Preliminary Study on the Thermal Behavior and Chemical-Physical Characteristics of Woody Biomass as Solid Biofuels
Abstract
1. Introduction
2. Materials and Methods
3. Results
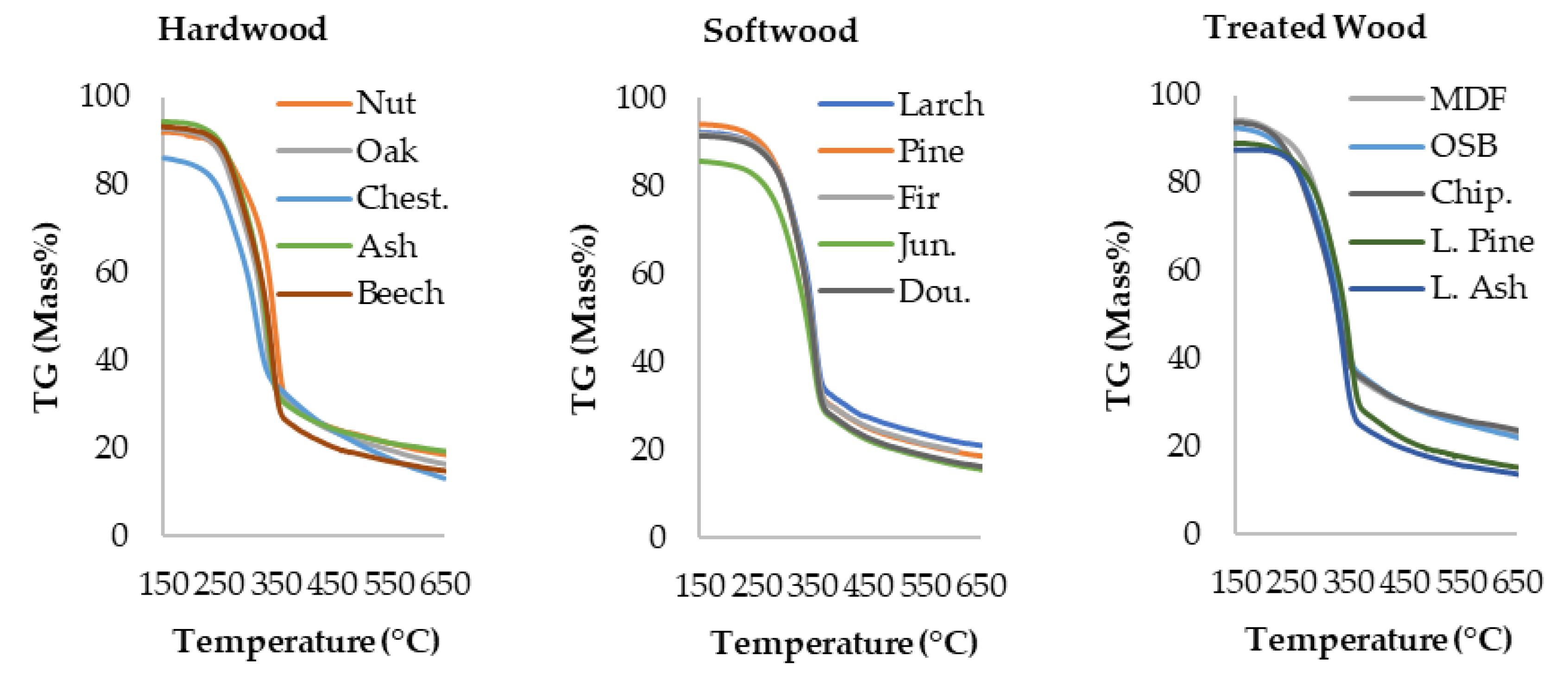
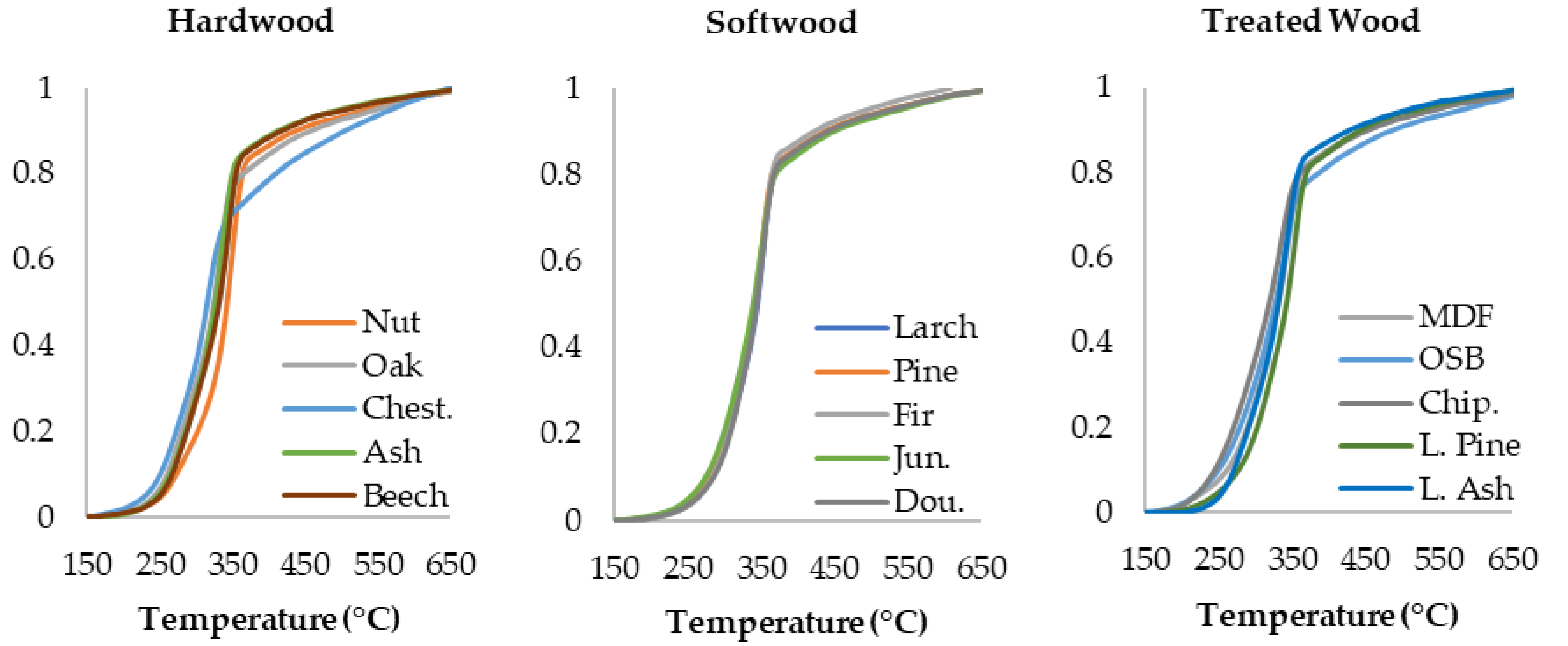
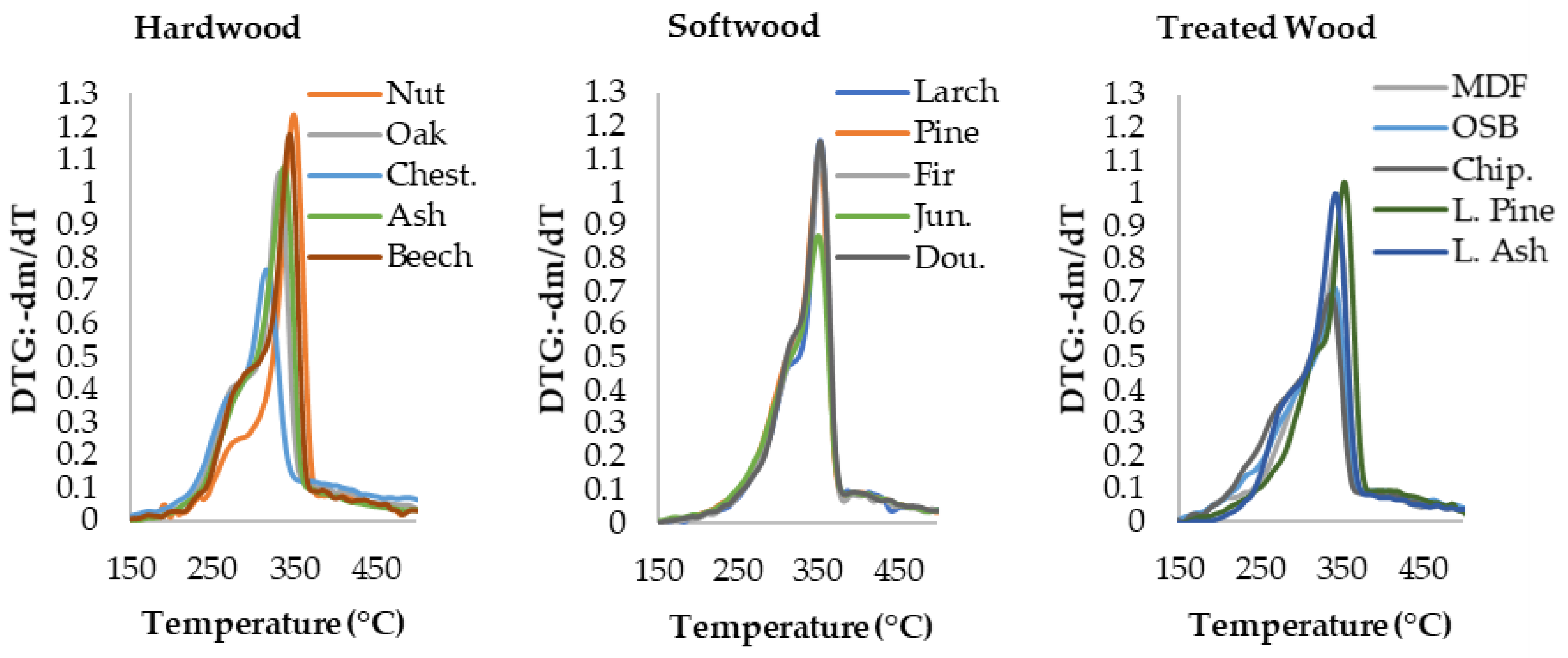
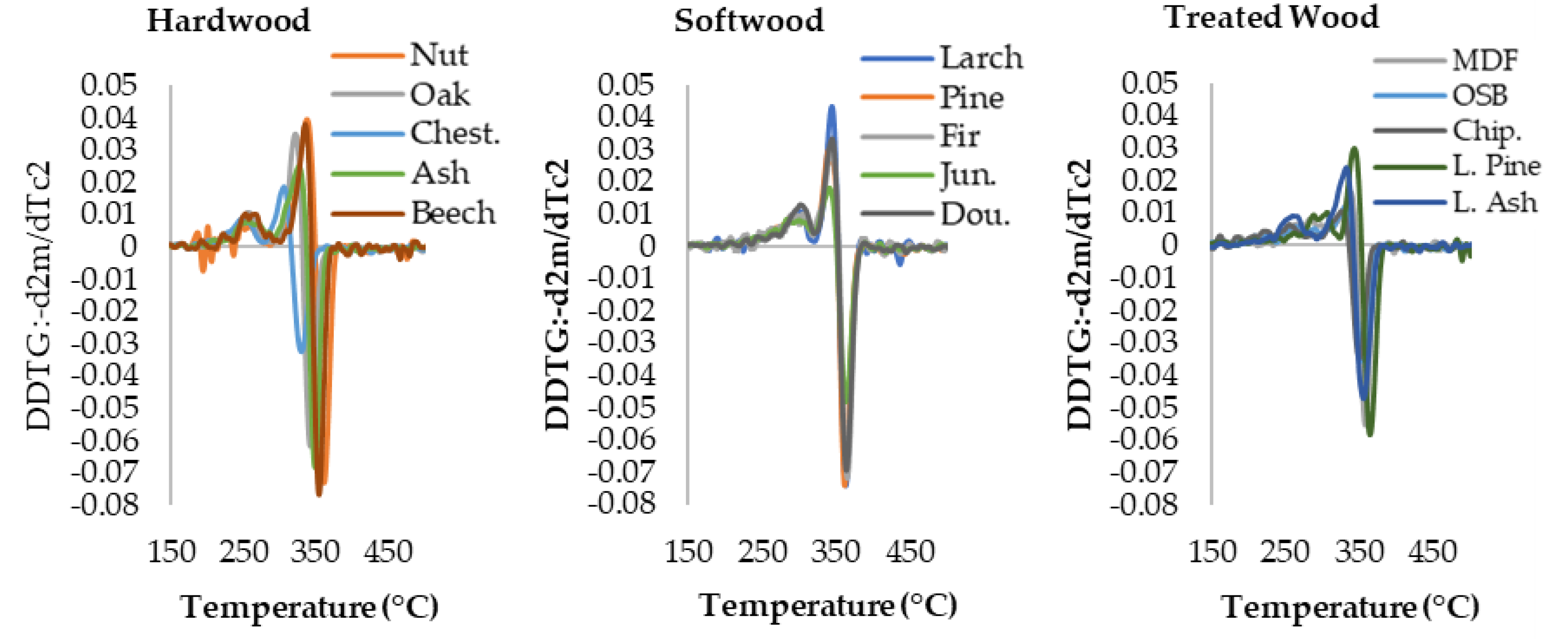
3.1. Thermogravimetric Analysis Results
3.2. Physical and Chemical Characterization Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IEA. World Energy Outlook 2021; IEA: Paris, France, 2021. [Google Scholar]
- Kopetz, H. Build a biomass energy market. Nature 2013, 494, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Balat, M.; Balat, H. Recent trends in global production and utilization of bio-ethanol fuel. Appl. Energy 2009, 86, 2273–2282. [Google Scholar] [CrossRef]
- ISO 17225-2:2021; Solid Biofuels—Fuel Specifications and Classes—Part 1: General Requirements. International Organization for Standardization: Geneva, Switzerland, 2020.
- Mancini, M.; Taavitsainen, V.M.; Toscano, G. Comparison of three different classification methods performance for the determination of biofuel quality by means of NIR spectroscopy. J. Chemom. 2019, 33, e3145. [Google Scholar] [CrossRef]
- Agovino, M.; D’Uva, M.; Garofalo, A.; Marchesano, K. Waste management performance in Italian provinces: Efficiency and spatial effects of local governments and citizen action. Ecol. Indic. 2018, 89, 680–695. [Google Scholar] [CrossRef]
- Brown, M.; Herrera, M. Combined heat and power as a platform for clean energy systems. Appl. Energy 2021, 304, 117686. [Google Scholar] [CrossRef]
- Jimenez-Navarro, J.; Kavvadias, K.; Filippidou, F.; Pavičević, M.; Quoilin, S. Coupling the heating and power sectors: The role of centralised combined heat and power plants and district heat in a European decarbonised power system. Appl. Energy 2020, 270, 115134. [Google Scholar] [CrossRef]
- Villarino, Y.; Rial, L.; Rodríguez-Abalde, A. Assessment of a residual biomass micro-combined heat and power system based on an organic Rankine Cycle coupled to a boiler. J. Environ. Manag. 2022, 301, 113832. [Google Scholar] [CrossRef]
- Martinez, S.; Michaux, G.; Salagnac, P.; Bouvier, J. Micro-combined heat and power systems (micro-CHP) based on renewable energy source. Energy Convers. Manag. 2017, 154, 262–285. [Google Scholar] [CrossRef]
- Malico, I.; Pereira, R.N.; Gonçalves, A.C.; Sousa, A.M.O. Current status and future perspectives for energy production from solid biomass in the European industry. Renew Sust. Energy Rev. 2019, 112, 960–977. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Joo, H.S.; Yang, Y.H. Biowaste-to-bioenergy using biological methods–a mini-review. Energy Convers. Manag. 2018, 177, 640–660. [Google Scholar] [CrossRef]
- Romero, M.J.A.; Capuano, D.; Miranda, C. Economic and Environmental Performance of Biowaste-to-energy Technologies for Small-scale Electricity Generation. J. Mod. Power Syst. Clean Energy 2022, 10, 12–18. [Google Scholar]
- Situmorang, Y.A.; Zhao, Z.; Yoshida, A.; Abudula, A.; Guan, G. Small-scale biomass gasification systems for power generation (<200 kW class): A review. Renew. Sustain. Energy Rev. 2020, 117, 109486. [Google Scholar]
- Safarian, S.; Unnthorsson, R.; Richter, C. Techno-economic and environmental assessment of power supply chain by using waste biomass gasification in Iceland. Biophys. Econ. Sust. 2020, 5, 7. [Google Scholar] [CrossRef]
- Riva, C.; Schievano, A.; D’Imporzano, G.; Adani, F. Production costs and operative margins in electric energy generation from biogas: Full-scale case studies in Italy. J. Waste Manag. 2014, 34, 1429–1435. [Google Scholar] [CrossRef]
- No, S.Y. Application of straight vegetable oil from triglyceride based biomass to IC engines—A review. J. Environ. Manag. 2017, 216, 235–245. [Google Scholar] [CrossRef]
- Dabi, M.; Saha, U.K. Application potential of vegetable oils as alternative to diesel fuels in compression ignition engines: A review. J. Energy Inst. 2019, 92, 1710–1726. [Google Scholar] [CrossRef]
- Mat, S.C.; Idroas, M.Y.; Hamid, M.F.; Zainal, Z.A. Performance and emissions of straight vegetable oils and its blends as a fuel in diesel engine: A review. Renew. Sustain. Energy Rev. 2018, 82, 808–823. [Google Scholar] [CrossRef]
- Biobrent. Impianti a Olio Vegetale. Available online: https://www.biobrent.it/it/impianti_olio.php (accessed on 4 June 2022).
- Altertecno. Impianti di Cogenerazione ad Olio Vegetale. Available online: http://www.altertecno.com/olio-vegetale/ (accessed on 4 June 2022).
- Compagnia Tecnica Motori. Sistemi di Generazione a Combustibili Vegetali. Available online: http://ctm.it/sistemi_generazione_combustibili_vegetali.php (accessed on 4 June 2022).
- Man Energy Solutions. Biofuels. Available online: https://www.man-es.com/marine/strategic-expertise/future-fuels/biofuel (accessed on 4 June 2022).
- Wärtsilä. Sustainable Fuels. Available online: https://wartsila.prod.sitefinity.fi/energy/sustainable-fuels/paper (accessed on 4 June 2022).
- Atelge, M.R.; Atabania, A.E.; Banuc, J.R.; Krisa, D.; Kaya, M.; Eskicioglu, C.; Kumar, G.; Lee, C.; Yildiz, Y.Ş.; Unalan, S.; et al. A critical review of pretreatment technologies to enhance anaerobic digestion and energy recovery. Fuel 2020, 270, 117494. [Google Scholar] [CrossRef]
- Ardolino, F.; Parrillo, F.; Arena, U. Biowaste-to-biomethane or biowaste-to-energy? An LCA study on anaerobic digestion of organic waste. J. Clean. Prod. 2018, 174, 462–476. [Google Scholar] [CrossRef]
- Valenti, F.; Zhong, Y.; Sun, M.; Porto, S.M.C.; Toscano, A.; Dale, B.E.; Sibilla, F.; Liao, W. Anaerobic co-digestion of multiple agricultural residues to enhance biogas production in southern Italy. J. Waste Manag. 2018, 78, 151–157. [Google Scholar] [CrossRef]
- Banja, M.; Jégard, M.; Motola, V.; Sikkema, R. Support for biogas in the EU electricity sector—A comparative analysis. Biomass Bioenergy 2019, 128, 105313. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Safarian, S.; Richter, C.; Unnthorsson, R. Waste biomass gasification simulation using aspen plus: Performance evaluation of wood chips, sawdust and mixed paper wastes. Int. J. Power Energy Eng. 2019, 7, 12–30. [Google Scholar] [CrossRef]
- Jungbluth, N.; Chudacoff, M.; Dauriat, A.; Dinkel, F.; Doka, G.; Faist, M.; Gnansounou, E.; Kljun, N.; Schleiss, K.; Spielmann, M.; et al. Life Cycle Inventories of Bioenergy: Ecoinvent Report No. 17, v2.0; Swiss Centre for Life Cycle Inventories: Dübendorf, Switzerland, 2007. [Google Scholar]
- ESPE. Il Cogeneratore a Biomassa ESPE CHiP50. Available online: https://www.espegroup.com/biomassa/cogeneratore-a-biomassa/ (accessed on 5 June 2022).
- Gruppo, R.M. Unità di Gassificazione. Available online: https://www.rmimpiantisrl.it/gassificazione/gassificazione_impianto (accessed on 5 June 2022).
- CMD. Microcogeneratore ECO20. Available online: https://eco20cmd.com/ (accessed on 5 June 2022).
- Spanner Re² GmbH. Impianti legno-energia. Available online: https://www.holz-kraft.com/it/prodotti/impianti-legno-energia.html (accessed on 5 June 2022).
- Ankur. Ankur Gasifiers. Available online: https://www.ankurscientific.com/ankur-gasifiers-msw-rdf.html (accessed on 5 June 2022).
- Wang, F.; Ouyang, D.; Zhou, Z.; Page, S.J.; Liu, D.; Zhao, X. Lignocellulosic biomass as sustainable feedstock and materials for power generation and energy storage. J. Energy Chem. 2021, 57, 247–280. [Google Scholar] [CrossRef]
- Ouyang, D.; Wang, F.; Gao, D.; Han, W.; Hu, X.; Qiao, D.; Zhao, X. Light-driven lignocellulosic biomass conversion for production of energy and chemicals. iScience 2022, 25, 105221. [Google Scholar] [CrossRef]
- Renders, T.; Van den Bosch, S.; Koelewijn, S.; Schutyser, W.; Sels, B. Lignin-first biomass fractionation: The advent of active stabilization strategies. Energy Environ. Sci. 2017, 10, 1551–1557. [Google Scholar] [CrossRef]
- Renders, T.; Den Bossche, G.V.; Vangeel, T.; Van Aelst, K.; Sels, B. Reductive catalytic fractionation: State of the art of the lignin-first biorefinery. Curr. Opin. Biotechnol. 2019, 56, 193–201. [Google Scholar] [CrossRef]
- Paone, E.; Tabanelli, T.; Mauriello, F. The rise of lignin biorefinery. Curr. Opin. Green Sustain. Chem. 2020, 24, 1–6. [Google Scholar] [CrossRef]
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Saldarriaga-Hernández, S.; Velasco-Ayala, C.; Leal-Isla Flores, P.; Rostro-Alanis, M.J.; Parra-Saldivar, R.; Iqbal, H.M.N.; Carrillo-Nieves, D. Biotransformation of lignocellulosic biomass into industrially relevant products with the aid of fungi-derived lignocellulolytic enzymes. Int. J. Biol. Macromol. 2020, 161, 1099–1116. [Google Scholar] [CrossRef]
- Poveda-Giraldo, J.A.; Solarte-Toro, J.C.; Cardona-Alzate, C.A. The potential use of lignin as a platform product in biorefineries: A review. Renew. Sust. Energy Rev. 2021, 138, 110688. [Google Scholar] [CrossRef]
- Bilal, M.; Wang, Z.; Cui, J.; Romanholo Ferreira, L.F.; Bharagava, R.N.; Iqbal, H.M.N. Environmental impact of lignocellulosic wastes and their effective exploitation as smart carriers—A drive towards greener and eco-friendlier biocatalytic systems. Sci. Total Environ. 2022, 722, 137903. [Google Scholar] [CrossRef]
- Hoang, A.T.; Ong, H.C.; Fattah, I.M.R.; Chong, C.T.; Cheng, C.K.; Sakthivel, R.; Ok, Y.S. Progress on the lignocellulosic biomass pyrolysis for biofuel production toward environmental sustainability. Fuel Process. Technol. 2021, 223, 106997. [Google Scholar] [CrossRef]
- Romero, M.J.A.; Duca, D.; Toscano, G. Advancements in the Conversion of Lipid-Rich Biowastes and Lignocellulosic Residues into High-Quality Road and Jet Biofuels Using Nanomaterials as Catalysts. Processes 2022, 10, 187. [Google Scholar] [CrossRef]
- Ding, Y.; Ezekoye, O.; Lu, S.; Wang, C.; Zhou, R. Comparative pyrolysis behaviors and reaction mechanisms of hardwood and softwood. Energy Convers. Manag. 2017, 132, 102–109. [Google Scholar] [CrossRef]
- Yao, F.; Wu, Q.; Lei, Y.; Guo, W.; Xu, Y. Thermal decomposition kinetics of natural fibers: Activation energy with dynamic thermogravimetric analysis. Polym. Degrad. Stab. 2008, 93, 90–98. [Google Scholar] [CrossRef]
- Xu, X.; Pan, R.; Chen, R. Comparative Thermal Degradation Behaviors and Kinetic Mechanisms of Typical Hardwood and Softwood in Oxygenous Atmosphere. Processes 2021, 9, 1598. [Google Scholar] [CrossRef]
- Gil, M.; Casal, D.; Pevida, C.; Pis, J.; Rubiera, F. Thermal behaviour and kinetics of coal/biomass blends during co-combustion. Bioresour. Technol. 2010, 101, 5601–5608. [Google Scholar] [CrossRef]
- Kong, B.; Wang, E.; Li, Z.; Lu, W. Study on the feature of electromagnetic radiation under coal oxidation and temperature rise based on multi-fractal theory. Fractals 2019, 27, 1950038. [Google Scholar] [CrossRef]
- Cai, P.; Nie, W.; Chen, D.; Yang, S.; Liu, Z. Effect of air flowrate on pollutant dispersion pattern of coal dust particles at fully mechanized mining face based on numerical simulation. Fuel 2019, 239, 623–635. [Google Scholar] [CrossRef]
- Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J.M.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bioenergy 2011, 35, 298–307. [Google Scholar] [CrossRef]
- Rego, F.; Soares Dias, A.P.; Casquilho, M.; Rosa, F.C.; Rodrigues, A. Fast determination of lignocellulosic composition of poplar biomass by Thermogravimetry. Biomass Bioenergy 2019, 122, 375–380. [Google Scholar] [CrossRef]
- Sanchez-Silva, L.; López-González, D.; Villaseñor, J.; Sánchez, P.; Valverde, J.L. Thermogravimetric–mass spectrometric analysis of lignocellulosic and marine biomass pyrolysis. Bioresour. Technol. 2012, 109, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.L.B.; Rodrigues, B.G.; Canettieri, E.V.; Martinez, E.A.; Palladino, F.; Wisniewski, A., Jr.; Rodrigues, D., Jr. Comprehensive approach of methods for microstructural analysis and analytical tools in lignocellulosic biomass assessment—A review. Bioresour. Technol. 2022, 348, 126627. [Google Scholar] [CrossRef]
- Valente, M.; Brillard, A.; Schönnenbeck, C.; Brilhac, J.F. Investigation of grape marc combustion using thermogravimetric analysis. Kinetic modeling using an extended independent parallel reaction (EIPR). Fuel Process. Technol. 2015, 131, 297–303. [Google Scholar] [CrossRef]
- Ikegwu, U.M.; Okoro, N.M.; Ozonoh, M.; Daramola, M.O. Thermogravimetric properties and degradation kinetics of biomass during its thermochemical conversion process. Mater. Today 2022, 65, 2163–2171. [Google Scholar] [CrossRef]
- Mancini, M.; Toscano, G.; Feliciangeli, G.; Leoni, E.; Duca, D. Investigation on woodchip quality with respect to ISO standards and relationship among quality parameters. Fuel 2020, 279, 118559. [Google Scholar] [CrossRef]
- Leoni, E.; Mancini, M.; Duca, D.; Toscano, G. Rapid Quality Control of Woodchip Parameters Using a Hand-Held Near Infrared Spectrophotometer. Processes 2020, 8, 1413. [Google Scholar] [CrossRef]
- Felix, C.B.; Chen, W.H.; Ubando, A.T.; Park, Y.K.; Lin, K.Y.A.; Pugazhendhi, A.; Nguyen, T.B.; Dong, C.D. A comprehensive review of thermogravimetric analysis in lignocellulosic and algal biomass gasification. Chem. Eng. J. 2022, 445, 136730. [Google Scholar] [CrossRef]
- Cao, W.; Li, J.; Lue, L. Study on the ignition behavior and kinetics of combustion of biomass. Energy Procedia 2017, 142, 136–141. [Google Scholar] [CrossRef]
- Kok, M.V.; Özgür, E. Thermal analysis and kinetics of biomass samples. Fuel Process. Technol. 2013, 106, 739–743. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, M.; Zhu, X.; Guo, D.; Liu, S.; Hu, Z.; Xiao, B.; Wang, J.; Laghar, M. Characteristics and kinetic study on pyrolysis of five lignocellulosic biomass via thermogravimetric analysis. Bioresour. Technol. 2015, 192, 441–450. [Google Scholar] [CrossRef]
- Musa, Y.; Bwatanglang, I.B. Current role and future developments of biopolymers in green and sustainable chemistry and catalysis. In Micro and Nano Technologies, Sustainable Nanocellulose and Nanohydrogels from Natural Sources; Elsevier: Amsterdam, The Netherlands, 2020; pp. 131–154. [Google Scholar]
- Kumar, N.; Dixit, A. Management of Biomasss. In Micro and Nano Technologies, Nanotechnology for Rural Development; Elsevier: Amsterdam, The Netherlands, 2021; pp. 97–140. [Google Scholar]
- Koch, G. Raw Material for Pulp. In Handbook of Pulp; WILEY-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2006; Volume 1, pp. 21–68. [Google Scholar]
- Li, L.; Rowbotham, J.; Greenwell, H.; Dyer, P. An Introduction to Pyrolysis and Catalytic Pyrolysis: Versatile Techniques for Biomass Conversion. In Catalytic Biomass Conversion, New and Future Developments in Catalysis; Elsevier: Amsterdam, The Netherlands, 2013; pp. 173–208. [Google Scholar]
- Parsell, T.; Yohe, S.; Degenstein, J.; Jarrell, T.; Klein, I.; Gencer, E.; Hewetson, B.; Hurt, M.; Kim, J.; Choudhari, H.; et al. A synergistic biorefinery based on catalytic conversion of lignin prior to cellulose starting from lignocellulosic biomass. Green Chem. 2015, 17, 1492. [Google Scholar] [CrossRef]
- Carrozza, C.F.; Leonardi, G.; Vasso, M.; Gelfi, C.; Serafini, A.; Gambarotti, C.; Citterio, A.; Sebastiano, R. Novel in-situ preparation of nano sized Ni (0) catalyst for depolymerization of lignin-rich waste from industrial biorefinery. Bioresour. Technol. Rep. 2020, 10, 100355. [Google Scholar] [CrossRef]
- Kouris, P.; Oevering, H.; Boot, M.D.; Hensen, E.J.M. An integrated lignin biorefinery: Scaling-up lignin depolymerization technology for biofuels and chemicals. In Proceedings of the NOVACAM Winter School, Padua, Italy, 22–23 February 2017. [Google Scholar]
- Busca, G. Production of Gasolines and Monocyclic Aromatic Hydrocarbons: From Fossil Raw Materials to Green Processes. Energies 2021, 14, 4061. [Google Scholar] [CrossRef]
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From lignin to valuable products–strategies, challenges, and prospects. Bioresour. Technol. 2019, 271, 449–461. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Barbash, V.A.; Yaschenko, O.V.; Shniruk, O.M. Preparation and Properties of Nanocellulose from Organosolv Straw Pulp. Nanoscale Res. Lett. 2017, 12, 241. [Google Scholar] [CrossRef]
- Romero, M.; Pizzi, A.; Toscano, G.; Busca, G.; Bosio, B.; Arato, E. Deoxygenation of waste cooking oil and non-edible oil for the production of liquid hydrocarbon biofuels. Waste Manag. 2016, 47, 62–68. [Google Scholar] [CrossRef]
- Romero, M.; Pizzi, A.; Toscano, G.; Casazza, A.; Busca, G.; Bosio, B.; Arato, E. Deoxygenation of non-edible vegetable oil to produce hydrocarbons over Mg-Al mixed oxydes. Chem. Eng. Trans. 2018, 64, 121–126. [Google Scholar]
- Romero, M.; Pizzi, A.; Toscano, G.; Casazza, A.; Busca, G.; Bosio, B.; Arato, E. Preliminary experimental study on biofuel production by deoxygenation of Jatropha oil. Fuel Process. Technol. 2015, 137, 31–37. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Magdziarz, A.; Dziok, T.; Sieradzka, M.; Nowak, W. Laboratory studies on the influence of biomass particle size on pyrolysis and combustion using TG GC/MS. Fuel 2019, 252, 635–645. [Google Scholar] [CrossRef]
- Da Silva, S.B.; Arantes, M.D.C.; De Andrade, J.K.B.; Andrade, C.R.; Carneiro, A.C.O.; Protásio, T.P. Influence of physical and chemical compositions on the properties and energy use of lignocellulosic biomass pellets in Brazil. Renew. Energy 2020, 147, 1870–1879. [Google Scholar] [CrossRef]
- Nanda, S.; Reddy, S.N.; Dalai, A.K.; Kozinski, J.A. Subcritical and supercritical water gasification of lignocellulosic biomass impregnated with nickel nanocatalyst for hydrogen production. Int. J. Hydrog. Energy 2016, 41, 4907–4921. [Google Scholar] [CrossRef]
- Frihart, C.R. Introduction to Special Issue: Wood Adhesives: Past, Present, and Future. For. Prod. J. 2015, 65, 4–8. [Google Scholar] [CrossRef]
- Pizzi, A. Synthetic adhesives for wood panels. Rev. Adhes. Adhes. 2014, 2, 85–126. [Google Scholar] [CrossRef]
- Cesprini, E.; Resente, G.; Causin, V.; Urso, T.; Cavalli, R.; Zanetti, M. Energy recovery of glued wood waste—A review. Fuel 2020, 262, 116520. [Google Scholar] [CrossRef]
- Mishra, R.; Mohanty, K. Kinetic analysis and pyrolysis behavior of low-value waste lignocellulosic biomass for its bioenergy potential using thermogravimetric analyzer. Mater. Sci. Energy Technol. 2021, 4, 136–147. [Google Scholar]
- Watson, J.; Zhang, Y.; Si, B.; Chen, W.T.; De Souza, R. Gasification of biowaste: A critical review and outlooks. Renew. Sust. Energy Rev. 2018, 83, 1–17. [Google Scholar] [CrossRef]
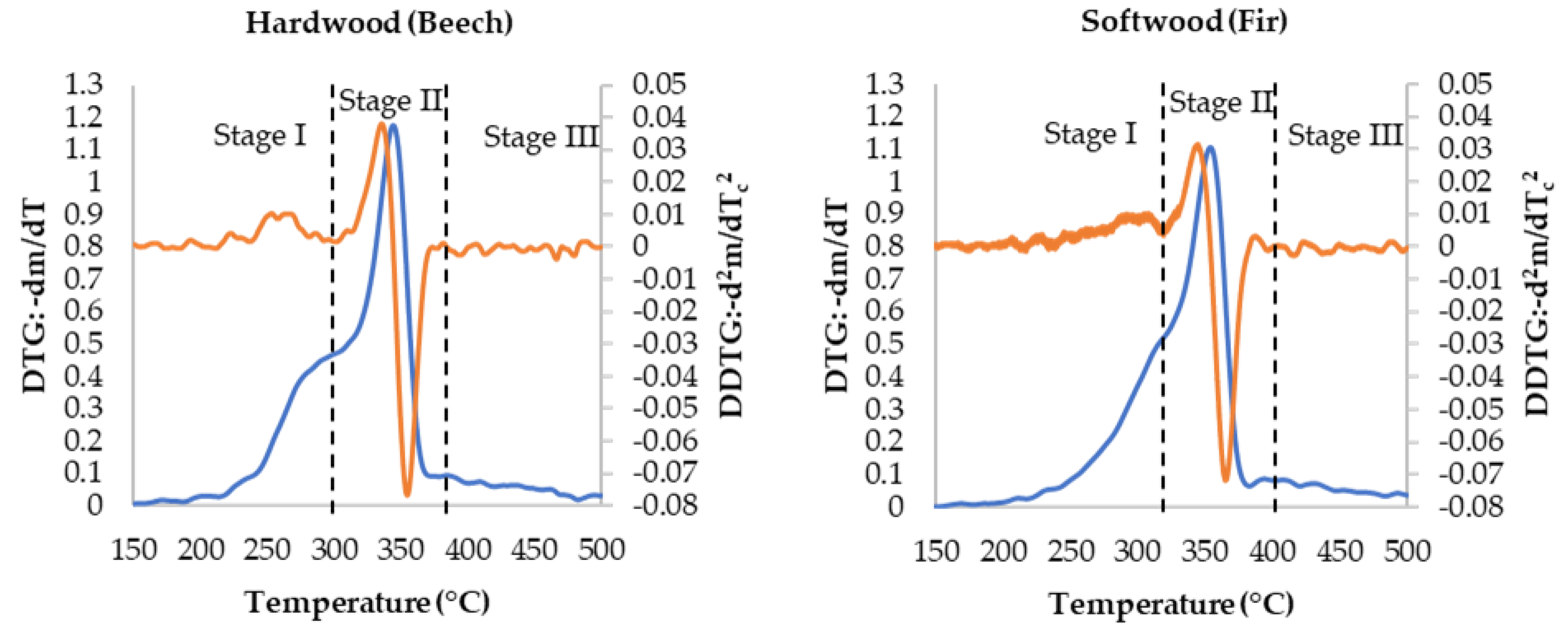
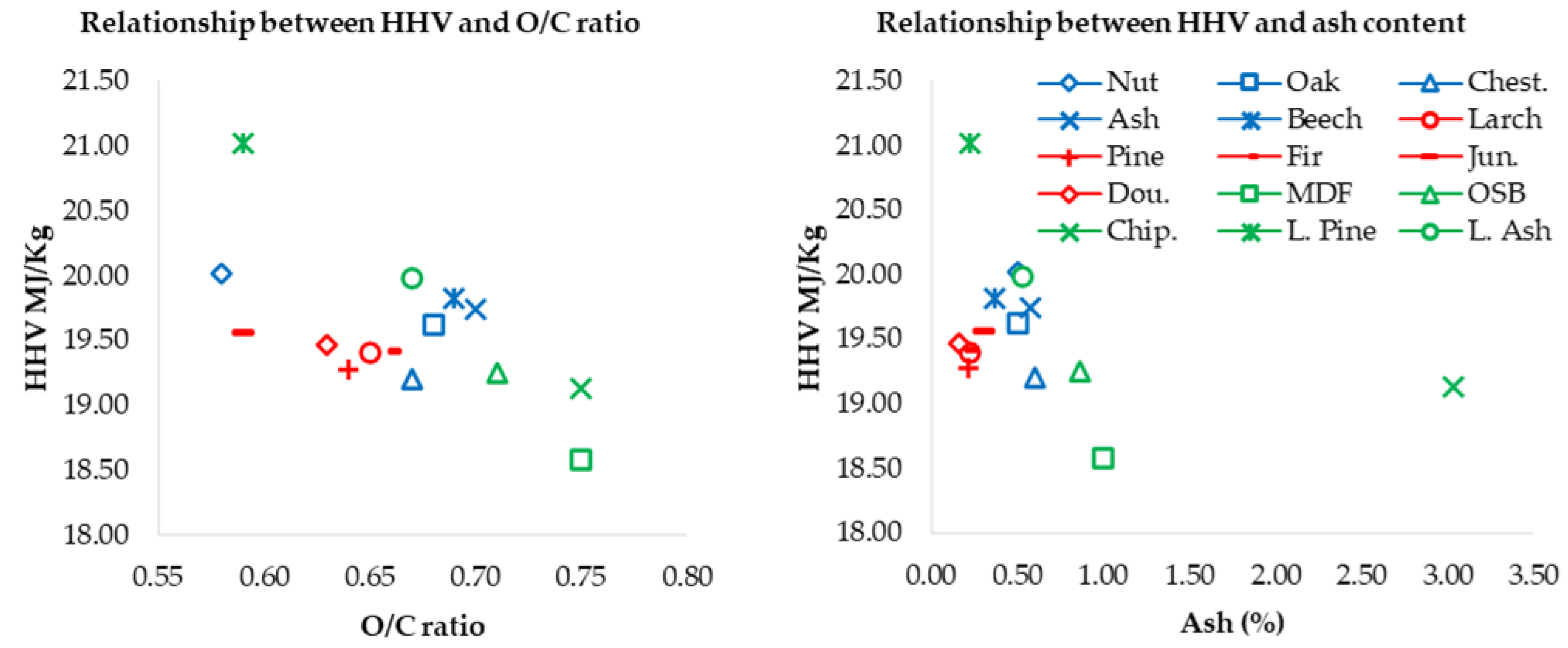
| Category | Woody Biomasses | |||||
|---|---|---|---|---|---|---|
| Hardwood | Common name | Walnut | Sessile Oak | Chestnut | Ash | Beech |
| Scientific name | Juglans regia | Quercus petraea | Castanea sativa | Fraxinus | Fagus sylvatica | |
| Abbreviation | Nut. | Oak | Chest. | Ash | Beech | |
| Softwood | Common name | Larch | Pine | Fir | Juniper | Douglas Fir |
| Scientific name | Larix Decidua | Pinus | Abies | Juniperus communis | Pseudotsuga menziesii | |
| Abbreviation | Larch | Pine | Fir | Jun. | Dou. | |
| Chemically-Treated Wood | Common name | Medium density Fibreboard | Oriented Strand Board | Chipboard | Laminated Pine | Laminated Ash |
| Scientific name | - | - | - | - | - | |
| Abbreviation | MDF | OSB | Chip. | L. Pine | L. Ash | |
| Analyses | Instrument | Method |
|---|---|---|
| Thermogravimetry | TGA LINSEIS (STA PT-1600) | - |
| Elemental Composition (CHNO) | Elemental analyser (TruSpec CHN, Leco) | ISO16948 |
| Calorific Value | Isoperibolic calorimeter mod.C200 basic, IKA | ISO18125 |
| Ash Content | TGA Leco 701 | ISO18122 |
| Hardwood | Softwood | Chemically-Treated Wood | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp. (°C) | Nut | Oak | Chest. | Ash | Beech | Larch | Pine | Fir | Jun. | Dou. | MDF | OSB | Chip. | L. Pine | L. Ash |
| 150 | 92 | 93 | 86 | 94 | 93 | 92 | 94 | 92 | 86 | 91 | 94 | 93 | 94 | 89 | 88 |
| 500 | 23 | 22 | 21 | 23 | 19 | 25 | 23 | 23 | 20 | 21 | 28 | 27 | 28 | 19 | 17 |
| 650 | 19 | 16 | 13 | 19 | 15 | 21 | 19 | 19 | 16 | 16 | 23 | 22 | 24 | 15 | 14 |
| Hardwood | Softwood | Chemically-Treated Wood | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nut | Oak | Chest. | Ash | Beech | Larch | Pine | Fir | Jun. | Dou. | MDF | OSB | Chip. | L. Pine | L. Ash | |
| C (%) | 52.10 | 49.10 | 49.20 | 48.24 | 48.40 | 50.07 | 50.55 | 49.86 | 52.49 | 50.70 | 45.20 | 46.10 | 44.50 | 52.36 | 49.16 |
| H (%) | 6.53 | 6.05 | 5.80 | 5.98 | 5.95 | 5.96 | 6.08 | 6.16 | 6.14 | 6.26 | 6.45 | 6.70 | 6.48 | 6.21 | 6.12 |
| N (%) | 0.33 | 0.11 | 0.20 | 0.21 | 0.17 | 0.23 | 0.12 | 0.12 | 0.15 | 0.11 | 3.28 | 2.47 | 2.87 | 0.10 | 0.13 |
| O (%) | 40.57 | 44.25 | 44.2 | 45.00 | 45.13 | 43.52 | 43.04 | 43.66 | 40.92 | 42.77 | 45.07 | 43.87 | 43.12 | 41.11 | 44.06 |
| Ash (%) | 0.50 | 0.50 | 0.60 | 0.57 | 0.35 | 0.22 | 0.20 | 0.20 | 0.30 | 0.16 | 1.00 | 0.86 | 3.03 | 0.22 | 0.53 |
| Hardwood | Softwood | Chemically-Treated Wood | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nut | Oak | Chest. | Ash | Beech | Larch | Pine | Fir | Jun. | Dou. | MDF | OSB | Chip. | L. Pine | L. Ash | |
| H/C | 1.49 | 1.47 | 1.40 | 1.47 | 1.46 | 1.41 | 1.43 | 1.47 | 1.39 | 1.47 | 1.70 | 1.73 | 1.73 | 1.41 | 1.48 |
| N/C | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 | 0.05 | 0.09 | 0.00 | 0.00 |
| O/C | 0.58 | 0.68 | 0.67 | 0.70 | 0.70 | 0.65 | 0.64 | 0.66 | 0.59 | 0.63 | 0.75 | 0.71 | 0.75 | 0.59 | 0.67 |
| HHV (MJ/Kg) | 20.02 | 19.62 | 19.20 | 19.74 | 19.82 | 19.40 | 19.27 | 19.42 | 19.56 | 19.47 | 18.58 | 19.25 | 19.13 | 21.02 | 19.98 |
| LHV (MJ/Kg) | 18.60 | 18.30 | 17.88 | 18.44 | 18.51 | 18.10 | 17.92 | 18.08 | 18.23 | 18.10 | 17.34 | 17.98 | 17.78 | 18.57 | 18.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero, M.J.A.; Duca, D.; Maceratesi, V.; Di Stefano, S.; De Francesco, C.; Toscano, G. Preliminary Study on the Thermal Behavior and Chemical-Physical Characteristics of Woody Biomass as Solid Biofuels. Processes 2023, 11, 154. https://doi.org/10.3390/pr11010154
Romero MJA, Duca D, Maceratesi V, Di Stefano S, De Francesco C, Toscano G. Preliminary Study on the Thermal Behavior and Chemical-Physical Characteristics of Woody Biomass as Solid Biofuels. Processes. 2023; 11(1):154. https://doi.org/10.3390/pr11010154
Chicago/Turabian StyleRomero, Max J. A., Daniele Duca, Vittorio Maceratesi, Sara Di Stefano, Carmine De Francesco, and Giuseppe Toscano. 2023. "Preliminary Study on the Thermal Behavior and Chemical-Physical Characteristics of Woody Biomass as Solid Biofuels" Processes 11, no. 1: 154. https://doi.org/10.3390/pr11010154
APA StyleRomero, M. J. A., Duca, D., Maceratesi, V., Di Stefano, S., De Francesco, C., & Toscano, G. (2023). Preliminary Study on the Thermal Behavior and Chemical-Physical Characteristics of Woody Biomass as Solid Biofuels. Processes, 11(1), 154. https://doi.org/10.3390/pr11010154










