Memory-Improving Activity of the Flower Extract from Chrysanthemum boreale (Makino) Maskino in Scopolamine-Treated Rodents
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Plant Resource and Extract Preparation
2.1.2. Chemicals
2.2. Cell Lines and Cell Culture
2.3. In Vitro Assay
2.3.1. Analysis of NO Levels, IL-6 Levels, and Cell Proliferation in BV2
2.3.2. Analysis of p-Tau Production in SH-SY5Y Cells
2.4. In Vivo Experiments
2.4.1. Animal Experiments Using Rats Chronically Treated with Sco
2.4.2. Analysis of Biomarkers in Rats Chronically Treated with Sco
2.4.3. Animal Experiments Using Mice Acutely Treated with Sco
2.5. Analysis of Phenolic Composition
2.6. Statistical Analysis
3. Results
3.1. Effects of the CB Extract on NO Production and Cell Proliferation in BV2 Cells
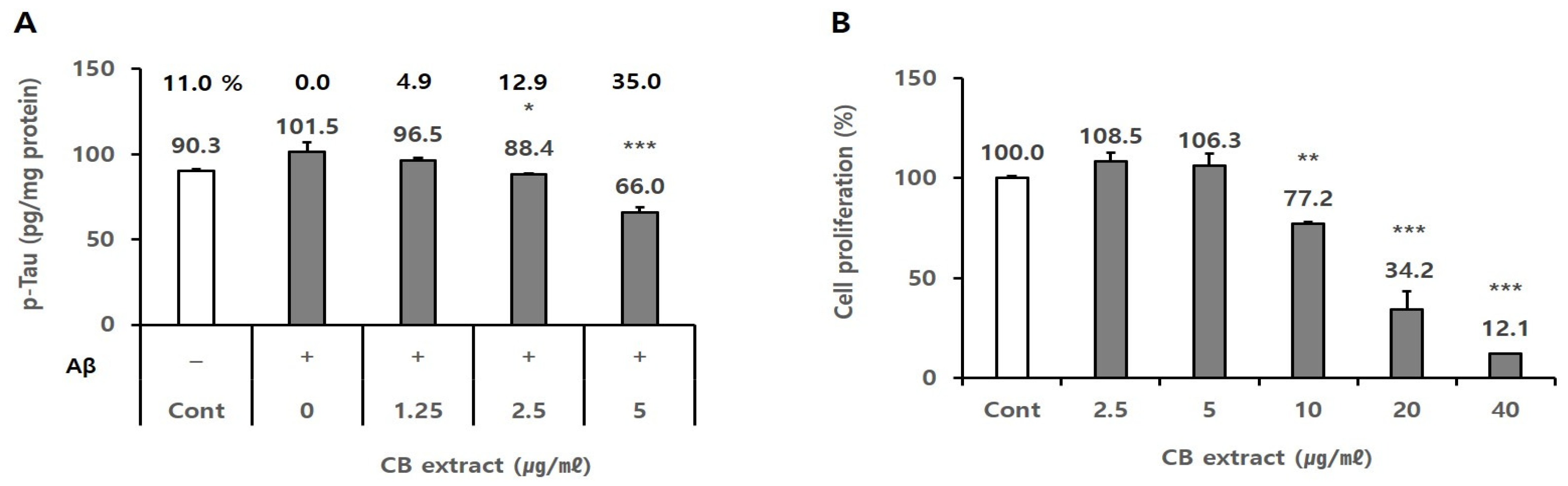
3.2. Inhibitory Effect of the CB Extract on p-Tau Production in SH-SY5Y Cells
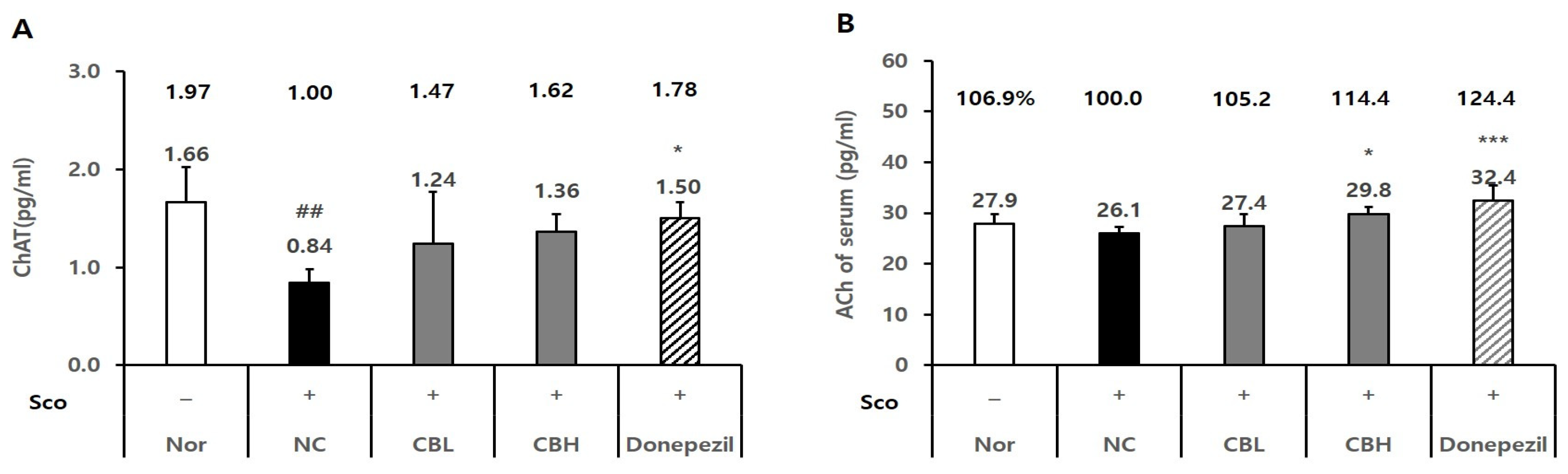
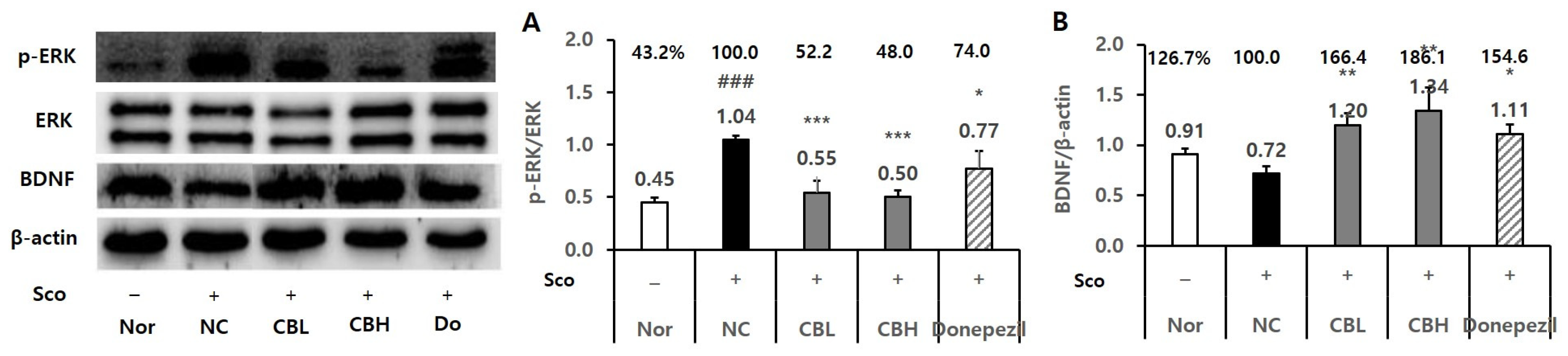
3.3. Effect of the CB Extract on the Biomarker Levels in Rats Treated Chronically with Sco
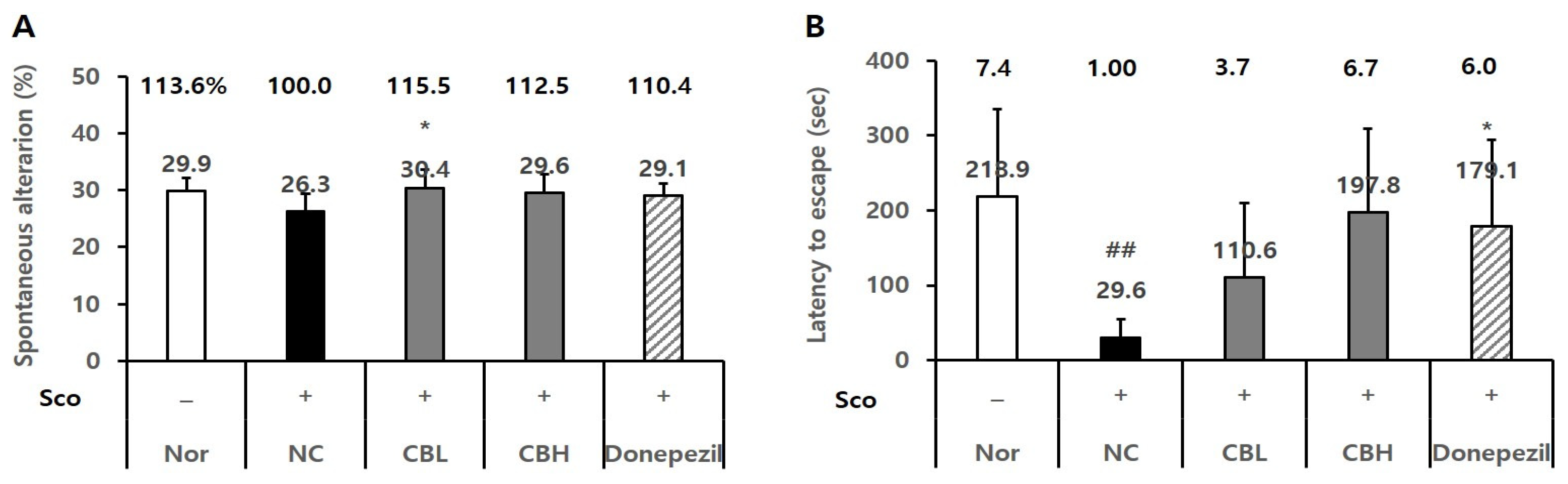
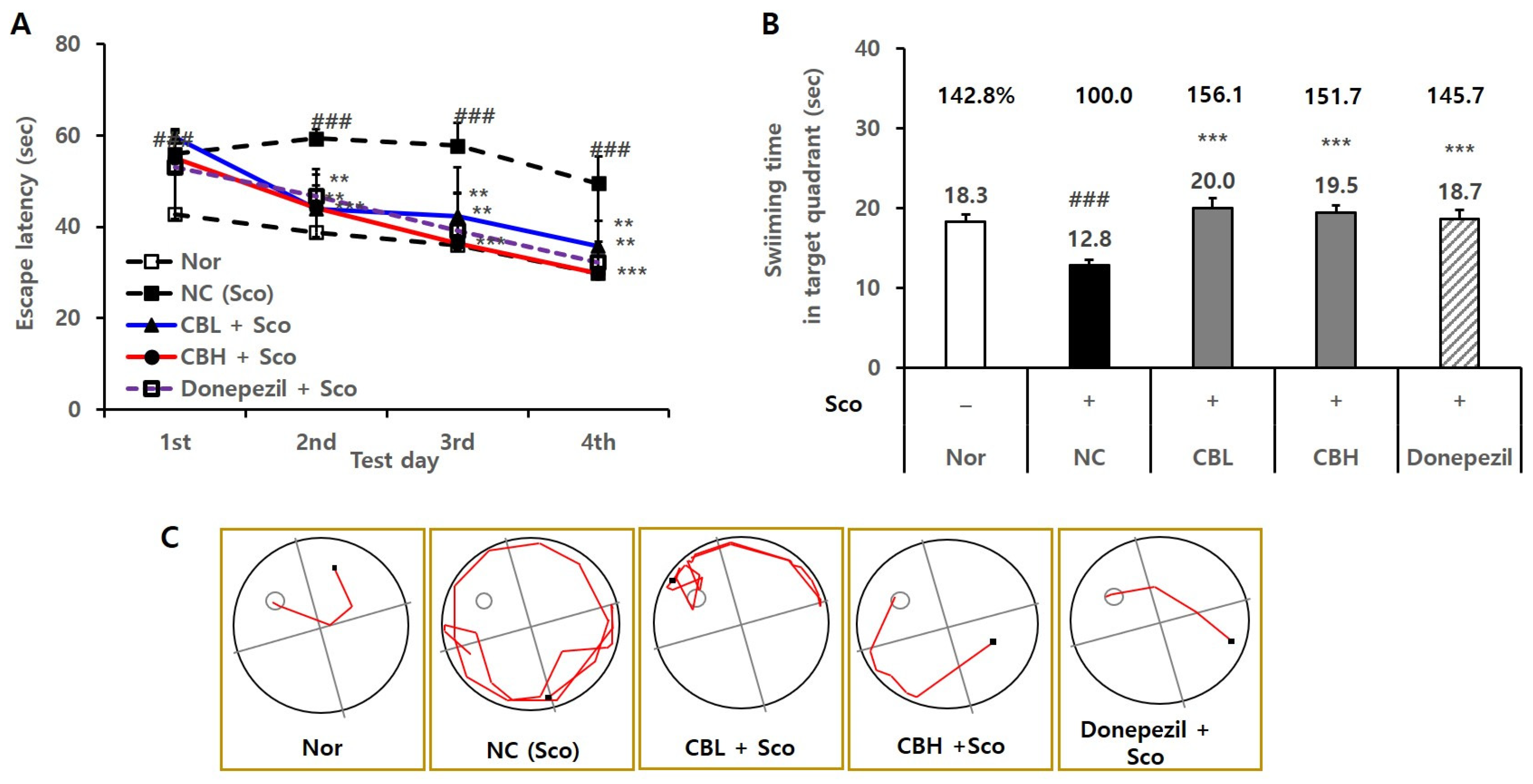
3.4. Effects of the CB Extract on the Behavior of Animals Treated Chronically and Acutely with Sco
3.5. Phenolic Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, Y.Y.; Ni, D.F.; Xu, C.M. Gene expression profiles in the olfactory bulb from a rat model of Alzheimer’s disease. J. Alzheimers Dis. 2009, 18, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J.; Anderson, L. Dementia/Alzheimer’s disease. BMC Womens Health. 2004, 4, S20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soria Lopez, J.A.S.; González, H.M.; Léger, G.C. Alzheimer’s disease. Handb. Clin. Neurol. 2019, 167, 231–255. [Google Scholar] [CrossRef]
- Burns, A.; Iliffe, S. Alzheimer’s disease. BMJ 2009, 338, b158. [Google Scholar] [CrossRef]
- Singhal, G.; Jaehne, E.J.; Corrigan, F.; Toben, C.; Baune, B.T. Inflammasomes in neuroinflammation and changes in brain function: A focused review. Front. Neurosci. 2014, 8, 315. [Google Scholar] [CrossRef]
- Scheltens, P.; Blennow, K.; Breteler, M.M.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Ozben, T.; Ozben, S. Neuro-inflammation and anti-inflammatory treatment options for Alzheimer’s disease. Clin. Biochem. 2019, 72, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Hou, J.; Ping, J.; Cai, D. Advances in developing novel therapeutic strategies for Alzheimer’s disease. Mol. Neurodegener. 2018, 13, 64. [Google Scholar] [CrossRef]
- Howes, M.J.R.; Perry, N.S.L.; Houghton, P.J. Plants with traditional uses and activities, relevant to the management of Alzheimer’s disease and other cognitive disorders. Phytother. Res. 2003, 17, 1–18. [Google Scholar] [CrossRef]
- Ansari, N.; Khodagholi, F. Natural products as promising drug candidates for the treatment of Alzheimer’s disease: Molecular mechanism aspect. Curr. Neuropharmacol. 2013, 11, 414–429. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.S.; Hong, J.Y.; Lee, J.H.; Lee, H.J.; Park, J.Y.; Choi, J.H.; Park, H.J.; Hong, J.; Lee, K.T. β-caryophyllene in the essential oil from Chrysanthemum boreale induces G1 phase cell cycle arrest in human lung cancer cells. Molecules 2019, 24, 3754. [Google Scholar] [CrossRef]
- Cha, J.D.; Jeong, M.R.; Lee, Y.E. Induction of apoptosis in human oral epidermoid carcinoma cells by essential oil of Chrysanthemum boreale Makino. Food Sci. Biotechnol. 2005, 14, 350–354. [Google Scholar]
- Song, P.; Choi, S.Y.; Hwang, J.S.; Park, H.C.; Kim, K.K.; Son, H.J.; Hong, C.O.; Kim, Y.J.; Kim, W.; Lee, K.M. Chrysanthemum boreale Makino inhibits oxidative stress-induced neuronal damage in human neuroblastoma SH-SY5Y cells by suppressing MAPK-regulated apoptosis. Molecules 2022, 27, 5498. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, J. Antioxidant activity of water extract of Chrysanthemum boreale against MPTP-induced mice models. Korean J. Physiol. Pathol. 2013, 27, 46–56. [Google Scholar]
- Lee, J.R.; Park, M.K. Isolation of guaianolides with ACAT inhibitory activity from the leaves and stems of Chrysanthemum boreale Makino. J. Environ. Sci. Int. 2017, 26, 1275–1284. [Google Scholar] [CrossRef]
- Park, S.J.; Park, M.K.; Lee, J.R. Isolation and characterization of constituent compounds from leaves and stems of Chrysanthemum boreale Makino. J. Environ. Sci. Int. 2019, 28, 993–1004. [Google Scholar] [CrossRef]
- Nam, S.H.; Yang, M.S. Antibacterial Activities of Extracts From Chrysanthemun boreale M. Assoc. Clin. Biochem. 1995, 38, 269–272. [Google Scholar]
- Nam, S.H.; Choi, S.D.; Choi, J.S.; Jang, D.S.; Choi, S.U.; Yang, M.S. Effects of sesquiterpene lactones isolated from Chrysanthemum boreale M. against sarcoma180 implanted in ICR mice. J. Korean Soc. Food Sci. Nutr. 1997, 26, 144–147. [Google Scholar]
- Jang, D.S.; Park, K.H.; Lee, J.R.; Ha, T.J.; Park, Y.B.; Nam, S.B.; Yang, M.S. Antimicrobial activities of sesquiterpene lactones isolated from Hemisteptia lyrata, Chrysanthemum zawadskii and Chrysanthemum boreale. J. Korean Agric. Chem. Biotechnol. 1999, 42, 176–179. [Google Scholar]
- You, K.; Bang, C.; Lee, K.; Ham, I.; Choi, H.Y. Anti-inflammatory effects of Chrysanthemun boreale flower. Korean J. Herbol. 2011, 26, 31–37. [Google Scholar]
- Kim, D.Y.; Won, K.J.; Yoon, M.S.; Hwang, D.I.; Yoon, S.W.; Park, J.H.; Kim, B.; Lee, H.M. Chrysanthemum boreale Makino essential oil induces keratinocyte proliferation and skin regeneration. Nat. Prod. Res. 2015, 29, 562–564. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Won, K.J.; Yoon, M.S.; Yu, H.J.; Park, J.H.; Kim, B.; Lee, H.M. Chrysanthemum boreale flower floral water inhibits platelet-derived growth factor-stimulated migration and proliferation in vascular smooth muscle cells. Pharm. Biol. 2015, 53, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Won, K.J.; Hwang, D.I.; Park, S.M.; Kim, B.; Lee, H.M. Chemical composition, antioxidant and anti-melanogenic activities of essential oils from Chrysanthemum boreale Makino at different harvesting stages. Chem. Biodiv. 2018, 15, e1700506. [Google Scholar] [CrossRef]
- Shin, K.H.; Kang, S.S.; Seo, E.A.; Shin, S.W. Isolation of aldose reductase inhibitors from the flowers of Chrysanthemum boreale. Arch. Pharm. Res. 1995, 18, 65–68. [Google Scholar] [CrossRef]
- Hong, Y.; Yang, M.S.; Pak, Y. Effect of cumambrin A treatment on blood pressure in spontaneously hypertensive rats. Korean J. Pharmacogn. 1999, 30, 226–230. [Google Scholar]
- Pyee, Y.; Chung, H.J.; Choi, T.J.; Park, H.J.; Hong, J.Y.; Kim, J.S.; Kang, S.S.; Lee, S.K. Suppression of inflammatory responses by handelin, a guaianolide dimer from Chrysanthemum boreale, via downregulation of NF-κB signaling and proinflammatory cytokine production. J. Nat. Prod. 2014, 77, 917–924. [Google Scholar] [CrossRef]
- Yang, G.; Lee, K.; Lee, D.G.; An, M.H.; Lee, M.H.; Ham, I.H.; Choi, H.Y. Effect of Chrysanthmi boreales fos on atopic dermatitis induced by 1-Choro 2,4-dinitrobenzene in NC/Nga mouse. Immunopharmacol. Immunotoxicol. 2012, 34, 413–418. [Google Scholar] [CrossRef]
- Kim, D.Y.; Won, K.J.; Hwang, D.I.; Kim, N.Y.; Kim, B.; Lee, H.M. 1-Indolehexadecane alleviates 2,4-dinitrochlorobebzene-induced atopic dermatitis in mice: Possible involvements of the skin barrier and mast cell SNARE proteins. Molecules 2022, 27, 1560. [Google Scholar] [CrossRef]
- Nugroho, A.; Lim, S.C.; Choi, J.; Park, H.J. Identification and quantification of the sedative and anticonvulsant flavone glycoside from Chrysanthemum boreale. Arch. Pharm. Res. 2013, 36, 51–60. [Google Scholar] [CrossRef]
- Kwon, Y.W.; Cheon, S.Y.; Park, S.Y.; Song, J.; Lee, J.H. Tryptnthrin suppresses the activation of the LPS-treated BV2 microglial cell line via Nrf2/HO-1 antioxidant signaling. Front. Cell Neurosci. 2017, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, G.; Brack, C.; Müller-Spahn, F.M.; Stählin, H.B.; Herrmann, M.; Renard, P.; Brockhaus, M.; Hock, C. Mercury induces cell cytotoxicity and oxidative stress and increases β-amyloid seretion and tau phosphorylation in SHSY5Y neuroblastoma cells. J. Neurochem. 2000, 74, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Bhuvanendran, S.; Kumari, Y.; Othman, L.; Shaikh, M.F. Amelioration of cognitive deficit by embelin in a scopolamine-induced alzheimer’s disease-like condition in a rat model. Fron. Pharmacol. 2018, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Sarter, M.; Bodewitz, G.; Stephens, D.N. Attenuation of scopolamine-induced impairment of spontaneous alteration behavior by antagonist but not inverse agonist and agonist β-carbolines. Psychopharmacology 1988, 94, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Pury, A.; Srivastava, P.; Pandey, P.; Yadav, R.S.; Bhatt, P.C. Scopolamine induced behavior and biochemical modifications and protective effect of Celastrus paniculatous and Angelica glauca in rats. IJNPND 2014, 4, 158–169. [Google Scholar] [CrossRef]
- Weizner, D.S.; Engler-Chiurazzi, E.B.; Kotilinek, L.A.; Ashe, K.H.; Reed, M.N. Morris water maze test: Optimization for mouse strain and testing environment. J. Vis. Exp. 2015, 100, 52706. [Google Scholar] [CrossRef]
- Mottaghipisheh, J.; Tghrir, H.; Dehsheikh, A.B.; Zomorodian, K.; Irajie, C.; Sourestani, M.M.; Iraji, A. Linarin, a glycosylated flavonoid, with potential therapeutic attributes: A comprehensive review. Pharmaceuticals 2021, 14, 1104. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.P.; Lim, Y.H.; Su, J.; Shen, H.M.; Ong, C.N. Identification and characterization of major flavonoids and caffeoylquinic acids in three compositae plants. J. Chromatogr. B 2007, 848, 215–325. [Google Scholar] [CrossRef]
- Maurer, S.V.; Williams, C.L. The cholinergic system modulates memory and hippocampal plasticity via its interactions with non-neuronal cells. Front. Immunol. 2017, 8, 1489. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, M.; Yin, X.; Chen, K.; Hu, Z.; Zhou, Q.; Cao, X.; Chen, Z.; Liu, D. The role of pathological tau in synaptic dysfunction in Alzheimer’s diseases. Transl. Neurodegener. 2021, 10, 45. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [PubMed]
- DeKosky, S.T.; Ikonomovic, M.D.; Styren, S.D.; Beckett, L.; Wisniewski, S.; Bennett, D.A.; Cochran, E.J.; Kordower, J.H.; Mufson, E.J. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann. Neurol. 2002, 51, 145–155. [Google Scholar] [CrossRef]
- Yu, T.X.; Zhang, P.; Guan, Y.; Wang, M.; Zhen, M.Q. Protective effects of luteolin against cognitive impairment induced by infusion of Aß peptide in rats. Int. J. Clin. Exp. Pathol. 2015, 8, 6740–6747. [Google Scholar] [PubMed]
- Sun, J.; Nan, G. The extracellular signal-regulated kinase 1/2 pathway neurological diseases: A potential therapeutic target [Review]. Int. J. Mol. Med. 2017, 39, 1338–1346. [Google Scholar] [CrossRef]
- Lee, J.E.; Song, H.S.; Park, M.N.; Kim, S.H.; Shim, B.S.; Kim, B. Ethanol extract of Oldenlandia diffusa herba attenuates scopolamine-induced cognitive impairments in mice via activation of BDNF, P-CREB and inhibition of acetylcholinesterase. Int. J. Mol. Sci. 2018, 19, 363. [Google Scholar] [CrossRef] [PubMed]
- Hock, C.; Heese, K.; Hulette, C.; Rosenberg, C.; Otten, U. Region-specific neurotrophin imbalances in Alzheimer disease: Decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch. Neurol. 2000, 57, 846–851. [Google Scholar] [CrossRef]
- Elrod, K.; Buccafusco, J.J. An evaluation of the mechanism of scopolamine-induced impairment in two passive avoidance protocols. Pharmacol. Biochem. Behav. 1988, 29, 15–21. [Google Scholar] [CrossRef]
- Hong, S.; Joo, T.; Jhoo, J.W. Antioxidant and anti-inflammatory activities of 3,5-dicaffeoylquinic acid isolated from Ligularia ficheri Leaves. Food Sci. Biotechnol. 2015, 24, 257–263. [Google Scholar] [CrossRef]
- Gao, L.; Li, X.; Meng, S.; Ma, T.; Wan, L.; Xu, S. Chlorogenic acid alleviates Aβ25-35-induced autophagy and cognitive impairment via the mTOR/TFEB signaling pathway. Drug Des. Dev. Ther. 2020, 14, 1705–1716. [Google Scholar] [CrossRef]
- Saitou, K.; Ochiai, R.; Kozuma, K.; Sato, H.; Koikeda, T.; Osaki, N.; Katsuragi, Y. Effect of chlorogenic acids on cognitive function: A randomized, double-blind, placebo-controlled trial. Nutrients 2018, 10, 1337. [Google Scholar] [CrossRef]
- Kato, M.; Ochiai, R.; Kozuma, K.; Sato, H.; Katsuragi, Y. Effect of chlorogenic acid intake on cognitive function in the elderly: A pilot study. Evid. Based Complement. Alternat. Med. 2018, 2018, 8608497. [Google Scholar] [CrossRef]
- Miyamae, Y.; Kurisu, M.; Murakami, K.; Han, J.; Isoda, H.; Irie, K.; Shigemori, H. Protective effects of caffeoylquinic acids on the aggregation and neurotoxicity of the 42-residue amyloid β-protein. Bioorg. Med. Chem. 2012, 20, 5844–5849. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, J.H.; Seo, M.J.; Eom, S.H.; Kim, W. Linarin down-regulates phagocytosis, pro-inflammatory cytokine production, and activation marker expression in RAW264.7 macrophages. Food Sci. Biotechnol. 2016, 25, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, X.; Liu, Y.; Di, X. Linarin inhibits the acetylcholinesterase activity in-vitro and ex-vivo. Iran. J. Pharm. Res. 2015, 14, 949–954. [Google Scholar] [PubMed]
- Fan, P.; Hay, A.E.; Marston, A.; Hostettmann, K. Acetylcholinesterase-inhibitory activity of linarin from Buddleja davidii, structure-activity relationships of related flavonoids, and chemical investigation of Buddleja nitida. Pharm. Biol. 2008, 46, 596–601. [Google Scholar] [CrossRef]

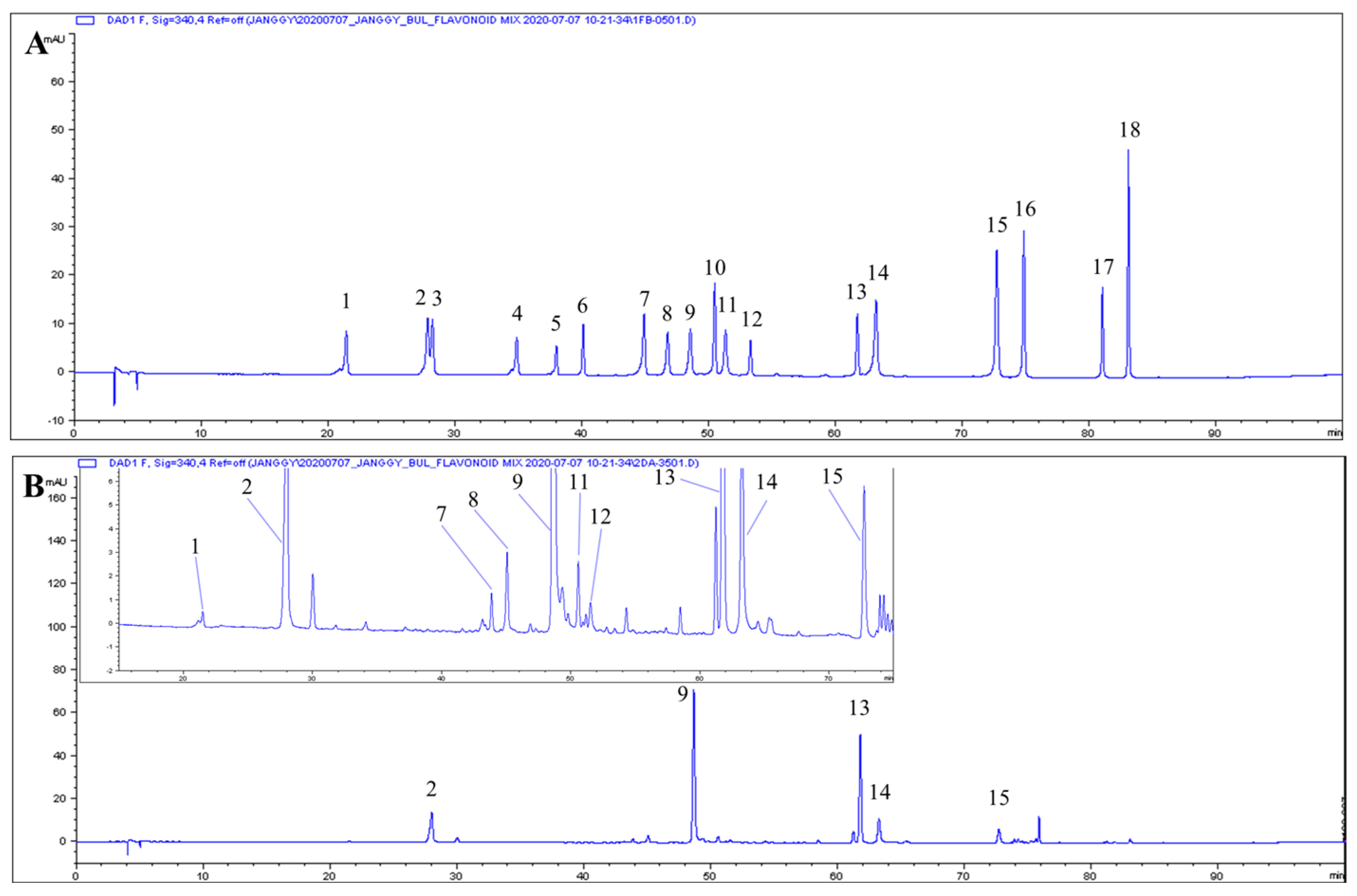
| RT (min) | Phenolic | Content (mg/g Extract, d.b.) |
|---|---|---|
| 21.40 | Neochlorogenic acid | 0.14 ± 0.002 |
| 27.88 | Chlorogenic acid | 7.37 ± 0.286 |
| 44.92 | Luteolin 7-O-glucoside | 1.39 ± 0.082 |
| 46.78 | Isochlorogenic acid B | 0.21 ± 0.018 |
| 48.58 | Isochlorogenic acid A | 34.79 ± 1.043 |
| 50.46 | Apigenin 7-O-glucoside | 0.88 ± 0.060 |
| 51.38 | Isochlorogenic acid C | 0.72 ± 0.013 |
| 53.28 | Diosmetin 7-O-glucoside | 0.14 ± 0.007 |
| 61.67 | Linarin | 24.03 ± 1.853 |
| 63.16 | Luteolin | 3.85 ± 0.137 |
| 72.65 | Apigenin | 1.53 ± 0.173 |
| 74.79 | Diosmetin | 0.15 ± 0.015 |
| 83.02 | Acacetin | 0.15 ± 0.020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Jang, G.; Jung, J.; Park, S.; Lee, J.; Lee, Y.; Lee, J.; Ji, Y.; Choi, J.; Kim, G. Memory-Improving Activity of the Flower Extract from Chrysanthemum boreale (Makino) Maskino in Scopolamine-Treated Rodents. Processes 2023, 11, 159. https://doi.org/10.3390/pr11010159
Lee S, Jang G, Jung J, Park S, Lee J, Lee Y, Lee J, Ji Y, Choi J, Kim G. Memory-Improving Activity of the Flower Extract from Chrysanthemum boreale (Makino) Maskino in Scopolamine-Treated Rodents. Processes. 2023; 11(1):159. https://doi.org/10.3390/pr11010159
Chicago/Turabian StyleLee, Seungeun, Gwiyeong Jang, Jiwook Jung, Saetbyeol Park, Jeonghoon Lee, Yunji Lee, Jihye Lee, Yunjeong Ji, Jehun Choi, and Geumsoog Kim. 2023. "Memory-Improving Activity of the Flower Extract from Chrysanthemum boreale (Makino) Maskino in Scopolamine-Treated Rodents" Processes 11, no. 1: 159. https://doi.org/10.3390/pr11010159
APA StyleLee, S., Jang, G., Jung, J., Park, S., Lee, J., Lee, Y., Lee, J., Ji, Y., Choi, J., & Kim, G. (2023). Memory-Improving Activity of the Flower Extract from Chrysanthemum boreale (Makino) Maskino in Scopolamine-Treated Rodents. Processes, 11(1), 159. https://doi.org/10.3390/pr11010159






