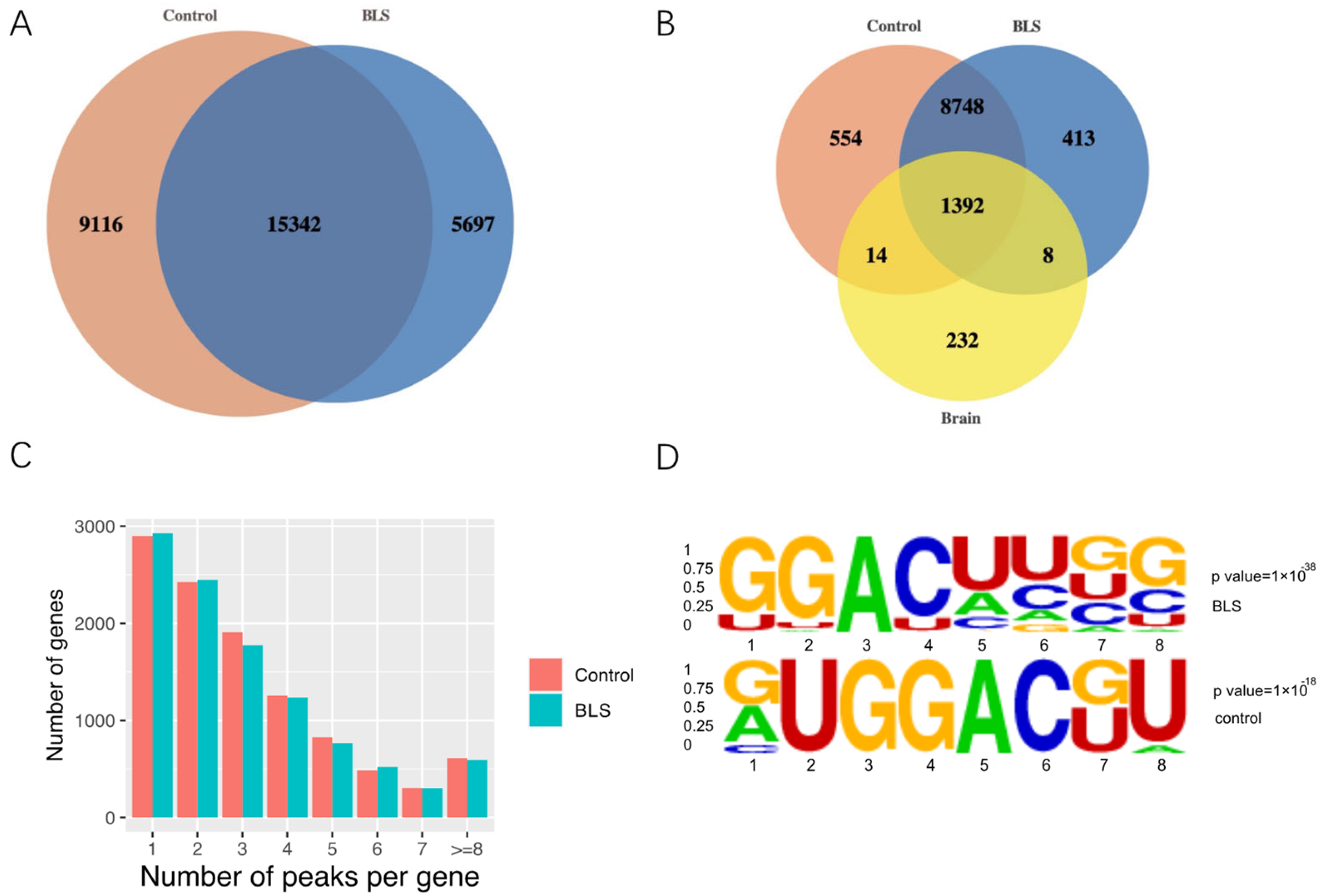
3.1. Transcriptome-Wide Detection of m6A Modification in LHb
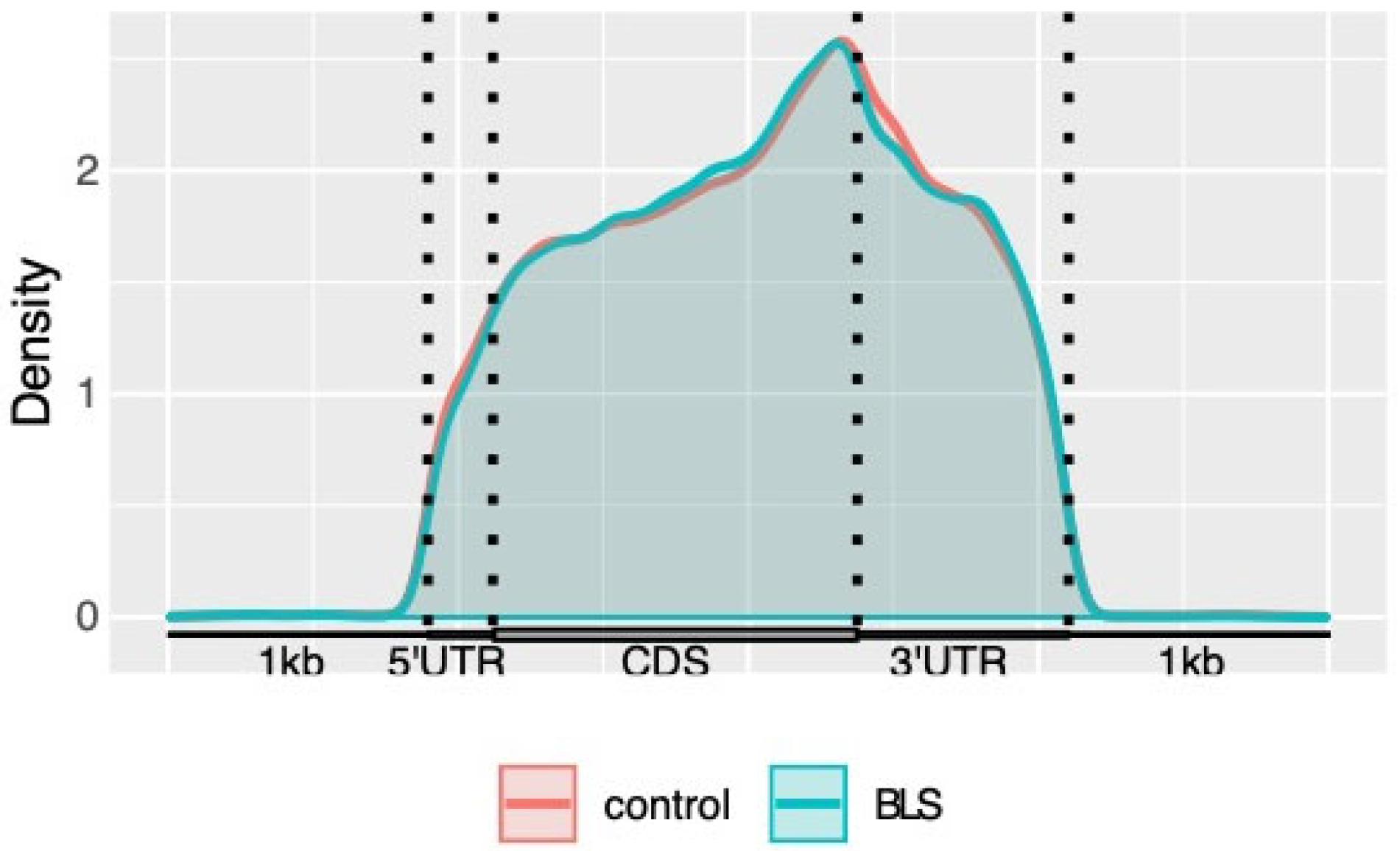
3.2. Distribution of m6A Modification in Transcriptome
3.3. Differentially Methylated Genes and Differentially Expressed Genes
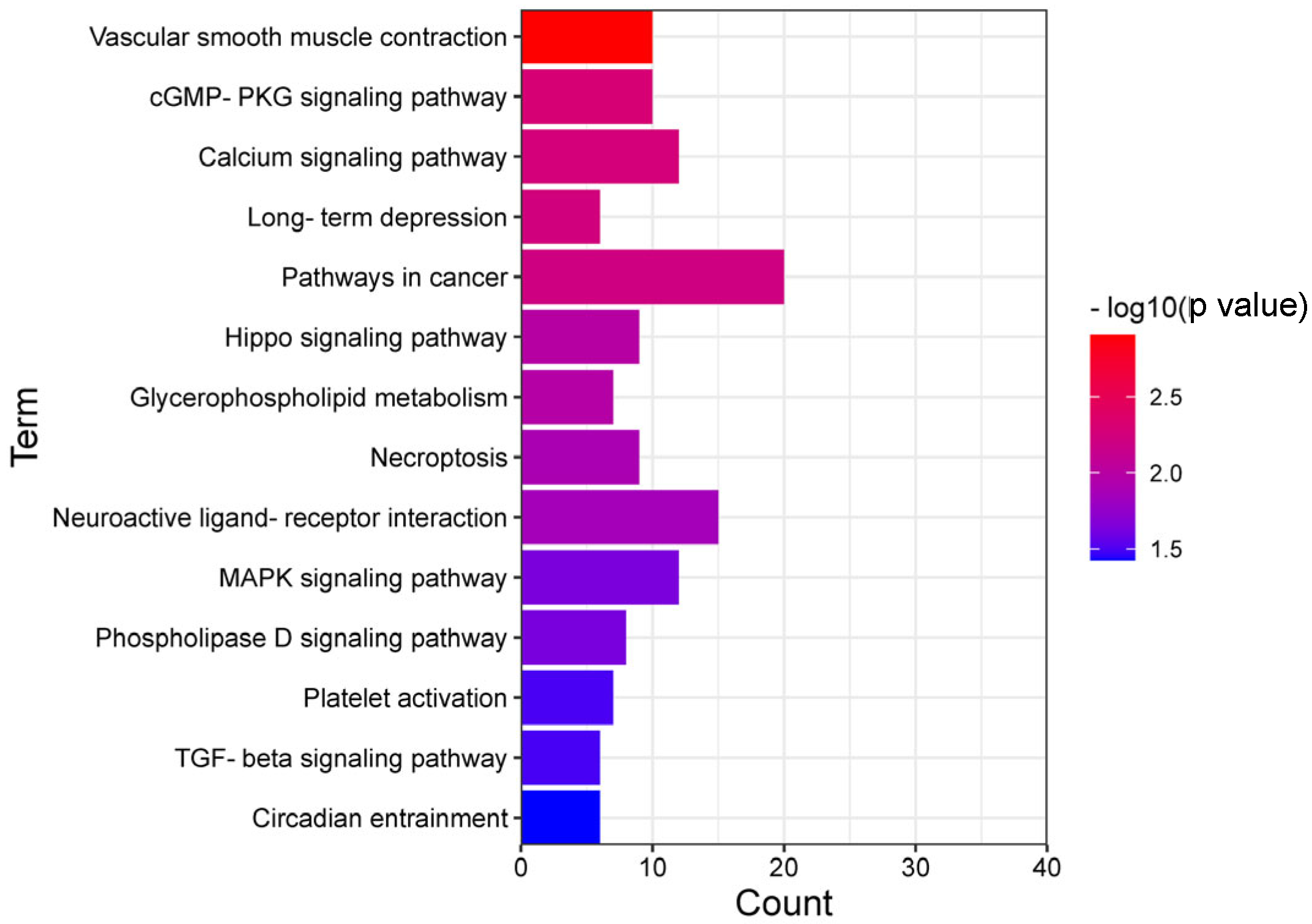
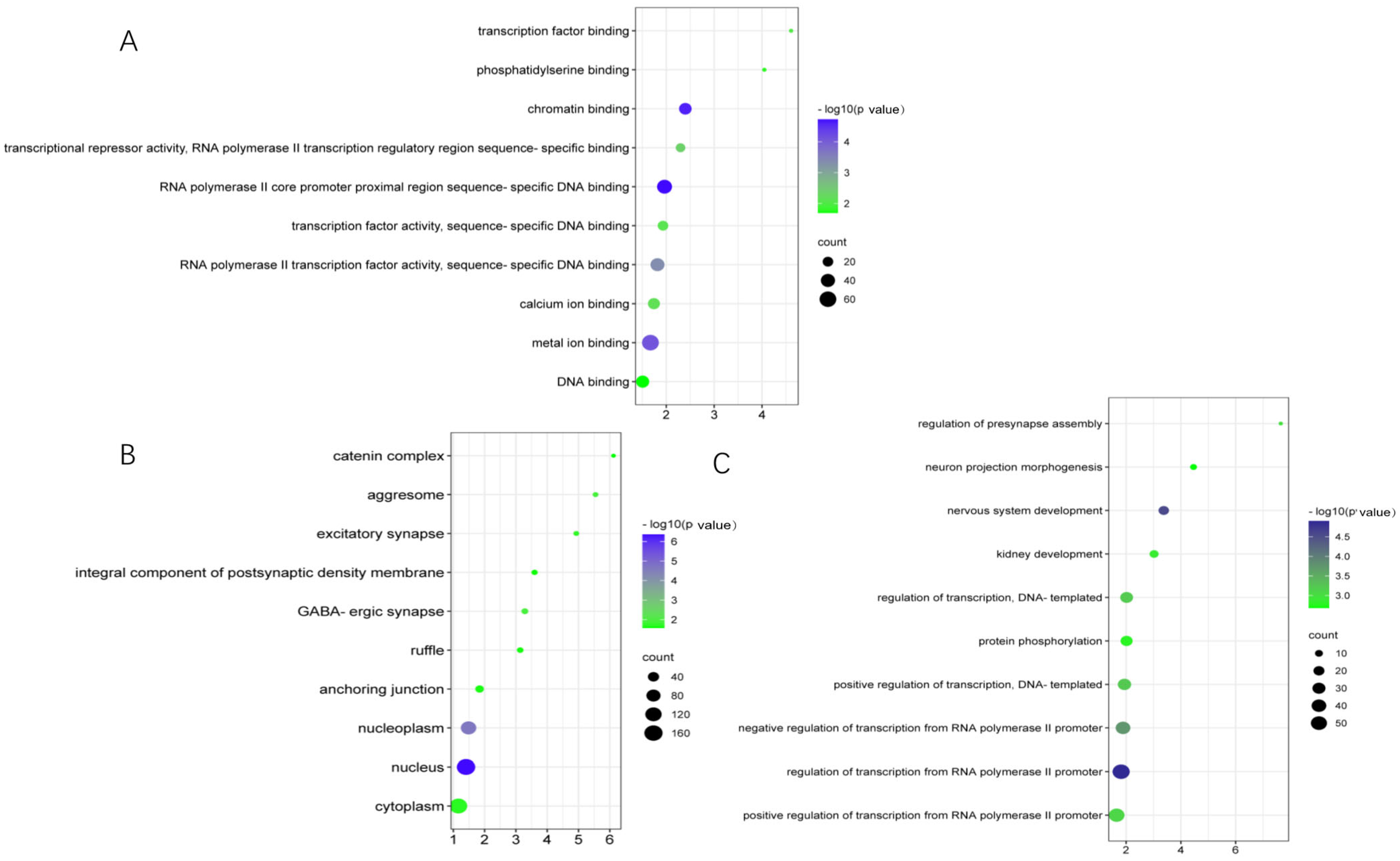
3.4. KEGG and GO Annotation of the Overlap of Differentially m6A-Modified mRNA and Differentially Expressed mRNA
3.5. Neuroactive Ligand Receptor Interaction, Circadian Rhythm Entrainment, cGMP-PKG Signal Pathway and Regulation of Potential Regulators
3.6. Potential RNA m6A Regulators of Different Methylation Genes
3.7. Molecular Interactions of Melatonin with Differently Expressed Regulators of RNA m6A Modification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LHb | lateral habenula |
| cGMP-PKG | cyclic guanosine monophosphate-protein kinase |
| BLS | Blue light during the sleep period |
| NMDAR | N-methyl-D-aspartate receptor |
| GABA | γ-2Aminobutiric acid |
| LED | light emitting diode |
| LAN | light-at-night |
| ipRGCs | intrinsically photosensitive retinal ganglion cells |
| 3′UTR 3′ | untranslated regions |
| 5′UTR 5′ | untranslated regions |
| CDS | coding sequence (CDS) |
| GO | Gene Ontology |
| LEGG | Kyoto Encyclopedia of Genes and Genomes |
| m6A | N6-methyladenosine |
| MeRIP-seq | methylated RNA immunoprecipitation sequencing |
References
- Bedrosian, T.A.; Nelson, R.J. Influence of the modern light environment on mood. Mol. Psychiatry 2013, 18, 751–757. [Google Scholar] [CrossRef] [PubMed]
- LeGates, T.A.; Fernandez, D.C.; Hattar, S. Light as a central modulator of circadian rhythms, sleep and affect. Nat. Rev. Neurosci. 2014, 15, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Vandewalle, G.; Maquet, P.; Dijk, D.J. Light as a modulator of cognitive brain function. Trends Cogn. Sci. 2009, 13, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.D.; Burkhart, E.M.; Nagai, A.; Aizawa, Y.; Kato, T.A. Cytotoxicity and genotoxicity of blue LED light and protective effects of AA2G in mammalian cells and associated DNA repair deficient cell lines. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2021, 872, 503416. [Google Scholar] [CrossRef] [PubMed]
- Nickla, D.L.; Rucker, F.; Taylor, C.P.; Sarfare, S.; Chen, W.; Elin-Calcador, J.; Wang, X. Effects of morning and evening exposures to blue light of varying illuminance on ocular growth rates and ocular rhythms in chicks. Exp. Eye Res. 2022, 217, 108963. [Google Scholar] [CrossRef]
- Zielinska-Dabkowska, K.M. Make lighting healthier. Nature 2018, 553, 274–276. [Google Scholar] [CrossRef]
- Brainard, G.C.; Hanifin, J.P. Photons, clocks, and consciousness. J. Biol. Rhythm 2005, 20, 314–325. [Google Scholar] [CrossRef]
- Prayag, A.S.; Najjar, R.P.; Gronfier, C. Melatonin suppression is exquisitely sensitive to light and primarily driven by melanopsin in humans. J. Pineal Res. 2019, 66, e12562. [Google Scholar] [CrossRef]
- Jenkins, A.; Muñoz, M.; Tarttelin, E.E.; Bellingham, J.; Foster, R.G.; Hankins, M.W. VA opsin, melanopsin, and an inherent light response within retinal interneurons. Curr. Biol. 2003, 13, 1269–1278. [Google Scholar] [CrossRef]
- Dacey, D.M.; Liao, H.W.; Peterson, B.B.; Robinson, F.R.; Smith, V.C.; Pokorny, J.; Yau, K.W.; Gamlin, P.D. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 2005, 433, 749–754. [Google Scholar] [CrossRef]
- An, K.; Zhao, H.; Miao, Y.; Xu, Q.; Li, Y.F.; Ma, Y.Q.; Shi, Y.M.; Shen, J.W.; Meng, J.J.; Yao, Y.G.; et al. A circadian rhythm-gated subcortical pathway for nighttime-light-induced depressive-like behaviors in mice. Nat. Neurosci. 2020, 23, 869–880. [Google Scholar] [CrossRef]
- Zhu, Y.; Qi, S.; Zhang, B.; He, D.; Teng, Y.; Hu, J.; Wei, X. Connectome-Based Biomarkers Predict Subclinical Depression and Identify Abnormal Brain Connections with the Lateral Habenula and Thalamus. Front. Psychiatry 2019, 10, 371. [Google Scholar] [CrossRef]
- Jhou, T.C.; Geisler, S.; Marinelli, M.; Degarmo, B.A.; Zahm, D.S. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J. Comp. Neurol. 2009, 513, 566–596. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, Y.; Sang, K.; Dong, Y.; Ni, Z.; Ma, S.; Hu, H. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 2018, 554, 317–322. [Google Scholar] [CrossRef]
- Barker, D.J.; Miranda-Barrientos, J.; Zhang, S.; Root, D.H.; Wang, H.L.; Liu, B.; Calipari, E.S.; Morales, M. Lateral Preoptic Control of the Lateral Habenula through Convergent Glutamate and GABA Transmission. Cell Rep. 2017, 21, 1757–1769. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, Y.; Ni, Z.; Dong, Y.; Cai, G.; Foncelle, A.; Ma, S.; Sang, K.; Tang, S.; Li, Y.; et al. Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature 2018, 554, 323–327. [Google Scholar] [CrossRef]
- Arjmand, S.; Landau, A.M.; Varastehmoradi, B.; Andreatini, R.; Joca, S.; Wegener, G. The intersection of astrocytes and the endocannabinoid system in the lateral habenula: On the fast-track to novel rapid-acting antidepressants. Mol. Psychiatry 2022, 27, 3138–3149. [Google Scholar] [CrossRef]
- Czarny, P.; Białek, K.; Ziółkowska, S.; Strycharz, J.; Barszczewska, G.; Sliwinski, T. The Importance of Epigenetics in Diagnostics and Treatment of Major Depressive Disorder. J. Pers. Med. 2021, 11, 167. [Google Scholar] [CrossRef]
- Ma, J.; Song, B.; Wei, Z.; Huang, D.; Zhang, Y.; Su, J.; de Magalhães, J.P.; Rigden, D.J.; Meng, J.; Chen, K. m5C-Atlas: A comprehensive database for decoding and annotating the 5-methylcytosine (m5C) epitranscriptome. Nucleic Acids Res. 2022, 50, D196–D203. [Google Scholar] [CrossRef]
- Dominissini, D.; Nachtergaele, S.; Moshitch-Moshkovitz, S.; Peer, E.; Kol, N.; Ben-Haim, M.S.; Dai, Q.; Di Segni, A.; Salmon-Divon, M.; Clark, W.C.; et al. The dynamic N1-methyladenosine methylome in eukaryotic messenger RNA. Nature 2016, 530, 441–446. [Google Scholar] [CrossRef]
- Shu, L.; Huang, X.; Cheng, X.; Li, X. Emerging Roles of N6-Methyladenosine Modification in Neurodevelopment and Neurodegeneration. Cells 2021, 10, 2694. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Tang, Y.; Chen, K.; Wei, Z.; Rong, R.; Lu, Z.; Su, J.; de Magalhães, J.P.; Rigden, D.J.; Meng, J. m7GHub: Deciphering the location, regulation and pathogenesis of internal mRNA N7-methylguanosine (m7G) sites in human. Bioinformatics 2020, 36, 3528–3536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ding, C.; Zuo, Y.; Peng, Y.; Zuo, L. N6-methyladenosine and Neurological Diseases. Mol. Neurobiol. 2022, 59, 1925–1937. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hsu, P.J.; Chen, Y.S.; Yang, Y.G. Dynamic transcriptomic m(6)A decoration: Writers, erasers, readers and functions in RNA metabolism. Cell Res. 2018, 28, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Wang, Q. The Progression of N6-methyladenosine Study and Its Role in Neuropsychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 5922. [Google Scholar] [CrossRef]
- Mathoux, J.; Henshall, D.C.; Brennan, G.P. Regulatory Mechanisms of the RNA Modification m(6)A and Significance in Brain Function in Health and Disease. Front. Cell. Neurosci. 2021, 15, 671932. [Google Scholar] [CrossRef]
- Du, T.; Rao, S.; Wu, L.; Ye, N.; Liu, Z.; Hu, H.; Xiu, J.; Shen, Y.; Xu, Q. An association study of the m6A genes with major depressive disorder in Chinese Han population. J. Affect. Disord. 2015, 183, 279–286. [Google Scholar] [CrossRef]
- Shen, J.; Yang, L.; Wei, W. Role of Fto on CaMKII/CREB signaling pathway of hippocampus in depressive-like behaviors induced by chronic restraint stress mice. Behav. Brain Res. 2021, 406, 113227. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, K.; Song, B.; Ma, J.; Wu, X.; Xu, Q.; Wei, Z.; Su, J.; Liu, G.; Rong, R.; et al. m6A-Atlas: A comprehensive knowledgebase for unraveling the N6-methyladenosine (m6A) epitranscriptome. Nucleic Acids Res. 2020, 49, D134–D143. [Google Scholar] [CrossRef]
- Meng, J.; Cui, X.; Rao, M.K.; Chen, Y.; Huang, Y. Exome-based analysis for RNA epigenome sequencing data. Bioinformatics 2013, 29, 1565–1567. [Google Scholar] [CrossRef]
- Bailey, T.L. STREME: Accurate and versatile sequence motif discovery. Bioinformatics 2021, 37, 2834–2840. [Google Scholar] [CrossRef]
- Jiao, X.; Sherman, B.T.; Huang, D.W.; Stephens, R.; Baseler, M.W.; Lane, H.C.; Lempicki, R.A. DAVID-WS: A stateful web service to facilitate gene/protein list analysis. Bioinformatics 2012, 28, 1805–1806. [Google Scholar] [CrossRef]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, J.; Ma, J.; Wei, Z.; Wang, Y.; Song, B.; Meng, J.; Jia, G.; de Magalhães, J.P.; Rigden, D.J.; et al. DirectRMDB: A database of post-transcriptional RNA modifications unveiled from direct RNA sequencing technology. Nucleic Acids Res. 2022, 51, D106–D116. [Google Scholar] [CrossRef]
- Huang, D.; Chen, K.; Song, B.; Wei, Z.; Su, J.; Coenen, F.; de Magalhães, J.P.; Rigden, D.J.; Meng, J. Geographic encoding of transcripts enabled high-accuracy and isoform-aware deep learning of RNA methylation. Nucleic Acids Res. 2022, 50, 10290–10310. [Google Scholar] [CrossRef]
- Song, B.; Huang, D.; Zhang, Y.; Wei, Z.; Su, J.; Pedro de Magalhães, J.; Rigden, D.J.; Meng, J.; Chen, K. m6A-TSHub: Unveiling the Context-specific m(6)A Methylation and m6A-affecting Mutations in 23 Human Tissues. Genom. Proteom. Bioinform. 2022; in press. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.; Wei, Z.; Coenen, F.; Su, J.; Meng, J. MetaTX: Deciphering the distribution of mRNA-related features in the presence of isoform ambiguity, with applications in epitranscriptome analysis. Bioinformatics 2021, 37, 1285–1291. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, S.; Zhu, Y.; Xi, X.; Bao, P.; Ma, Z.; Kapral, T.H.; Chen, S.; Zagrovic, B.; Yang, Y.T.; et al. POSTAR3: An updated platform for exploring post-transcriptional regulation coordinated by RNA-binding proteins. Nucleic Acids Res. 2022, 50, D287–D294. [Google Scholar] [CrossRef]
- Li, J.H.; Liu, S.; Zhou, H.; Qu, L.H.; Yang, J.H. starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014, 42, D92–D97. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, Z.; Wang, Q.; Zhang, J.; Wang, L.; Zhang, Q.; Li, H.; Wu, S. Manganese chloride induces histone acetylation changes in neuronal cells: Its role in manganese-induced damage. Neurotoxicology 2018, 65, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, L.; Zhu, A.; Liu, X.; Wang, T.; Wan, M.; Yang, X.; Zhang, Y.; Zhang, Y. Metabolic Profiling of Nuciferine In Vivo and In Vitro. J. Agric. Food Chem. 2020, 68, 14135–14147. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Daut, R.A.; Hartsock, M.J.; Tomczik, A.C.; Watkins, L.R.; Spencer, R.L.; Maier, S.F.; Fonken, L.K. Circadian misalignment has differential effects on affective behavior following exposure to controllable or uncontrollable stress. Behav. Brain Res. 2019, 359, 440–445. [Google Scholar] [CrossRef]
- Kecklund, G.; Axelsson, J. Health consequences of shift work and insufficient sleep. Br. Med. J. 2016, 355, i5210. [Google Scholar] [CrossRef]
- Baño-Otálora, B.; Piggins, H.D. Contributions of the lateral habenula to circadian timekeeping. Pharmacol. Biochem. Behav. 2017, 162, 46–54. [Google Scholar] [CrossRef]
- Popoli, M.; Gennarelli, M.; Racagni, G. Modulation of synaptic plasticity by stress and antidepressants. Bipolar Disord. 2002, 4, 166–182. [Google Scholar] [CrossRef]
- Krzystyniak, A.; Baczynska, E.; Magnowska, M.; Antoniuk, S.; Roszkowska, M.; Zareba-Koziol, M.; Das, N.; Basu, S.; Pikula, M.; Wlodarczyk, J. Prophylactic Ketamine Treatment Promotes Resilience to Chronic Stress and Accelerates Recovery: Correlation with Changes in Synaptic Plasticity in the CA3 Subregion of the Hippocampus. Int. J. Mol. Sci. 2019, 20, 1726. [Google Scholar] [CrossRef]
- Park, H.; Rhee, J.; Park, K.; Han, J.S.; Malinow, R.; Chung, C. Exposure to Stressors Facilitates Long-Term Synaptic Potentiation in the Lateral Habenula. J. Neurosci. 2017, 37, 6021–6030. [Google Scholar] [CrossRef]
- Mayr, C. What Are 3′ UTRs Doing? Cold Spring Harb. Perspect. Biol. 2019, 11, a034728. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, Y.; Shen, H.; Xie, W. m(6)A-binding proteins: The emerging crucial performers in epigenetics. J. Hematol. Oncol. 2020, 13, 35. [Google Scholar] [CrossRef]
- Hoernes, T.P.; Hüttenhofer, A.; Erlacher, M.D. mRNA modifications: Dynamic regulators of gene expression? RNA Biol. 2016, 13, 760–765. [Google Scholar] [CrossRef]
- Eom, T.; Antar, L.N.; Singer, R.H.; Bassell, G.J. Localization of a beta-actin messenger ribonucleoprotein complex with zipcode-binding protein modulates the density of dendritic filopodia and filopodial synapses. J. Neurosci. 2003, 23, 10433–10444. [Google Scholar] [CrossRef]
- Tiruchinapalli, D.M.; Oleynikov, Y.; Kelic, S.; Shenoy, S.M.; Hartley, A.; Stanton, P.K.; Singer, R.H.; Bassell, G.J. Activity-dependent trafficking and dynamic localization of zipcode binding protein 1 and beta-actin mRNA in dendrites and spines of hippocampal neurons. J. Neurosci. 2003, 23, 3251–3261. [Google Scholar] [CrossRef]
- Lüscher, C.; Malenka, R.C. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb. Perspect. Biol. 2012, 4, a005710. [Google Scholar] [CrossRef]
- Wang, Q.; Mergia, E.; Koesling, D.; Mittmann, T. Nitric oxide/cGMP signaling via guanylyl cyclase isoform 1 modulates glutamate and GABA release in somatosensory cortex of mice. Neuroscience 2017, 360, 180–189. [Google Scholar] [CrossRef]
- McIntyre, I.M.; Norman, T.R.; Burrows, G.D.; Armstrong, S.M. Human melatonin suppression by light is intensity dependent. J. Pineal Res. 1989, 6, 149–156. [Google Scholar] [CrossRef]


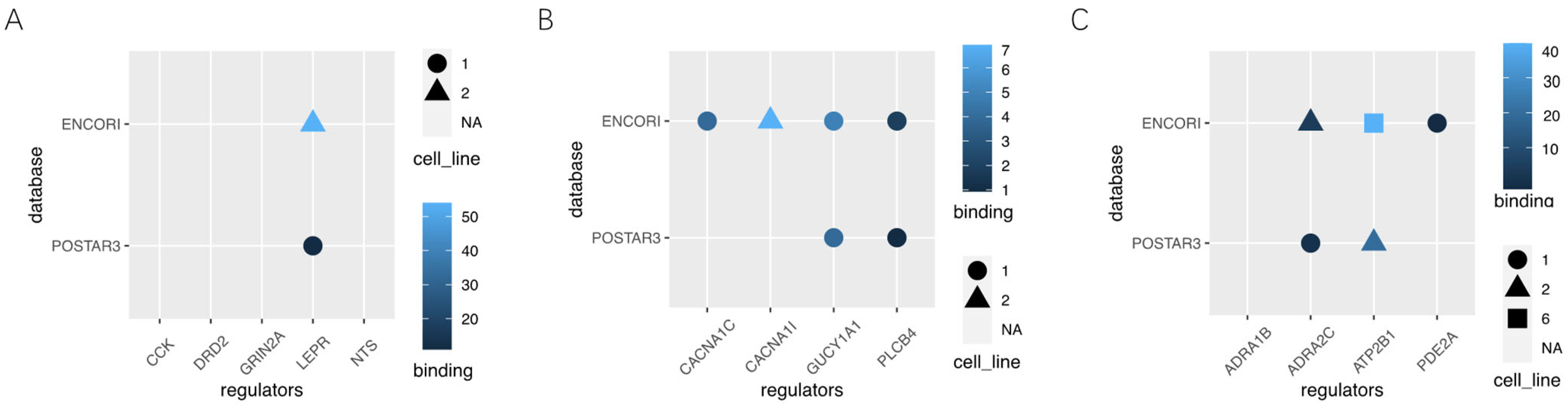
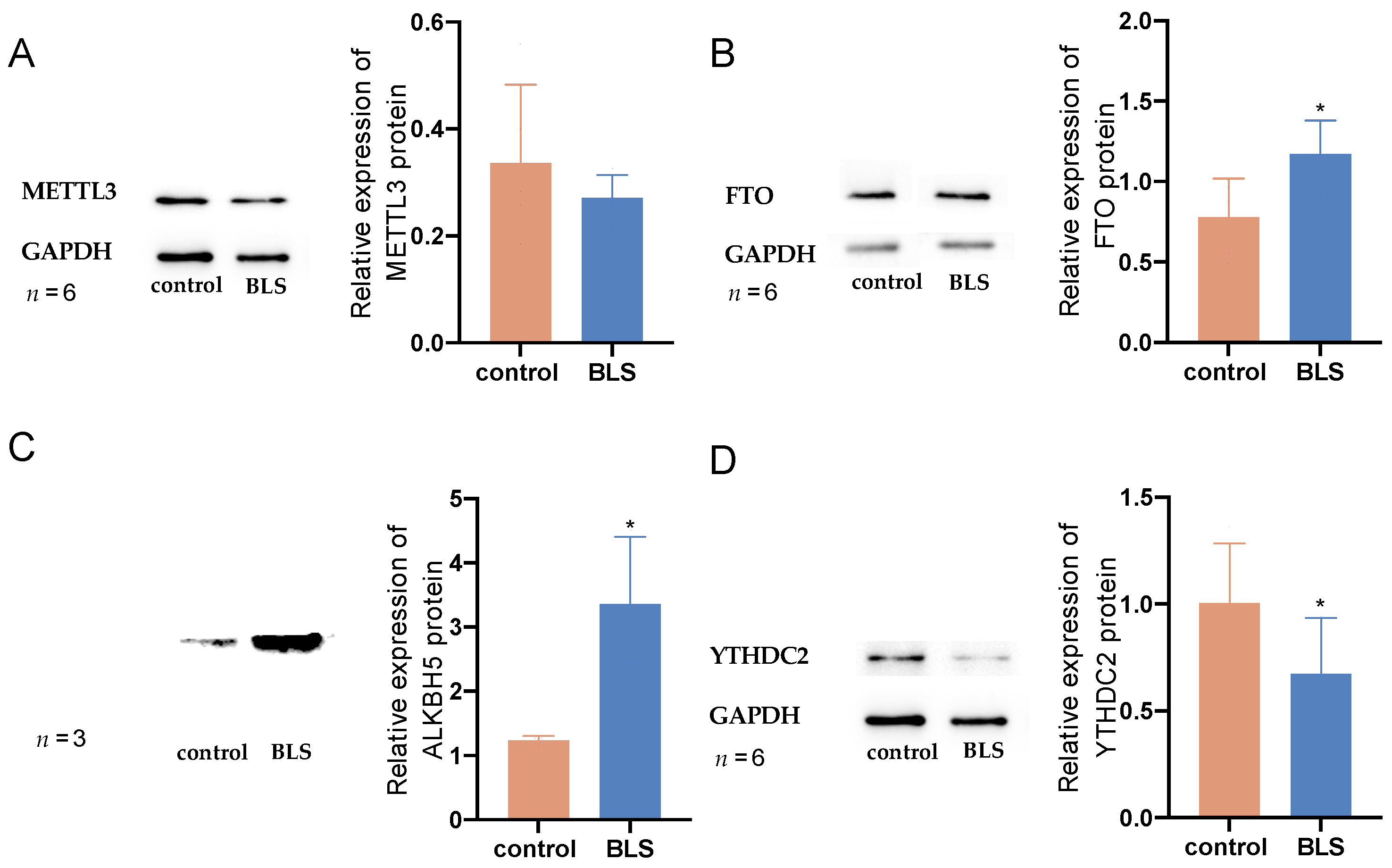
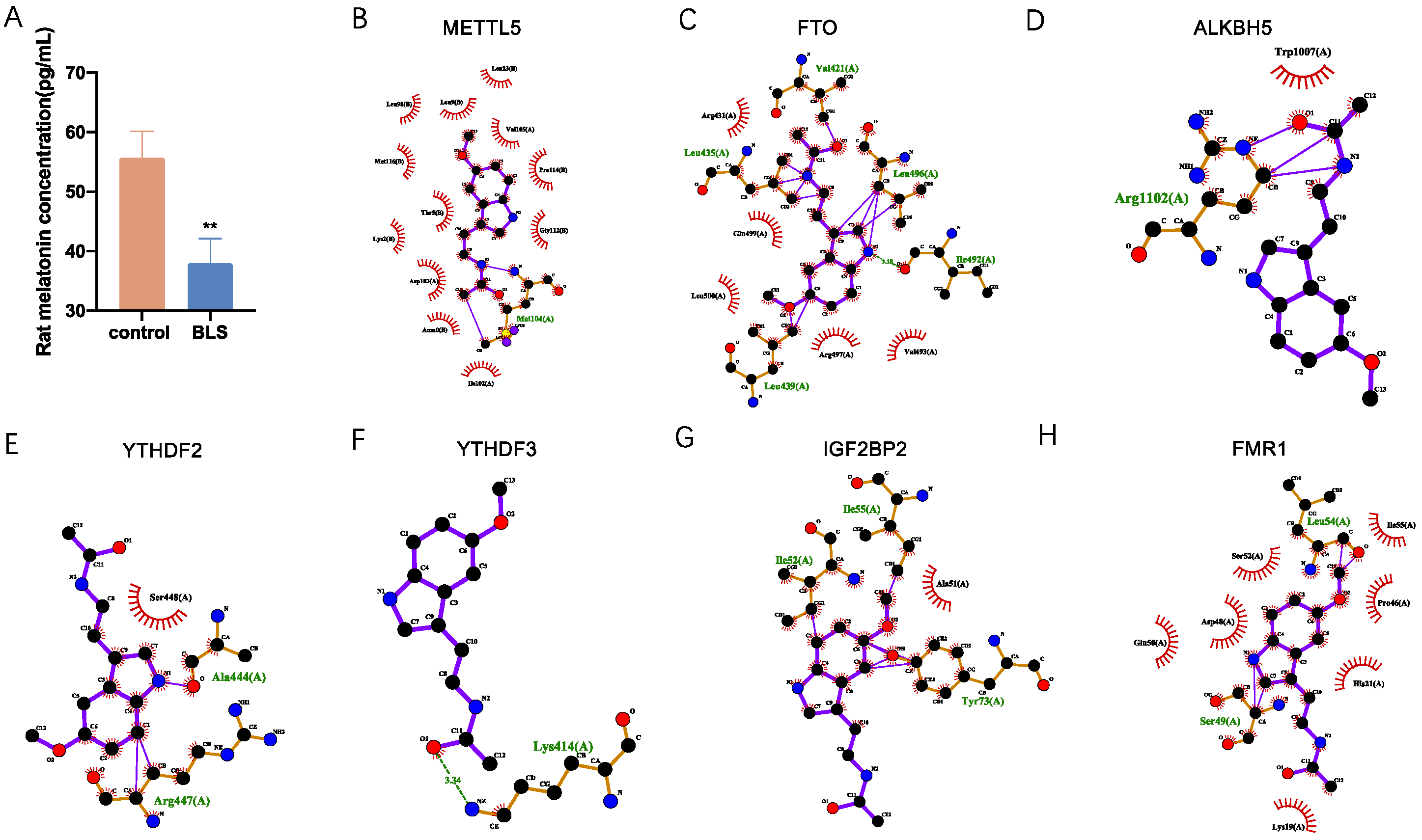

| Gene | log2FoldChange | p Value | IGF2BP1 | ||
|---|---|---|---|---|---|
| POSTAR3 | ENCORI | ||||
| Neuroactive ligand-receptor interaction | DRD21 | 2.410717348 | 0.000718739 | 0 | 0 |
| NTS | 2.289483776 | 0.000814387 | 0 | 0 | |
| CCK | −3.891976901 | 0.002732121 | 0 | 0 | |
| LEPR | −1.544900237 | 0.018075531 | 1 | 1 | |
| GRIN2A | −1.383616013 | 0.048896803 | 0 | 0 | |
| Circadian entrainment | CACNA1C | −1.325997265 | 0.002641267 | 0 | 1 |
| PLCB4 | −1.769449948 | 0.003986134 | 1 | 1 | |
| GUCY1A1 | −.359242738 | 0.032953756 | 1 | 1 | |
| CACNA1I | 0.077654698 | 0.85792273 | 0 | 1 | |
| cGMP-PKG signaling pathway | PDE2A | 1.63634444 | 0.000483644 | 0 | 1 |
| ADRA1B | −2.789275777 | 0.000878733 | 0 | 0 | |
| ATP2B1 | −1.753044611 | 0.006740132 | 1 | 1 | |
| ADRA2C | 1.717512915 | 0.017494168 | 1 | 1 | |
| Gene | Regulation | Base Mean | log2FoldChange | p Value |
|---|---|---|---|---|
| ALKBH5 | eraser | 341.9384302 | −0.109170657 | 0.771338043 |
| CBLL1 | writer | 346.9551446 | 0.116591986 | 0.640932024 |
| FMR1 | reader | 1847.917149 | −0.071575928 | 0.756365327 |
| FTO | eraser | 1766.661301 | 0.156834515 | 0.728325104 |
| HNRNPA2B1 | reader | 23816.12242 | 0.04414699 | 0.831082269 |
| HNRNPC | reader | 5475.18213 | 0.188188685 | 0.223239932 |
| IGF2BP1 | reader | 1.168447989 | 3.574733353 | 0.364559468 |
| IGF2BP2 | reader | 53.11726013 | 0.147512464 | 0.760550998 |
| IGF2BP3 | reader | 8.376292315 | 0.613618984 | 0.540055918 |
| METTL14 | writer | 1787.917122 | 0.116946577 | 0.522853135 |
| METTL3 | writer | 2373.613619 | −0.098001527 | 0.667618896 |
| METTL5 | writer | 1275.304982 | −0.179998413 | 0.419116968 |
| VIRMA | writer | 1113.272801 | −0.226631023 | 0.341256528 |
| WTAP | writer | 1863.746967 | 0.106961708 | 0.735133477 |
| YTHDC1 | reader | 7452.265261 | −0.077633436 | 0.679592938 |
| YTHDF1 | reader | 1043.115245 | −0.087632591 | 0.633796561 |
| YTHDF2 | reader | 1082.137679 | 0.153862012 | 0.481990904 |
| YTHDF3 | reader | 732.4864141 | 0.064147425 | 0.865441311 |
| ZC3H13 | writer | 13329.50949 | −0.284321826 | 0.402424357 |
| Protein | Regulation | Total Score | H-Bond Number | Residues Involved in H-Bond Formation | Hydrophobic Contacts Number | Residues Involved in Hydrophobic Contacts |
|---|---|---|---|---|---|---|
| METTL5 | writer | 10 | 1 | Met104 | 12 | Ile102, Amn0, Asp103, Lys2, Thr5, Met116, Leu98, Leu9, Leu23, Val105, Pro114, Gly112 |
| FTO | eraser | 9.13 | 5 | Leu435, Va1421, Leu496, Ile492, Leu439 | 5 | Arg431, Gln499, Leu500, Leu439, Ile492 |
| ALKBH5 | eraser | 4.39 | 1 | Arg1102 | 1 | Trp1007 |
| YTHDF3 | reader | 8.152 | 1 | Lys414 | 0 | |
| YTHDF2 | reader | 6.401 | 2 | Arg447, Ala444 | 1 | Ser448 |
| IGF2BP2 | reader | 4.891 | 3 | Ile55, Ile52, Tyr73 | 1 | Ala51 |
| FMR1 | reader | 3.19 | 2 | Leu54, Ser49 | 7 | Glu50, Asp48, Ser52, Ile55, Pro46, His21, Lys19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Ren, J.; Zhang, Z.; Weng, Y.; Zhang, J.; Zou, X.; Wu, S.; Hu, H. Modification and Expression of mRNA m6A in the Lateral Habenular of Rats after Long-Term Exposure to Blue Light during the Sleep Period. Genes 2023, 14, 143. https://doi.org/10.3390/genes14010143
Li Y, Ren J, Zhang Z, Weng Y, Zhang J, Zou X, Wu S, Hu H. Modification and Expression of mRNA m6A in the Lateral Habenular of Rats after Long-Term Exposure to Blue Light during the Sleep Period. Genes. 2023; 14(1):143. https://doi.org/10.3390/genes14010143
Chicago/Turabian StyleLi, Yinhan, Jinjin Ren, Zhaoting Zhang, Yali Weng, Jian Zhang, Xinhui Zou, Siying Wu, and Hong Hu. 2023. "Modification and Expression of mRNA m6A in the Lateral Habenular of Rats after Long-Term Exposure to Blue Light during the Sleep Period" Genes 14, no. 1: 143. https://doi.org/10.3390/genes14010143
APA StyleLi, Y., Ren, J., Zhang, Z., Weng, Y., Zhang, J., Zou, X., Wu, S., & Hu, H. (2023). Modification and Expression of mRNA m6A in the Lateral Habenular of Rats after Long-Term Exposure to Blue Light during the Sleep Period. Genes, 14(1), 143. https://doi.org/10.3390/genes14010143







