1. Introduction
In recent years, several co-products from manufacturing agricultural have been used as ingredients to supplements to traditional animal feed and have prompted the development of new technologies for better utilization by animals, in addition to reducing their negative impact on the environment by discarding co-products. Nanotechnology studies the phenomena and manipulation of materials at the nanoscale and contributes to the use of co-products in the agricultural sector. This has benefitted the sustainable development of animal production and shown promising results in several research areas as food, biomedical, cosmetics, and pharmaceutical [
1,
2]. Nanofibers are stable atomic structures with biodegradable characteristics and low-production costs [
3,
4]. They are obtained from renewable natural sources [
5] and can improve animal production and food safety.
Nanofibers have long-chain and amorphous cellulose structures which increase their surface area and reactivity [
6]. Nanometric-scale fibers have greater potential for absorption, reaction [
7,
8] and can serve as substrates for the fermentation of volatile fatty acids (VFA) by intestinal bacteria to increase the energy supply in animals. Animals fed a nanofiber (scale: <100 nm) diet show better zootechnical performance and greater productive efficiency [
9,
10] than those co-products rich in fiber resistant to digestive processes in monogastric animals.
El-Ratel et al. observed higher performance in rabbits that were fed a turmeric-nanoparticle-supplemented diet [
11]. Xu et al. observed a 32% improvement in weight gain in piglets fed on a nanochitosan-supplemented diet [
12]. The use of a nanomineral (nano-selenium) enriched diet increased live weight and improved feed conversion in broilers [
13,
14], and increased nutrient digestibility and milk quality in sheep [
15,
16].
Nanofiber-supplemented diets in mice can also improve intestinal health and intestinal morphology by preventing villus shortening and intestinal injuries [
9,
10,
17]. Another highlighted benefit of this diet is an improved blood–lipid profile [
7]. In addition, the inclusion of 0.75 or 1.5% cellulose nanofibers in the murine diet results in no cytotoxic effects on small intestine (SI) epithelial cells [
7], which makes them a safe source of animal nutrition.
To the best of our knowledge, no study has been conducted to evaluate the effects of adding nanofibers from soybean hulls (Glycine max) and palm heart sheaths (Bactris gasipaes) into the diet of rabbits. We hypothesized that the inclusion of nanofibers from soybean hulls and pupunha peach palm sheaths in rabbit diets would have positive effects on the health of the gastrointestinal tract, absorption, and metabolism of nutrients and the intestinal microbiota. Therefore, the present study was conducted to investigate the effects of 7% soybean-hull and 7% palm-heart-sheath nanofiber diets on the zootechnical performance, frequency of diarrhea, relative organ weight, pH of the digestive tract content, structural and ultrastructural histology of the intestine, biochemical, and immunological parameters, and cecal microbiota in growing rabbits.
2. Materials and Methods
The present study was conducted at the Rabbit Farming Sector of the Pontificia Universidade Católica of Paraná (PUCPR) for 42 d. The experiment was approved by the Ethics Committee for the Use of Animals, PUCPR, under number 903—2nd version.
2.1. Experimental Animals
Twenty-four New Zealand White rabbits (male and female) were weaned at the age of 35 d at an initial body weight (IBW) of 0.932 ± 0.171 kg. The animals were distributed in a randomized block design according to weight, placed in individual cages (80 × 60 × 45 cm), and divided into three treatment groups, with eight animals per group (n = 8). Animals received water and food ad libitum throughout the experimental period.
2.2. Production of Nanofibers
Nanofibers from soybean hulls and the sheath of the pupunha peach palm were produced through mechanical methods at the Wood Technology Laboratory of the Brazilian Agricultural Research Corporation (EMBRAPA Florestas) located in Colombo/PR. The peach palm and soybean hull samples were fragmented in a 450 W blender for 5 min and subjected to microfibrillation in a microprocessor (Super Masscolloider; Masuko Sangyo Co. Ltd., Kawaguchi-city, Saitama-pref, Japan), which consisted of a rotating disk coupled with a fixed disk containing an adjustable opening where the sample was deposited. The equipment parameters to obtain a gel with a 7% nanofiber concentration were as follows: rotation = 1500 RPM; number of passes = 30; and distance between the discs = 0.1 mm.
The nanofiber was characterized in terms of their dimensions and chemical characteristics. For the analysis, the sample was submitted to a procedure of individualization of the cellulose fibers. For this process, the sample was diluted in 4 parts of PA ethyl alcohol in eppendordf. This diluted sample was subjected to sonication for 60 min. After this step, the sample was dripped onto a screen covered with Parlodion. Once dried at room temperature, the samples were analyzed using a Transmission Electron Microscope (TEM), brand JEOL, model JEM1200EX-II, located at the Center for Electronic Microscopy (CME) of UFPR. The resulting images were processed with the software Paint.net TM version 3.5.10, which allowed for an estimation of the fibril dimensioning (
Figure 1).
The bromatological analysis of the vegetable raw materials (soybean hulls, pupunha peach palm sheath) and the respective nanofiber gels were performed at the Nutrition Laboratory at PUCPR and expressed in % based on dry matter (m/m). For weighing the samples, an analytical precision balance was used (Mars, AY220). The total fiber content was determined using the enzymatic-gravimetric method modified according to the Chemical and Physical Methods methodology for food analysis of the Analytical Norms of the Instituto Adolfo Luz [
18].
After defibrillation, the gel was stored in a refrigerator with 93% humidity and added to the feed. The inclusion of Nanosoja and Nanopupunha gel with 7% of Nanofibers in the natural matter was calculated to obtain an increment of 7% of nanofibers in the dry matter of the feed, considering the formula: Suspended mass of nanofibers (MSN) = Final mass of feed (MF) × percentage of nanofibers in the feed (NF) ÷ percentage of nanofibers in the gel (NG). The gel was composed of nanofibers in suspension, containing the following fiber levels: (1) nanosoy gel (%) contained neutral detergent fibers (NDF) = 5.82 and acid detergent fibers (ADF) = 5.34; (2) nanopupunha gel (%) contained NDF = 9.40 and ADF = 6.76. The feed was then pelleted in an electric machine (Grinder CAF-22 Machines, Rio Claro, SP, Brazil) and dried at 55 °C for approximately 16 h in a forced ventilation oven (Solab, Primar—SL-102/1540) located in the Nutrition Laboratory of PUCPR. Drying was conducted to reduce humidity and to allow greater durability of the product in the animal feed.
2.3. Diets
Diets were formulated based on recommendations for the nutritional requirements in growing rabbits [
19] (
Table 1). Three diets were tested: Control, basal diet; Nanosoy, diet containing 7% nanofibers from soybean hulls; and Nanopupunha, a diet containing 7% nanofibers from the palm heart sheath.
2.4. Performance Variables
The performance variables, final body weight (FBW), daily feed intake (DFI), daily weight gain (DBWG), and feed conversion (FC) were calculated from the individual weight of the animals weighed at the beginning and end of the experiment and from the quantification of the feed consumed and left in the trough. The occurrence and frequency of diarrhea was recorded daily according to the methodology adapted from a previous study [
20].
2.5. Relative Organ Weight
At the end of the experiment, all animals were euthanized to measure the relative weights of their digestive tract organs (stomach, liver, SI, and large intestine (LI)), kidneys, and spleen. The relative weights of the organs were calculated according to the final body weight of the rabbits.
2.6. Determination of the pH of the Stomach, Jejunal, and Cecal Contents
Immediately after the animals were euthanized, the digestive organs (stomach, jejunum, and cecum) were removed to measure the pH of the digesta using a pH meter (HI 99163; Hanna Instruments, RI, USA) following the methodology adapted from Silveira et al. [
21]. In the stomach, an incision was made approximately 2 cm from the antropyloric region. In the jejunum, an incision was made in the median portion and the cecum incision was made in the median portion for the pH measured.
2.7. Structural Analysis of the Intestinal Epithelium
Immediately after pH measurement, 3-cm-long sections from the duodenum and jejunum were collected and fixed in 10% formaldehyde for 72 h. Subsequently, at the PUCPR Histopathology Laboratory, these samples were exposed to gradual concentrations of alcohol and embedded in paraffin. Using a microtome, 4-µm-thick sections were prepared and stained using the hematoxylin-eosin stain. Slides were analyzed using light microscopy. For structural analysis, the slides were scanned using Axio Scan Z1 equipment (Carl Zeiss AG, Jena, Germany). The measurement of villus height (VH), crypt depth (CD), VH/CD ratio, villus density (VD), villus width (VW), total mucosal thickness (TMT), and wall thickness (WT) were performed using the ZEN software (ZEISS).
2.8. Ultrastructural Analysis of the Intestinal Epithelium
Eight samples measuring 0.50 × 0.50 cm2 were obtained from the duodenum and jejunum of the animals for ultrastructural analysis. At the time of collection, the samples were carefully washed with saline solution (0.9%), immersed for 1 h in Karnovsky’s fixative solution that contains glutaraldehyde 2.5% (Exodo Casv—111-30-8) and sodium cacodylated 3.424% (013850; Sigma-Aldrich, Saint Louis, MO, USA), cut, and permanently stored in the same solution. For ultrastructural analysis, the samples were fixed for approximately 2 h in 2% osmium tetroxide, dehydrated in acetone, and dried in CO2 until they reached the critical point. The samples were sputtered with gold (an electron semiconductor) and examined under a scanning electron microscope (JSM-6010PLUS/LA, Jeol, Tokyo, Japan) at Fundação Oswaldo Cruz (Fiocruz, Curitiba, PR, Brazil) to evaluate the villi structure and villi count per determined area (0.922 mm2), with six replicates per treatment.
2.9. Blood Biochemical Parameters
On day 1 and 42 of the experiment, 5 mL of blood was collected through cardiac venipuncture for analysis of blood glucose, total cholesterol, and triglycerides. The Accu-Chek Guide kit (Basel, Switzerland) was used for blood glucose analysis. For the evaluation of plasma levels of total cholesterol (Cholesterol SL reagent; ELITechgroup Inc., Sées, France) and triglycerides (Triglycerides Mono; ELITechgroup Inc.), the blood was centrifuged at 5000× g for 5 min, and the plasma was collected and transported to the PUCPR Veterinary Hospital and stored at −20 °C until needed for measurement using the automated reader (EL80; ELITech-Clinical Systems, Cairo, Egypt).
2.10. Immunology
For the quantification of specific immunoglobulins, rabbit IgG (Immunoglobulin G—ERB0171; Wuhan Fine Biotech Co., Ltd., Wuhan, Hubei, China) and rabbit IgM (Immunoglobulin M—ERB0172; Wuhan Fine Biotech Co., Ltd., Wuhan, Hubei, China) ELISA kits were used. The blood samples were centrifuged at 10,000 RPM for 5 min. The serum was collected and stored at ×20 °C until the tests were carried out at the Laboratório Imunova Análises Biológicas LTDA, PUCPR.
Serum samples were prepared according to the methodology described for rabbit IgG and IgM ELISA kits, which were based on the sandwich technique (linked to an immuno-absorbent enzyme that reacts with the added substrate). The 96-well plates were pre-coated with biotin-conjugated anti-IgG and anti-IgM antibodies that were used as detection antibodies. To perform the tests, the samples for IgG were diluted to 1:10,000 and those for IgM to 1:30,000, according to the curve fit of the kit, which ranged from 0.313–20 ng/mL. The absorbance was measured at 450 nm using an ELISA reader (Epoch2T; BioTek Instruments, Winooski, VT, USA) at the Laboratory of Agro-Food Research and Innovation at PUCPR.
2.11. Microbiota in the Cecal Content
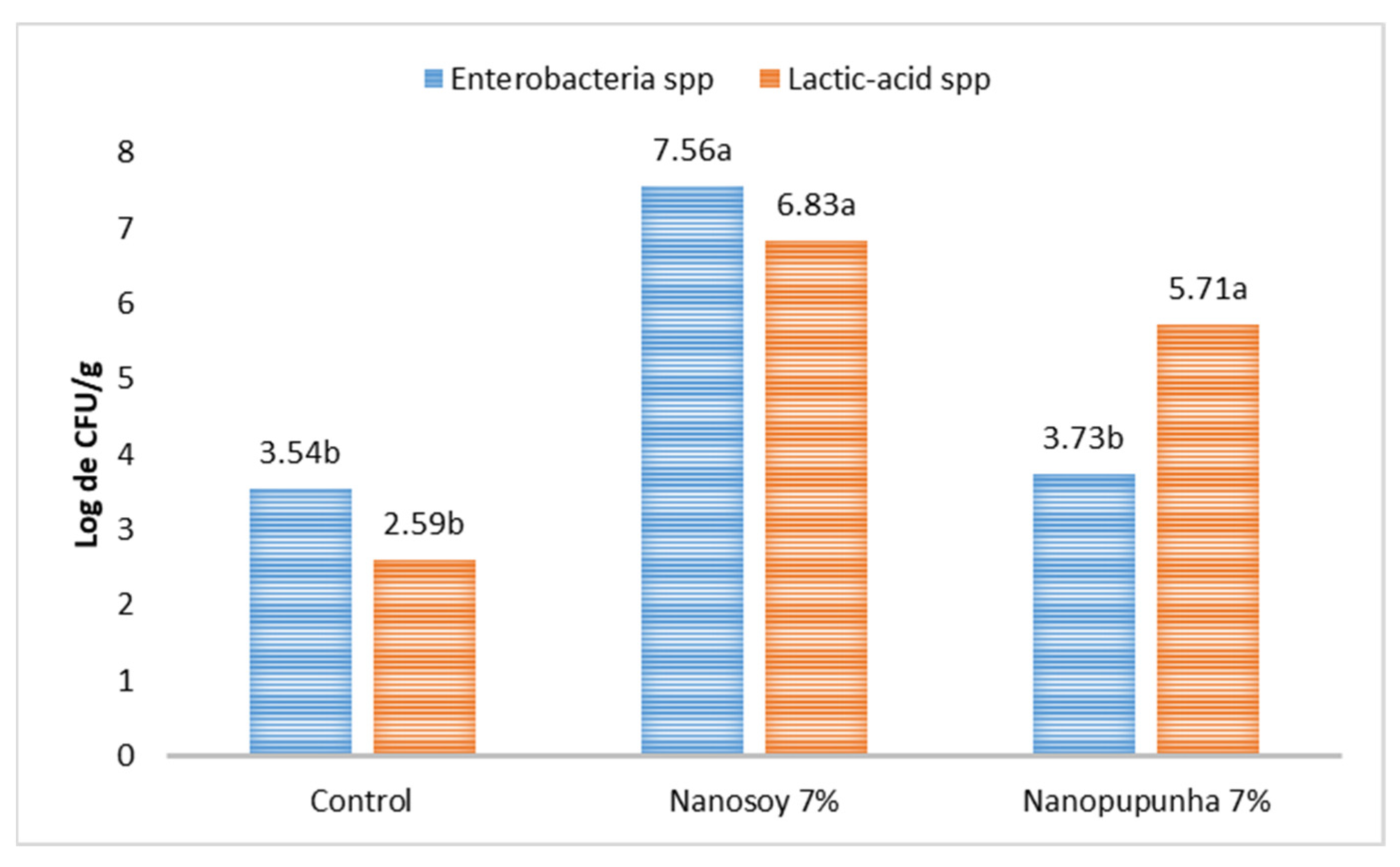
Samples of the cecal contents were collected and stored in sterile, hermetically sealed falcon tubes, and were refrigerated on ice for approximately 90 min. For the analysis of the concentrations of the different bacterial groups, approximately 1 g of digesta was weighed and decimal dilutions of the samples were performed in 1% peptone water solution (Kasvi, Conda, S.A., Madrid, Spain).
Colony counts were enumerated using the petri dish pour plate technique on MacConkey agar (Kasvi, Italy) for total Enterobacteriaceae (CBT) and on Man, Rogosa, and Sharpe agar (MRS; Sigma-Aldrich, Saint Louis, MO, USA) for lactic-acid bacteria. The plates were incubated under aerobic conditions for 24 and 48 h at 37 °C. The counts were determined according to the FDA-mandated method [
22]. Bacterial concentrations were subjected to log
10 transformation prior to analysis. After the colonies were isolated, the Enterobacteriaceae kit was used, which consisted of 10 biochemical tests associated with lactose fermentation (from the isolation medium) and allowed for the differentiation of gram-negative bacilli from other oxidase-negative bacteria.
2.12. Data Analysis
The data were submitted for testing the adequacy of the linear model using the statistical package Statgraphics 4.1. The initial live weight of the animals was used as a covariate for performance parameters. The averages of performance parameters, digestive content pH, relative organ weight, structural and ultrastructural histology of the intestine, biochemical and immunological parameters, and cecal microbiota of the treatment groups were compared using ANOVA type III, followed by Tukey’s test, and a Levene test whenever homoscedasticity was observed. A Shapiro–Wilk test was conducted to test for data normality. For all analyses,
p < 0.05 was considered statistically significant. All analyses were performed using SPSS 25 [
23].