1. Introduction
Periprosthetic fractures (PFs) can occur at multiple locations in both the upper and lower extremities, but they are significantly more common in the hip region (over 50%) [
1]. These fractures pose a serious challenge in orthopedic practice, highlighting the need for careful management and prevention strategies [
2]. The most common cause of these fractures is a fall from the same level, accounting for 76% of cases. In the upper limb, 73% of fractures occur during surgery. The risk of fractures also increases during the removal of implants, making the timing of the intervention critical and necessitating special attention. Additionally, spontaneous fractures are frequently seen after arthroplasty revision due to reduced bone density [
3].
PFs associated with total hip arthroplasty (THA) were first reported by Horwitz and Lenobel in 1954. The frequency of these fractures varies significantly, ranging from 0.045% to 4.1%. Several risk factors influence this variability, including gender, age (particularly for individuals over 70 years), the type of prosthesis used (cemented or cementless), the stability of the implant, femoral stem loosening, osteoporosis, and the use of bisphosphonates [
3]. Research on age and gender as risk factors for peri-prosthetic femoral fractures after total hip arthroplasty (THA) is inconsistent. Some studies indicate that the risk of periprosthetic fractures is higher, similar, or lower in women compared to men [
4].
One registry study suggests that those of age over 70 and female sex are at risk for these fractures, but most of the research is retrospective and small in size, unadjusted for confounding factors. Also, evidence about other risk factors, such as implant types or fracture history, is limited, and no previous studies have analyzed comorbidity (cardio-respiratory, neurological, musculoskeletal disorders, and diabetes mellitus) as a risk factor. Increasing comorbidity in the aging population is a health challenge. In the context of expanding indications for THA, it is essential to understand the impact of comorbidity and body mass index on periprosthetic fractures, which emphasizes the need for robust studies in this area [
5].
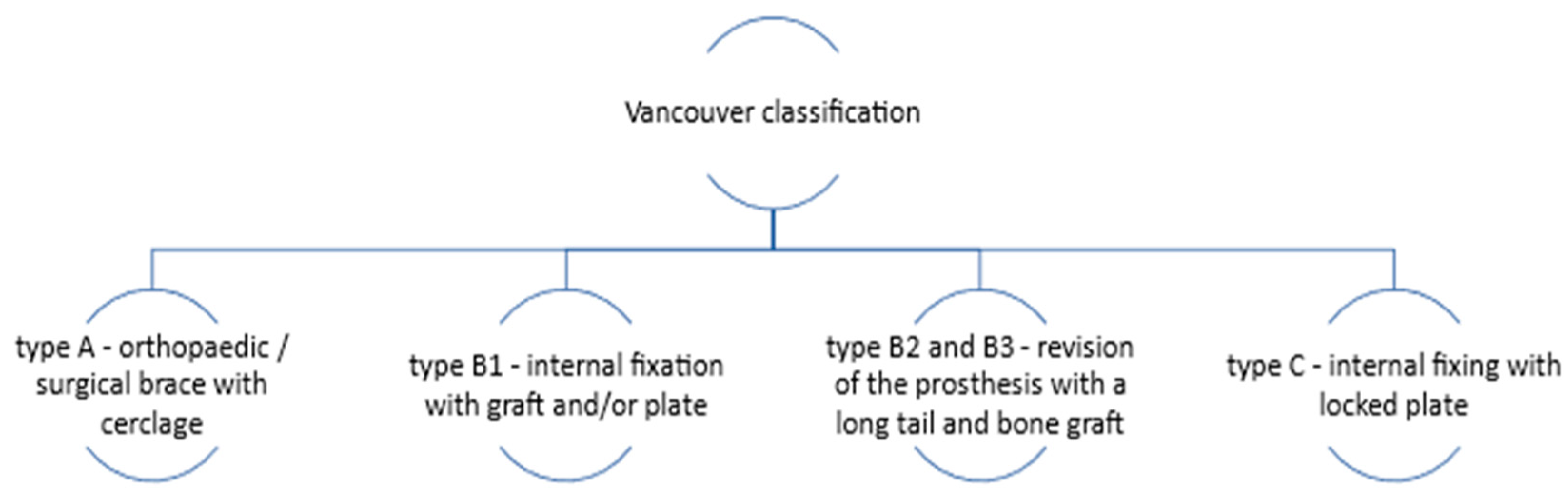
The standard treatment for THA-associated PFs involves surgical procedures such as internal fixation or revision arthroplasty (a therapeutic procedure used to replace a previous arthroplasty), which depend on the Vancouver classification of the fracture (
Figure 1). This classification is based on the location of the fracture, the stability of the prosthesis, and the quality of the surrounding bone. If the femoral component is stable, open reduction and internal fixation are recommended. An overview of the therapeutic approach according to the Vancouver classification is illustrated in
Figure 1 [
6,
7,
8].
The immediate complications associated with surgery include wound issues, which may be accompanied by infections (either superficial or deep), deep vein thrombosis, pulmonary embolism, sciatic nerve palsy, prosthesis dislocation, and refracture, as after primary THA intervention [
9]. The incidence of these complications tends to increase with age. The mortality associated with arthroplasty procedures is less than 2.5% during hospitalization. The literature suggests a significant increase in distant mortality (3.5% at 30 days, 4.8% at 90 days, and 13.4% at one year) [
10].
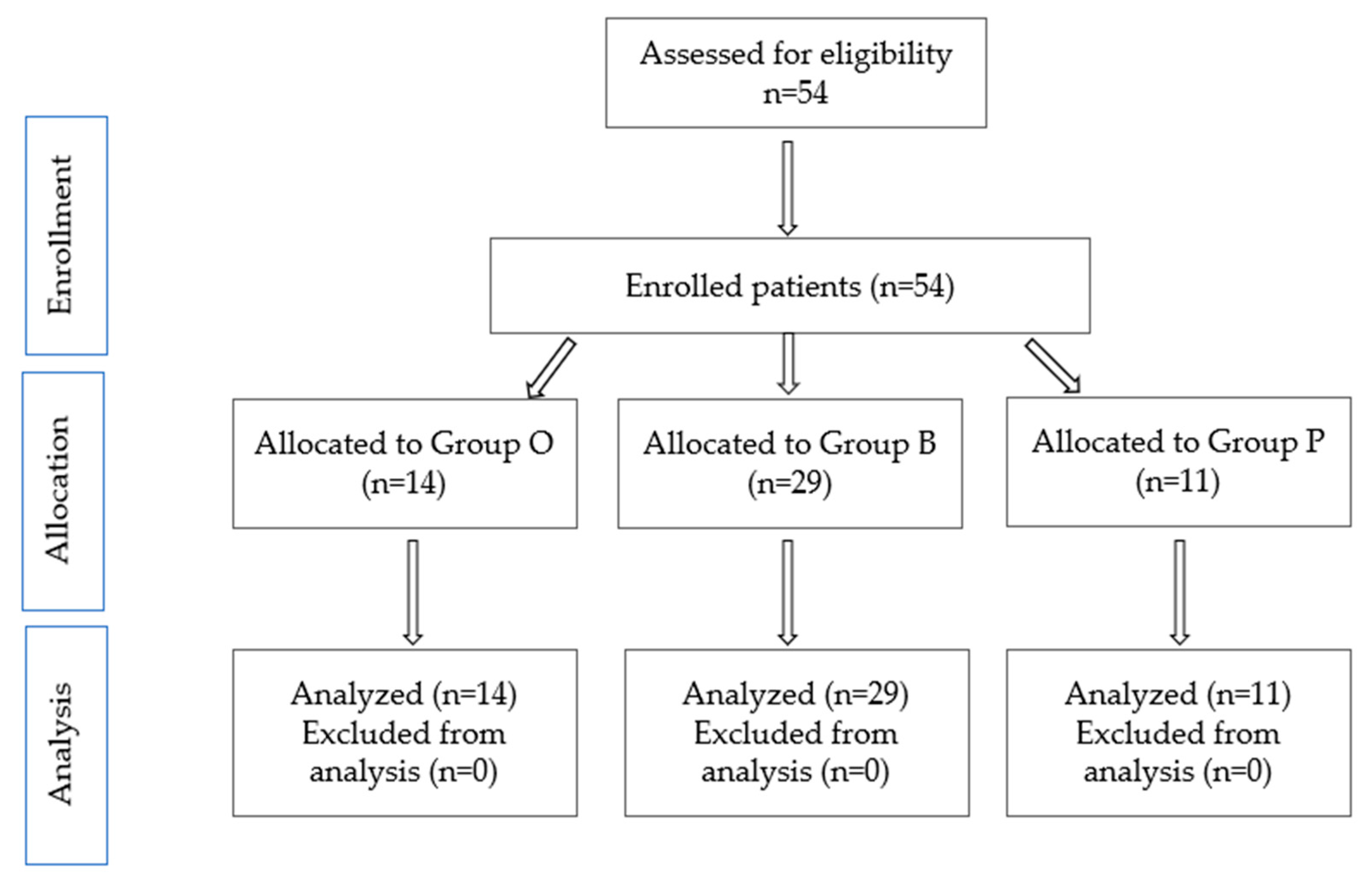
The study aims to analyze the incidence of THA-associated PFs, immediate postoperative complications, and comorbidities in patients with PFs, THA-associated, from three emergency hospitals in different areas of Romania. A secondary aim of the study is to identify the relationship between preoperative comorbidities, as assessed by the Deyo–Charlson Index, which is considered a valid measure for assessing comorbidities, and immediate postoperative complications [
11].
4. Discussion
The aim of the study was to describe the incidence of PFs, immediate postoperative complications, and comorbidities in patients with PFs from three emergency hospitals in Romania.
The prevalence of PFs is increasing, as predicted by Della Rocca et al., based on increasing life expectancy and the increasing number of total hip arthroplasties [
15]. The study published by Minutillo GT (2024) emphasizes the increase in the incidence of periprosthetic hip fractures from 2016 to 2021 by 38% and estimates an increase in periprosthetic fractures, in general, of 212% in 2032 (compared to 2016) (5). In our study, the frequency of these fractures is different according to the evaluated center. Thus, in Group O, the frequency is 2.82% vs. 7.75% (group B) and 5.39% (group P); the explanation could be the increased addressability in the university center Bucharest. At the cohort level, the prevalence of FPs is 5.02%—higher than in other studies [
3].
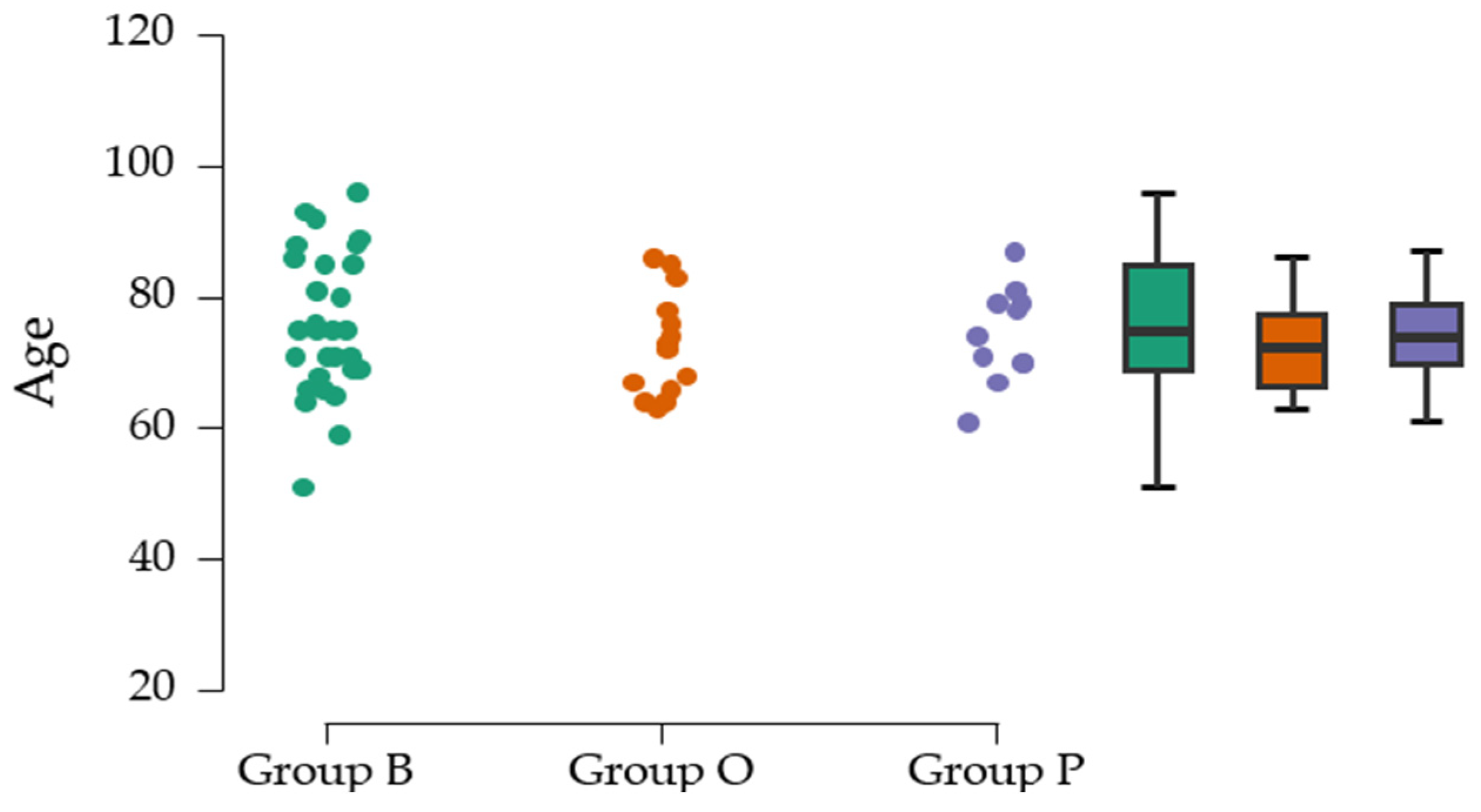
The mean age at the cohort level is 74.74 ± 9.52 years and is comparable with other results published in the literature [
4], with no significant differences in mean age between the three groups evaluated. The most common mechanism of periprosthetic fractures (PFs) in our study was falls, with a frequency of 88.89%. Literature data support this main mechanism but indicate a lower frequency of 76% [
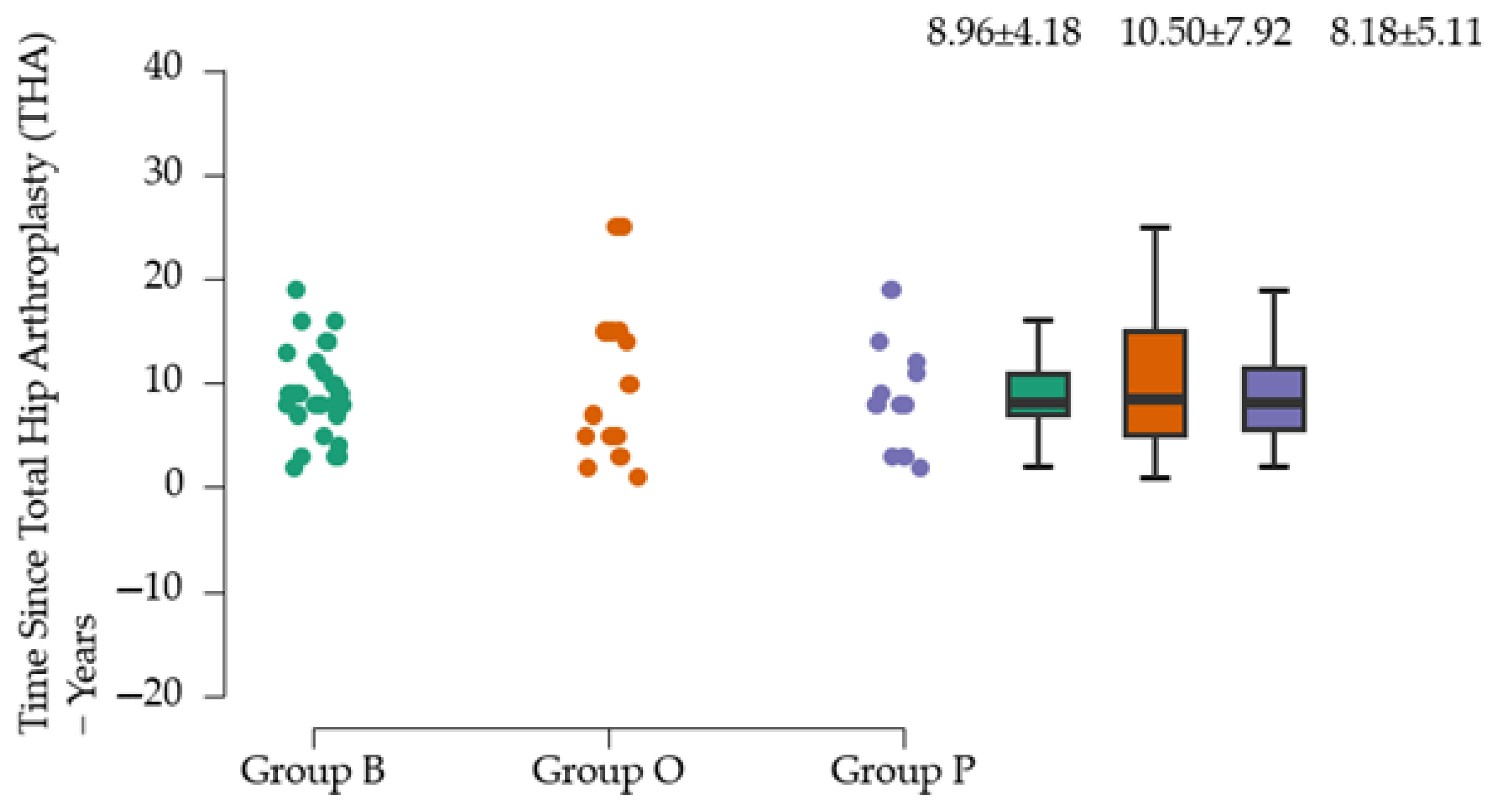
3]. In groups P and O, injuries caused by falls from the same level are the only cause identified. According to our analysis, motor vehicle accidents account for about 10% of all identified causes of PFs. Our study also suggests that about 50% of PFs occur within 3–10 years after THA. The incidence of PFs in women is higher in our study (62.94%) and in similar studies, where an incidence of 66% was reported [
16]. Group O has the highest total number of THA interventions, which can be explained by the high number of patients from neighboring counties. Group P has the lowest total number of interventions, which is due to the fact that it is located close to other hospitals in the capital city. The surgical approach for implanting the primary prosthesis was predominantly lateral; in group B, the posterior approach was chosen for intervention in 10%.
Data analysis suggests a low percentage of cemented prosthesis use (less than 5%), which can be explained by the fact that studies have shown that uncemented revision arthroplasty is more effective, as it reduces the risks related to complications caused by cement extrusion [
17]. Cement fixation was an important risk factor for periprosthetic fractures, as individuals with cemented implants had a reduced likelihood of experiencing such fractures [
5,
18]. However, the multicenter PIPPAS study published in 2024 (
n = 1387) suggests that THA-associated PFs occurred equally in cemented and uncemented strains [
1], in contrast to our study, in which fractures occurring on cemented prostheses were 5.55%. Also in this study, a higher rate of THA-associated PFs (6.2%) compared to PFs with other localizations is reported.
Most PFs belonged to the Vancouver B classification, similar to the results obtained by other authors [
16]. In terms of therapeutic management, changes in favor of open reduction and internal fixation versus revision arthroplasty have been reported [
19,
20]. Given this Vancouver classification, the predominant surgical technique approached in this study was internal fixation with cerclage (more than 50%). Data analysis suggests the absence of significant differences between the centers evaluated in terms of therapeutic approach technique, incidence of postoperative complications, and discharge status.
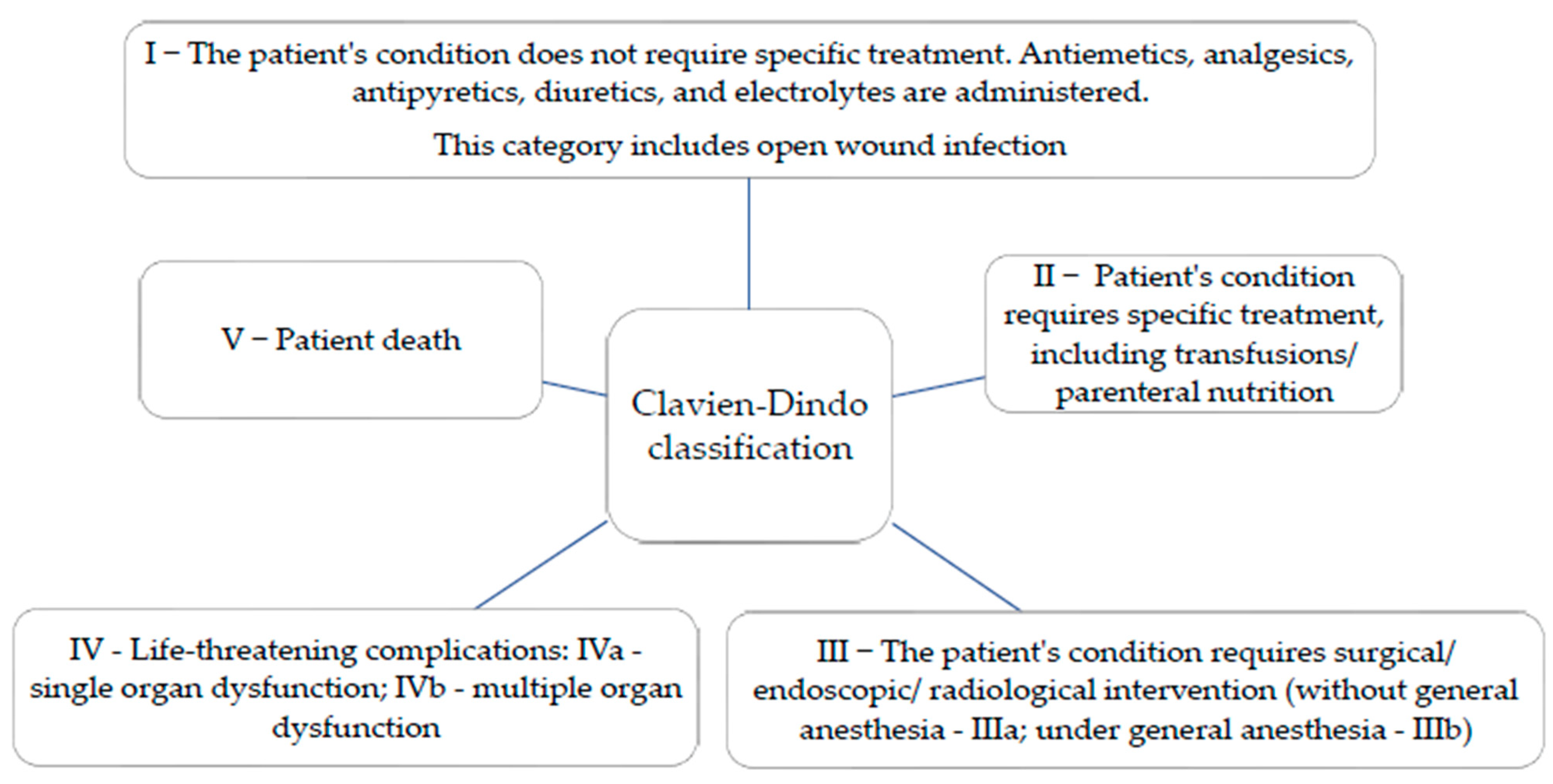
Most complications across all three groups are classified as low severity (index 1). However, Group O shows a higher incidence of moderate severity complications (indexes 2 and 3) compared to Group B. Group P exhibits a similar profile to Group B regarding minor complications, but with a slightly higher frequency of moderate complications. Indexes 4 and 5 are very rare, indicating effective management of complications within these groups.
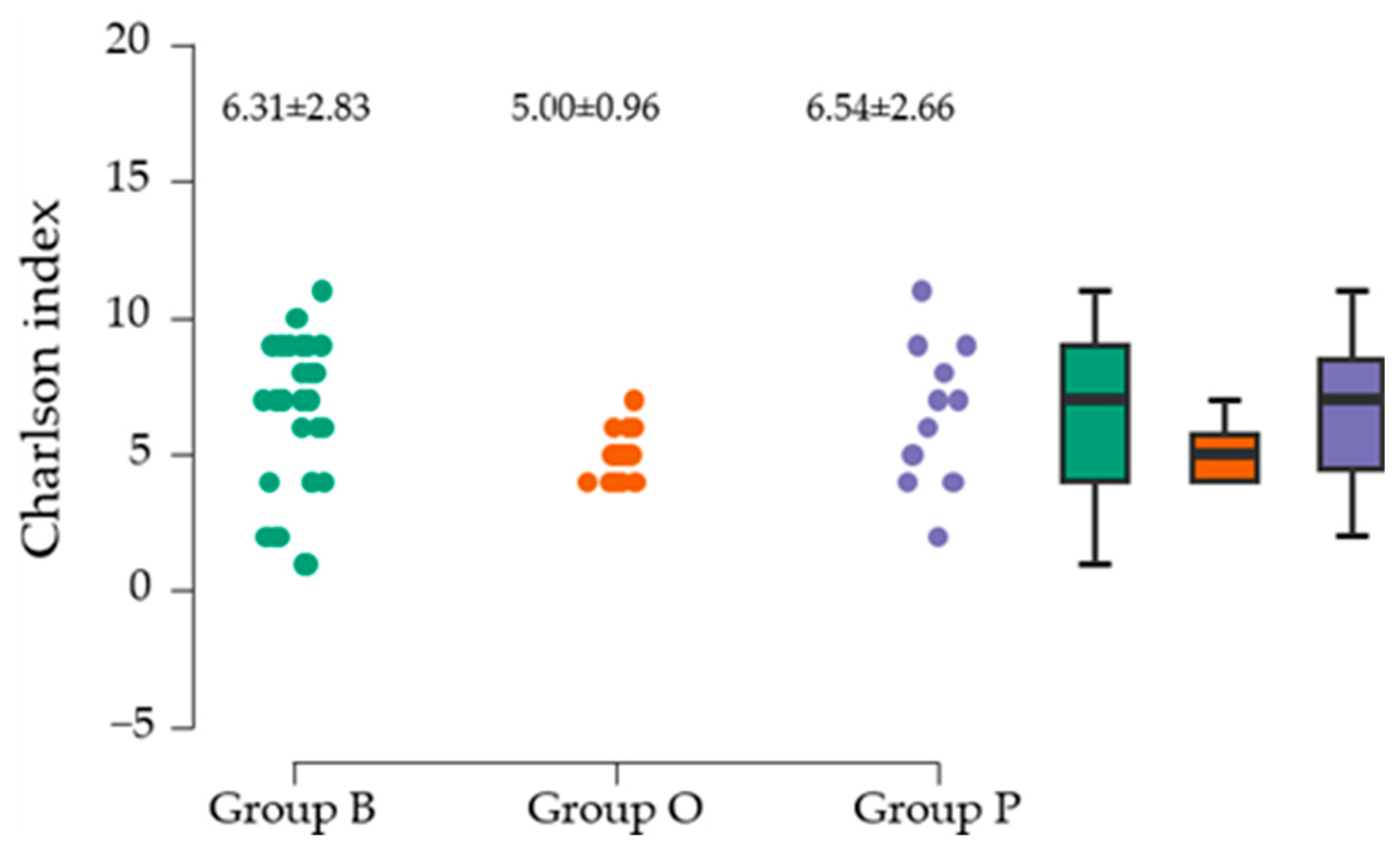
Another objective of the research was to establish the relationship between preoperative comorbidities, analyzed using the CCI, a tool recognized for its validity in assessing comorbidities, and immediate postoperative complications. The most common associated comorbidities were diabetes mellitus and cardiac disease. Cardiovascular diseases associated with PFs in our study are present in more than 90% of patients. The mean value of the CCI did not differ significantly between the three groups evaluated. All three deaths in the study were associated with a CCI above 5, which correlates with the results of the multicentric PIPPAS study [
1]. The study published by B.Y. Park (2019) [
21] aimed to correlate comorbidities with postoperative complications in 110 THA patients. The association of superficial infections in patients with diabetes was found [
21]. Our study reports a death incidence of 3.70%, which is higher than the 2.5% reported by JN Lamb et al. in 2022 (
n = 4841) [
6].
The three deaths (two women) all occurred in group B, and the age of the patients was over 85 years. This trend can be attributed to several factors, including the patients’ age (all over 85 years), the larger patient population, and the complexity of cases referred to the Bucharest Emergency Clinical Hospital, one of the largest hospitals in Romania. The incidence of death in group B (10.34%) is twice the value published by Nasser A.A. et al. (2023) [
22]. Associated comorbidities were heart failure, stroke sequelae, a pacemaker, and diabetes mellitus. A higher CCI suggests greater frailty, which may partly contribute to an increased risk of periprosthetic fracture [
23].
The study published by J. A. Singh and D.G. Lewallen (
n = 20,346) suggests an association between peptic ulcer disease and heart disease in patients with primary THA and revision THA with postoperative periprosthetic fracture [
24]. In our study, there is insufficient evidence to suggest that the CCI and the Clavien–Dindo Index are significantly associated. Longer-term studies are needed to follow these associations. The retrospective study published by S Märdian et al. (2017,
n = 151 patients with PFs) [
23] tracked the survival of patients with PFs relative to the general population. The results support the association of mortality in patients who had previous cardiovascular disease comorbidities (ischemic heart disease, cardiac arrhythmias, and heart failure) [
23].
The Strengths and Limitations of the Study
One of the main strengths of the study is that it is, to our knowledge, the only multicenter study in Romania to date with a significant number of cases, which makes it of high value in the research context. However, an important limitation is the varying sample sizes, which may influence the interpretation of the data, in particular in terms of standard error and coefficient of variation.