Effect of Exercise Training on Quality of Life, Symptoms, and Functional Status in Advanced-Stage Lung Cancer Patients: A Systematic Review
Abstract
1. Background
2. Methods
2.1. Eligibility Criteria
2.1.1. Types of Studies
2.1.2. Participants
2.1.3. Intervention and Comparison
2.1.4. Outcomes
2.2. Search Strategy
2.3. Study Selection
2.4. Bias Assessment
2.5. Data Extraction
3. Results
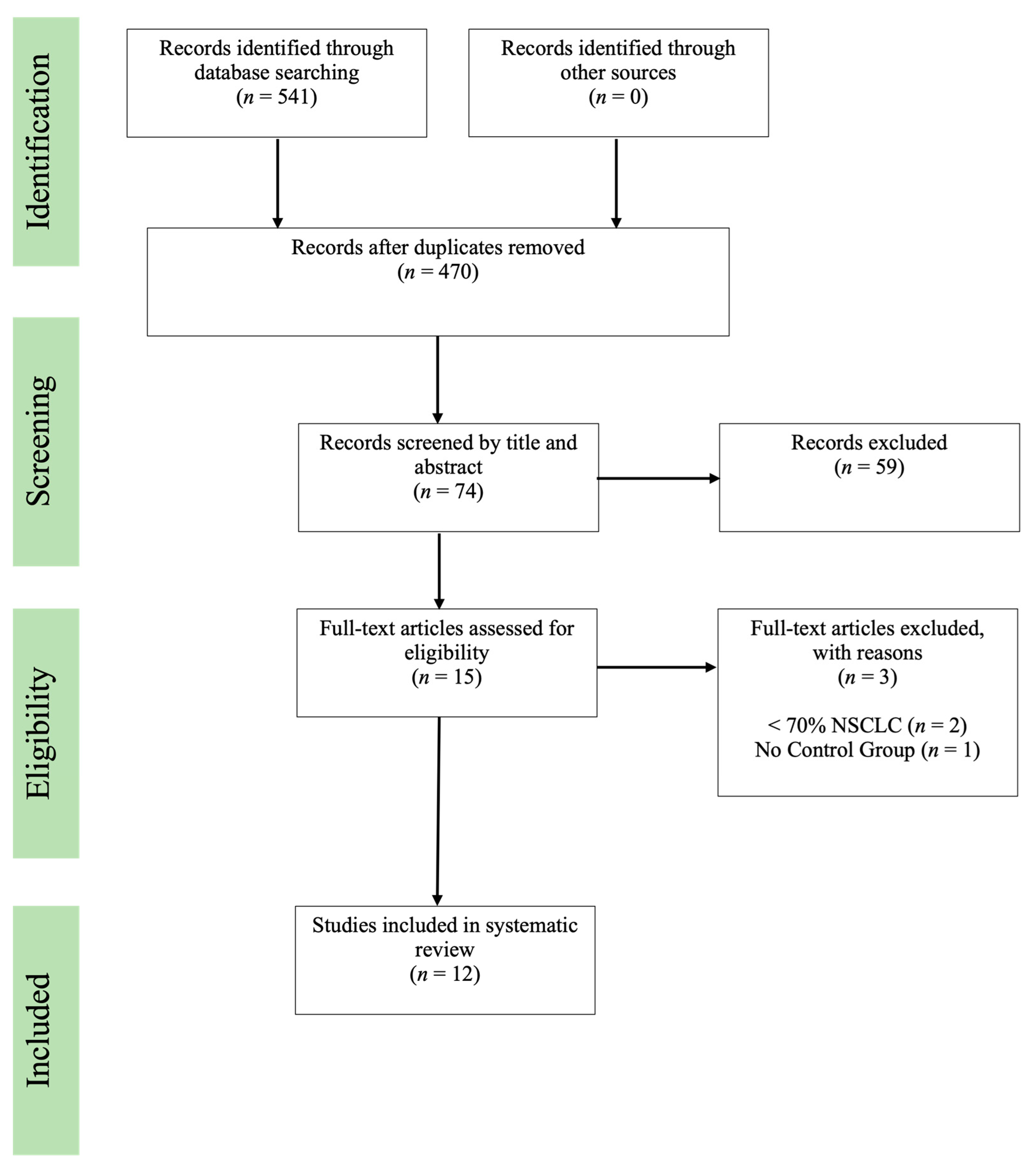
3.1. Study Search and Selection
3.2. Bias Assessment
3.3. Description of Studies
3.3.1. Patient Demographics
3.3.2. Timing of Antineoplastic Therapy with Regard to Exercise Intervention
3.3.3. Patient Performance Status
3.3.4. Characteristics of Exercise Intervention
3.4. Outcomes of Studies
3.4.1. Symptoms
3.4.2. Quality of Life
3.4.3. Anxiety and Depression
3.4.4. Functional Status
3.4.5. Physical Functioning
3.4.6. Spirometry
3.4.7. Biological Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statics. CA Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef]
- National Institutes of Health. SEER Stat Fact Sheets: Lung and Bronchus Cancer; National Institutes of Health: Rockville, MD, USA, 2016. [Google Scholar]
- Jakobsen, E.; Olsen, K.E.; Bliddal, M.; Hornbak, M.; Persson, G.F.; Green, A. Forecasting lung cancer incidence, mortality, and prevalence to year 2030. BMC Cancer 2021, 21, 985. [Google Scholar] [CrossRef]
- Hoffman, R.M.; Atallah, R.P.; Struble, R.D.; Badgett, R.G. Lung cancer screening with low-dose CT: A meta-analysis. J. Gen. Intern. Med. 2020, 35, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Jonas, D.E.; Reuland, D.S.; Reddy, S.M.; Nagle, M.; Clark, S.D.; Weber, R.P.; Enyioha, C.; Malo, T.L.; Brenner, A.T.; Armstrong, C. Screening for lung cancer with low-dose computed tomography: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2021, 325, 971–987. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.-Y.D.; Cramb, S.M.; Baade, P.D.; Youlden, D.R.; Nwogu, C.; Reid, M.E. The international epidemiology of lung cancer: Latest trends, disparities, and tumor characteristics. J. Thorac. Oncol. 2016, 11, 1653–1671. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.; Anggondowati, T.; Deviany, P.; Ryan, J.; Fetrick, A.; Bagenda, D.; Copur, M.; Tolentino, A.; Vaziri, I.; McKean, H. Patient preferences of chemotherapy treatment options and tolerance of chemotherapy side effects in advanced stage lung cancer. BMC Cancer 2019, 19, 835. [Google Scholar] [CrossRef]
- Iyer, S.; Roughley, A.; Rider, A.; Taylor-Stokes, G. The symptom burden of non-small cell lung cancer in the USA: A real-world cross-sectional study. Support. Care Cancer 2014, 22, 181–187. [Google Scholar] [CrossRef]
- Iyer, S.; Taylor-Stokes, G.; Roughley, A. Symptom burden and quality of life in advanced non-small cell lung cancer patients in France and Germany. Lung Cancer 2013, 81, 288–293. [Google Scholar] [CrossRef]
- Tanaka, K.; Akechi, T.; Okuyama, T.; Nishiwaki, Y.; Uchitomi, Y. Impact of Dyspnea, Pain, and Fatigue on Daily Life Activities in Ambulatory Patients with Advanced Lung Cancer. J. Pain Symptom Manag. 2002, 23, 417–423. [Google Scholar] [CrossRef]
- Choi, S.; Ryu, E. Effects of symptom clusters and depression on the quality of life in patients with advanced lung cancer. Eur. J. Cancer Care 2018, 27, e12508. [Google Scholar] [CrossRef]
- Sung, M.R.; Patel, M.V.; Djalalov, S.; Le, L.W.; Shepherd, F.A.; Burkes, R.L.; Feld, R.; Lin, S.; Tudor, R.; Leighl, N.B. Evolution of symptom burden of advanced lung cancer over a decade. Clin. Lung Cancer 2017, 18, 274–280.e6. [Google Scholar] [CrossRef]
- Vedadi, A.; Shakik, S.; Brown, M.C.; Lok, B.H.; Shepherd, F.A.; Leighl, N.B.; Sacher, A.; Bradbury, P.A.; Xu, W.; Liu, G.; et al. The impact of symptoms and comorbidity on health utility scores and health-related quality of life in small cell lung cancer using real world data. Qual. Life Res. 2021, 30, 445–454. [Google Scholar] [CrossRef]
- Morishima, T.; Matsumoto, Y.; Koeda, N.; Shimada, H.; Maruhama, T.; Matsuki, D.; Nakata, K.; Ito, Y.; Tabuchi, T.; Miyashiro, I. Impact of comorbidities on survival in gastric, colorectal, and lung cancer patients. J. Epidemiol. 2018, 29, 110–115. [Google Scholar] [CrossRef]
- Zhu, D.; Ding, R.; Ma, Y.; Chen, Z.; Shi, X.; He, P. Comorbidity in lung cancer patients and its association with hospital readmission and fatality in China. BMC Cancer 2021, 21, 557. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef]
- Avancini, A.; Sartori, G.; Gkountakos, A.; Casali, M.; Trestini, I.; Tregnago, D.; Bria, E.; Jones, L.W.; Milella, M.; Lanza, M.; et al. Physical Activity and Exercise in Lung Cancer Care: Will Promises Be Fulfilled? Oncologist 2020, 25, e555–e569. [Google Scholar] [CrossRef]
- Cavalheri, V.; Granger, C.L. Exercise training as part of lung cancer therapy. Respirology 2020, 25 (Suppl. 2), 80–87. [Google Scholar] [CrossRef]
- Codima, A.; das Neves Silva, W.; de Souza Borges, A.P.; de Castro, G. Exercise prescription for symptoms and quality of life improvements in lung cancer patients: A systematic review. Support. Care Cancer 2021, 29, 445–457. [Google Scholar] [CrossRef]
- Cavalheri, V.; Burtin, C.; Formico, V.R.; Nonoyama, M.L.; Jenkins, S.; Spruit, M.A.; Hill, K. Exercise training undertaken by people within 12 months of lung resection for non-small cell lung cancer. Cochrane Database Syst. Rev. 2019, 6, CD009955. [Google Scholar] [CrossRef]
- Gravier, F.-E.; Smondack, P.; Prieur, G.; Medrinal, C.; Combret, Y.; Muir, J.-F.; Baste, J.-M.; Cuvelier, A.; Boujibar, F.; Bonnevie, T. Effects of exercise training in people with non-small cell lung cancer before lung resection: A systematic review and meta-analysis. Thorax 2022, 77, 486–496. [Google Scholar] [CrossRef]
- Siddiqui, F.; Siddiqui, A. Lung Cancer; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Heywood, R.; McCarthy, A.L.; Skinner, T.L. Safety and feasibility of exercise interventions in patients with advanced cancer: A systematic review. Support. Care Cancer 2017, 25, 3031–3050. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, T.F.; Eriksen, M.B.; Hammer, D.M.G.; Christensen, J.B. PubMed coverage varied across specialties and over time: A large-scale study of included studies in Cochrane reviews. J. Clin. Epidemiol. 2019, 112, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Katchamart, W.; Faulkner, A.; Feldman, B.; Tomlinson, G.; Bombardier, C. PubMed had a higher sensitivity than Ovid-MEDLINE in the search for systematic reviews. J. Clin. Epidemiol. 2011, 64, 805–807. [Google Scholar] [CrossRef] [PubMed]
- The EndNote Team. EndNote, EndNote X9; Clarivate: Philadelphia, PA, USA, 2013. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, A.; Jastrzebski, D.; Rutkowski, S.; Żebrowska, A.; Stanula, A.; Szczegielniak, J.; Ziora, D.; Casaburi, R. Exercise Training in Patients with Non-Small Cell Lung Cancer During In-Hospital Chemotherapy Treatment: A randomized controlled trial. J. Cardiopulm. Rehabil. Prev. 2019, 39, 127–133. [Google Scholar] [CrossRef]
- Rutkowska, A.; Rutkowski, S.; Wrzeciono, A.; Czech, O.; Szczegielniak, J.; Jastrzębski, D. Short-Term Changes in Quality of Life in Patients with Advanced Lung Cancer during In-Hospital Exercise Training and Chemotherapy Treatment: A Randomized Controlled Trial. J. Clin. Med. 2021, 10, 1761. [Google Scholar] [CrossRef]
- Dhillon, H.M.; Bell, M.L.; van der Ploeg, H.P.; Turner, J.D.; Kabourakis, M.; Spencer, L.; Lewis, C.; Hui, R.; Blinman, P.; Clarke, S.J.; et al. Impact of physical activity on fatigue and quality of life in people with advanced lung cancer: A randomized controlled trial. Ann. Oncol. 2017, 28, 1889–1897. [Google Scholar] [CrossRef]
- Egegaard, T.; Rohold, J.; Lillelund, C.; Persson, G.; Quist, M. Pre-radiotherapy daily exercise training in non-small cell lung cancer: A feasibility study. Rep. Pract. Oncol. Radiother. 2019, 24, 375–382. [Google Scholar] [CrossRef]
- Hwang, C.-L.; Yu, C.-J.; Shih, J.-Y.; Yang, P.-C.; Wu, Y.-T. Effects of exercise training on exercise capacity in patients with non-small cell lung cancer receiving targeted therapy. Support. Care Cancer 2012, 20, 3169–3177. [Google Scholar] [CrossRef]
- Kirca, K.; Kutlutürkan, S. The effect of progressive relaxation exercises on treatment-related symptoms and self-efficacy in patients with lung cancer receiving chemotherapy. Complement. Ther. Clin. Pract. 2021, 45, 101488. [Google Scholar] [CrossRef]
- Quist, M.; Langer, S.W.; Lillelund, C.; Winther, L.; Laursen, J.H.; Christensen, K.B.; Rørth, M.; Adamsen, L. Effects of an exercise intervention for patients with advanced inoperable lung cancer undergoing chemotherapy: A randomized clinical trial. Lung Cancer 2020, 145, 76–82. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Wang, S.-Z.; Chen, H.-L.; Yuan, A.Z. Tai Chi Exercise for Cancer-Related Fatigue in Patients with Lung Cancer Undergoing Chemotherapy: A Randomized Controlled Trial. J. Pain Symptom Manag. 2016, 51, 504–511. [Google Scholar] [CrossRef]
- Molassiotis, A.; Charalambous, A.; Taylor, P.; Stamataki, Z.; Summers, Y. The effect of resistance inspiratory muscle training in the management of breathlessness in patients with thoracic malignancies: A feasibility randomised trial. Support. Care Cancer 2015, 23, 1637–1645. [Google Scholar] [CrossRef]
- Bade, B.C.; Gan, G.; Li, F.; Lu, L.; Tanoue, L.; Silvestri, G.A.; Irwin, M.L. Randomized trial of physical activity on quality of life and lung cancer biomarkers in patients with advanced stage lung cancer: A pilot study. BMC Cancer 2021, 21, 352. [Google Scholar] [CrossRef]
- Henke, C.C.; Cabri, J.; Fricke, L.; Pankow, W.; Kandilakis, G.; Feyer, P.C.; de Wit, M. Strength and endurance training in the treatment of lung cancer patients in stages IIIA/IIIB/IV. Support. Care Cancer 2014, 22, 95–101. [Google Scholar] [CrossRef]
- West, H.J.; Jin, J.O. Performance status in patients with cancer. JAMA Oncol. 2015, 1, 998. [Google Scholar] [CrossRef]
- Cheung, D.S.T.; Takemura, N.; Lam, T.C.; Ho, J.C.M.; Deng, W.; Smith, R.; Yan, Y.; Lee, A.W.M.; Lin, C.C. Feasibility of Aerobic Exercise and Tai-Chi Interventions in Advanced Lung Cancer Patients: A Randomized Controlled Trial. Integr. Cancer Ther. 2021, 20, 15347354211033352. [Google Scholar] [CrossRef]
- Fayers, P.; Bottomley, A.; EORTC Quality of Life Group and of the Quality of Life Unit. Quality of life research within the EORTC—The EORTC QLQ-C30. Eur. J. Cancer 2002, 38, 125–133. [Google Scholar] [CrossRef]
- Cella, D.F.; Bonomi, A.E.; Lloyd, S.R.; Tulsky, D.S.; Kaplan, E.; Bonomi, P. Reliability and validity of the Functional Assessment of Cancer Therapy—Lung (FACT-L) quality of life instrument. Lung Cancer 1995, 12, 199–220. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Li, X.-X.; Ma, H.-K.; Zhang, X.; Wang, B.-W.; Guo, T.-T.; Xiao, Y.; Bing, Z.-T.; Ge, L.; Yang, K.-H.; et al. Exercise training for improving patient-reported outcomes in patients with advanced-stage cancer: A systematic review and meta-analysis. J. Pain Symptom Manag. 2020, 59, 734–749.e10. [Google Scholar] [CrossRef]
- Lin, Y.-Y.; Liu, M.F.; Tzeng, J.-I.; Lin, C.-C. Effects of walking on quality of life among lung cancer patients: A longitudinal study. Cancer Nurs. 2015, 38, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, Q.; Zhang, J. Effect of exercise on fatigue in patients with lung cancer: A systematic review and meta-analysis of randomized trials. J. Palliat. Med. 2021, 24, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Polanski, J.; Jankowska-Polanska, B.; Rosinczuk, J.; Chabowski, M.; Szymanska-Chabowska, A. Quality of life of patients with lung cancer. OncoTargets Ther. 2016, 9, 1023. [Google Scholar]
- Zabora, J.; BrintzenhofeSzoc, K.; Curbow, B.; Hooker, C.; Piantadosi, S. The prevalence of psychological distress by cancer site. Psycho-Oncol. J. Psychol. Soc. Behav. Dimens. Cancer 2001, 10, 19–28. [Google Scholar] [CrossRef]
- Pirl, W.F.; Greer, J.A.; Traeger, L.; Jackson, V.; Lennes, I.T.; Gallagher, E.R.; Perez-Cruz, P.; Heist, R.S.; Temel, J.S. Depression and survival in metastatic non–small-cell lung cancer: Effects of early palliative care. J. Clin. Oncol. 2012, 30, 1310. [Google Scholar] [CrossRef]
- Danhauer, S.C.; Addington, E.L.; Cohen, L.; Sohl, S.J.; Van Puymbroeck, M.; Albinati, N.K.; Culos-Reed, S.N. Yoga for symptom management in oncology: A review of the evidence base and future directions for research. Cancer 2019, 125, 1979–1989. [Google Scholar] [CrossRef]
- Pinto, B.M.; Rabin, C.; Dunsiger, S. Home-based exercise among cancer survivors: Adherence and its predictors. Psycho-Oncol. J. Psychol. Soc. Behav. Dimens. Cancer 2009, 18, 369–376. [Google Scholar] [CrossRef]
- Midtgaard, J.; Hammer, N.M.; Andersen, C.; Larsen, A.; Bruun, D.-M.; Jarden, M. Cancer survivors’ experience of exercise-based cancer rehabilitation—A meta-synthesis of qualitative research. Acta Oncol. 2015, 54, 609–617. [Google Scholar] [CrossRef]
- Adamsen, L.; Stage, M.; Laursen, J.; Rørth, M.; Quist, M. Exercise and relaxation intervention for patients with advanced lung cancer: A qualitative feasibility study. Scand. J. Med. Sci. Sport. 2012, 22, 804–815. [Google Scholar] [CrossRef]
- Tai-Chi and Aerobic Exercise to Improve Emotional Symptom Cluster in Late-stage Lung Cancer Patients. Available online: https://ClinicalTrials.gov/show/NCT05778708 (accessed on 3 June 2023).
- Supportive Care Intervention (ROAR-LCT) for Patients with Stage IIIA, IIIB, and IV Lung Cancer, ROAR-LCT Trial. Available online: https://ClinicalTrials.gov/show/NCT05339022 (accessed on 3 June 2023).
| Category/ Study | Risk of Bias from Randomization Process | Effect of Assignment to Intervention | Effect of Adhering to Intervention | Risk of Bias due to Missing Outcome Data | Risk of Bias in Measurement of the Outcome | Risk of Bias in Selection of the Reported Result | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|
| Rutkowska et al., 2019 [28] | L | L | L | L | L | L | L |
| Rutkowska et al., 2021 [29] | L | L | L | L | L | L | L |
| Cheung et al., 2021 [30] | L | L | L | L | L | L | L |
| Dhillon et al., 2017 [31] | L | L | L | L | M | L | M |
| Egegaard et al., 2019 [32] | L | M | L | L | M | L | M |
| Hwang et al., 2012 [33] | L | M | L | L | M | L | M |
| Kirca et al., 2021 [34] | L | L | L | L | M | L | M |
| Quist et al., 2020 [35] | L | L | M | M | L | L | M |
| Zhang et al., 2016 [36] | L | L | L | L | M | L | M |
| Molassiotis et al., 2015 [37] | L | L | L | L | M | L | M |
| Bade et al., 2021 [38] | L | L | M | L | M | L | M |
| Henke et al., 2014 [39] | M | M | L | L | M | L | H |
| Study | Country | Study Population | Mean Age (Years) | Gender (n, %) | Cancer Stage(s) | Performance Status | Treatment Status |
|---|---|---|---|---|---|---|---|
| Rutkowska et al., 2019 [28] | Poland | n = 30 | IG 59.1 ± 6.8 CG 61.3 ± 8.8 | F (n = 3, 10%) M (n = 27, 90%) | NSCLC IIIb–IV; disqualified from surgery | WHO-PS 0-1 | During in-hospital chemotherapy |
| Rutkowska et al., 2021 [29] | Poland | n = 26 | IG 60.4 ± 7.2 CG 62.2 ± 9.0 | F (n = 2, 7.7%) M (n = 24, 92.3%) | NSCLC IIIb–IV; disqualified from surgery | ECOG-PS 0-1 | During in-hospital chemotherapy |
| Cheung et al., 2021 [30] | Hong Kong | n = 30 | AG 61.0 ± 12.12 TC 61.11 ± 7.01 CG 58.36 ± 9.32 | F (n = 14, 46.7%) M (n = 16, 53.3%) | NSCLC IIIb–IV | KPS score ≤ 80 | Chemotherapy, radiotherapy, targeted therapy, no treatment |
| Dhillon et al., 2017 [31] | Australia | n = 111 | IG 64 (range 38–80) CG 64 (range 34–76) | F (n = 50, 45%) M (n = 61, 55%) | NSCLC III–IV LS SCLC | ECOG-PS 0-2 | Chemotherapy, chemotherapy + targeted agent, targeted therapy only, no active treatment |
| Egegaard et al., 2019 [32] | Denmark | n = 15 | IG 64 ± 5.8 CG 65 ± 4.7 | F (n = 10, 66.7%) M (n = 5, 33.3%) | NSCLC III–IV | WHO-PS 0-1 | During chemoradiotherapy |
| Hwang et al., 2012 [33] | Taiwan | n = 24 | IG 60.4 ± 7.2 CG 62.2 ± 9.0 | F (n = 12, 50%) M (n = 12, 50%) | NSCLC III–IV | ECOG-PS 0-1 | During targeted therapy (EGFR inhibitors) for at least 4 weeks |
| Kirca et al., 2021 [34] | Turkey | n = 84 | IG 60.4 ± 7.2 CG 62.2 ± 9.0 | F (n = 69, 82.1%) M (n = 15, 17.9%) | NSCLC/SCLC III–IV | ECOG-PS 0-2 | During chemotherapy |
| Quist et al., 2020 [35] | Denmark | n = 218 | OG 64.4 ± 8.5 IG 65.2 ± 8.2 CG 63.5 ± 8.7 | F (n = 111, 50.9%) M (n = 107, 49.1%) | NSCLC III–IV SCLC LS/ES | WHO-PS 0-2 | During chemotherapy |
| Zhang et al., 2016 [36] | China | n = 91 | OG 62.8 | F (n = 23, 25.3%) M (n = 68, 74.7%) | 88% NSCLC/SCLC III–IV | ECOG-PS 0-3 | During cisplatin-based chemotherapy |
| Molassiotis et al., 2015 [37] | United Kingdom, Cyprus | n = 46 | Not reported | F (n = 9, 19.6%) M (n = 37, 80.4%) | 70% NSCLC III–IV and advanced thoracic malignancy | Not reported | Post chemotherapy only, radiotherapy only, chemoradiotherapy, surgery only, surgery + chemotherapy, surgery + chemotherapy + radiotherapy |
| Bade et al., 2021 [38] | USA | n = 40 | OG 64.88 ± 8.69 IG 66.55 ± 7.28 CG 63.20 ± 9.80 | F (n = 30, 75%) M (n = 10, 25%) | NSCLC III–IV | ECOG-PS 0-1 | Chemotherapy, immunotherapy, targeted therapy, post-treatment |
| Henke et al., 2014 [39] | Germany | n = 29 | Not reported | Not reported | NSCLC/SCLC III–IV | KPS score > 50 | During in-patient palliative platinum-based chemotherapy |
| Study | Study Design | Setting | Type/ Frequency/Length of Intervention | Assessment of Outcomes | Results | Adherence | Adverse Outcomes |
|---|---|---|---|---|---|---|---|
| Rutkowska et al., 2019 [28] | RCT | Supervised, hospital setting | Type: endurance, breathing, weight, and fitness training Frequency: 5 times per week for 30–45 min each session Length: 4 weeks in 2-week cycles; 6-week period between assessments | 6MWT, spirometry, mMRC, BDS, Fullerton Test | Statistically significant increase in IG when compared to baseline with 6MWD (p = 0.01), up-and-go test (p = 0.01), chair stand (p = 0.01), and arm curl (p = 0.001). CG showed significant decrease in the chair sit and reach and up-and-go tests. The up-and go-tests between groups showed statistical significance. Spirometry also improved significantly with FEV1 % predicted, FVC % predicted, and FEV1/FVC. No significant improvements in CG. mMRC showed no statistical significance in dyspnea improvement, but BDS showed significant improvement in perception of dyspnea (p = 0.04) in IG. No significant changes in CG. | 25% attrition rate. | No adverse outcomes reported. |
| Rutkowska et al., 2021 [29] | RCT | Supervised, hospital setting | Type: endurance, breathing, weight, and fitness training Frequency: 5 times per week Length: 4 weeks in 2-week cycles; 6-week period between assessments | SGRQ, SF-36, FACT-L | No statistically significant changes in SGRQ measuring QoL. However, intermediate effect size noted in symptom domain and impact of life domain, favoring IG. No statistically significant changes in IG for FACT-L with significant decrease in the CG’s physical wellbeing (p < 0.02). No significant changes in either group for SF-36. | 27% attrition rate. | No adverse outcomes reported. |
| Cheung et al., 2021 [30] | Assessor blinded, pilot feasibility RCT | Supervised aerobic or tai chi class | Type: aerobic, tai chi Frequency: 60 min 2× per week + 90 min self-practice (total 150 min/week) Length: 12 weeks (3 months) | PSQI, HADS, BFI, EORTC QLQ-C30, QLQ-LC13, physical performance, actigraph, and circadian rhythms (salivary cortisol) | Aerobic group showed statistically significant improvement post-intervention in time up-and-go (−2.26, 95% CI: −4.04, −0.48) and 30 s sit-to-stand tests (4.52, 95% CI: 2.19, 6.85) than the tai chi and control groups. Improvement in anxiety in tai chi group post-intervention (−1.45, 95% CI: −4.62, 1.72), 6-month (−2.13, 95% CI: −5.30, 1.04), and 1-year follow up (−1.98, 95% CI: −5.18, 1.22) relative to baseline. Aerobic and control groups reported smaller improvements. Tai chi showed more improvement in balance (28.25, 95% CI: −37.08, 93.58) and 6MWT (19.42, 95% CI: −44.83, 83.67) than the aerobic group. Control group showed improved anxiety, depression, sleep disturbance, and some aspects of physical performance (up-and-go, sit-to-stand tests). | Aerobic Group: 80% Tai Chi Group: 78% | No adverse events in the tai chi group. One participant in the aerobic group reported lip numbness during class, unrelated to intervention. |
| Dhillon et al., 2017 [31] | Open labeled RCT | Supervised with exercise provider and unsupervised sessions | Type: aerobic Frequency: 1 h/week Length: 8 weeks | FACT-F, EORTC-QLQ-C30-LC-13, ADL, iADL, GHQ-12, distress thermometer, physical performance FACT-Cognition, PSQI, spirometry, Glasgow Prognostic Score, biological blood samples; Questionnaires: Shortness of Breath, Active, Sedentary Behavior, Social Cognitive Determinants of Exercise | No difference in mean scores between groups in QoL. No significant difference between groups in fatigue, symptoms, dyspnea, stress, anxiety/depression, cognitive symptoms, sleep quality, activities of daily living, physical function, fitness, anthropometric measures, overall survival, Glasgow Prognostic Score, or biological biomarkers. | 69% in physical activity component; 75% in behavioral support sessions | No serious reported adverse events. Four participants in IG reported back/muscle soreness that resolved with no treatment. Four other participants had minor adverse events that were resolved with no treatment. |
| Egegaard et al., 2019 [32] | Feasibility RCT | Supervised, hospital setting | Type: aerobic Frequency: 20 min daily Length: 7 weeks | HADS, FACT-L, IPAQ-L, spirometry, 6MWD | No significant differences between groups for QoL via FACT-L. No significant differences within or between group differences with anxiety and depression. No significant differences between groups in steps, distance, or intensity minutes. No significant differences within or between groups from baseline to post-intervention in any cardiopulmonary endpoints. | 88.1% adherence to full exercise participation. No dropouts during intervention. | Two participants were hospitalized during the course due to chemotherapy adverse events. No reported events occurred during the exercise session. |
| Hwang et al., 2012 [33] | RCT | Supervised, outpatient clinic | Type: high intensity interval aerobic training Frequency: 3 time per week for 30–40 min each session Length: 8 weeks | CPET, NIRS, venous blood sample, isokinetic muscle testing, EORTC QLQ-C30 | Significant increase favoring IG found in VO2peak (p < 0.005) and %predVO2peak (p < 0.05). HOMA-IR and Hs-CRP unchanged in both groups. No between group differences in QoL. IG had a significant decrease in dyspnea and fatigue from baseline (p = 0.05). | Mean adherence rate of the exercise group was 71.2%. | No exercise-related adverse outcomes reported. |
| Kirca et al., 2021 [34] | RCT | Supervised hospital; at home setting | Type: relaxation exercises Frequency: 30 min daily Length: 3 courses of chemotherapy | MSAS, SUPPH, weekly telephone counseling | Statistically significant decrease in mean MSAS-GDI, MSAS-PSYCH and TMSAS in IG. Significant increase in SUPPH scores in IG. No statistically significant change among CG. Self-efficacy increased as frequency and severity of symptoms decreased (p = 0.000). | Not reported. | No adverse outcomes reported. |
| Quist et al., 2020 [35] | RCT | Hospital setting | Type: aerobic, strength, and fitness training Frequency: 1.5 h, 2 times/week Length: 12 weeks | VO2peak, 1RM, 6MWT, spirometry, FACT-L, HADS | No statistically significant difference in aerobic capacity based on VO2peak between IG and CG. Statistically significant increase in strength with leg press (p = 0.01), extension (p < 0.01), chest press (p < 0.01), and lat pull down (p = 0.04) in IG. Statistically significant difference between groups with decrease in social wellbeing (p = 0.04) and increase in anxiety (p = 0.02) and depression (p = 0.01) in CG. | 44% adherence rate. | No adverse outcomes reported. |
| Zhang et al., 2016 [36] | RCT | Unsupervised at home or supervised in community | Type: tai chi Frequency: every other day for 1 h sessions Length: 12 weeks | MSFI-SF | Significant decrease in IG when compared to CG in MFSI-SF total score, general fatigue scores, and physical fatigue scores (p < 0.05). Significant higher vigor score in IG than CG (p < 0.05). No significant differences in emotional or mental subscale. Results were similar at 6 weeks and 12 weeks. | Not reported. | No adverse outcomes reported. |
| Molassiotis et al., 2015 [37] | Feasibility RCT | Supervised in hospital; home setting | Type: inspiratory muscle training Frequency: 30 min sessions, 5 times/week Length: 12 weeks | CRDQ, MBS, HADS, spirometry | CG was statistically significantly higher than the IG group for the worst breathlessness over the past 24 h (p = 0.003 and average breathlessness over the past 24 h (p = 0.019). There was increasing distress experienced due to breathlessness in the CG from wk 4 to wk 12 (p = 0.035). Compared to the IG group, the mean distress for the CG was significantly higher (p = 0.018). Mean scores for coping ability were higher for the IG group at wk 4 (p = 0.012) and wk 8 (p = 0.023). Satisfaction with management of breathlessness was significantly higher in the IG group at wk 4, wk 8, wk 12 (p = 0.02, 0.001, 0.001). Mastery scores were also significantly lower in the CG group at wk 4, wk 8, wk 12 (p = 0.015, 0.028, 0.036). For the HADS scores, the IG group was significantly better than measured at baseline (p = 0.034) while the CG group was significantly worse than baseline (p = 0.026 and p = 0.035) as measured at wk 4 and wk 8. The CRDQ scale showed better fatigue scores in the IG group at wk 4, wk 8, wk 12. | Not reported. | No adverse outcomes reported. |
| Bade et al., 2021 [38] | Pilot open-labeled RCT | Home | Type: aerobic (walking) Length: 12 weeks (3 months) | MMRC Dyspnea Scale, Modifiable Activity Questionnaire, EORTC-QLQ-C30, PHQ-9, cancer biomarkers (insulin, leptin, CRP, sPD-1, sPD-L1) | Both groups reported higher QLQ summary scores though not significant. Significant between group differences in the role functioning domain of EORTC (p = 0.02). No statistically significant increase with both groups in MMRC dyspnea scale. Significant between group differences in sPD-1 compared to baseline (p < 0.001) with increases in IG and decreases in CG. | Individualized walking goals met in 21% of weeks. | Four serious adverse events unrelated to study (three hospitalizations, one ER visit); two minor adverse events unrelated to study. |
| Henke et al., 2014 [39] | RCT | Supervised, hospital setting | Type: strength, endurance, and breathing training Frequency/Length: 3 rounds of chemotherapy | ADL, EORTC-QLQ-LC-13, 6MWT, staircase walking, MBS | Statistically significant difference in EORTC-QLQ-C-30-L-13 for physical functioning (p = 0.025), hemoptysis (p = 0.019), arm/shoulder pain (p = 0.048), peripheral neuropathy (p = 0.050), cognitive functioning (p = 0.050). Significant differences favoring the IG found in ADL (p = 0.041), 6MWT, stair walking, strength capacity, patient’s dyspnea perception. Baseline differences in endurance capacity and strength between groups before and after intervention. | Originally started with 46 patients, but only 29 completed the intervention. | Six participants did not complete the trial due to death; otherwise, no reported adverse outcomes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.; Tracy, K.; Ullah, A.; Karim, N.A. Effect of Exercise Training on Quality of Life, Symptoms, and Functional Status in Advanced-Stage Lung Cancer Patients: A Systematic Review. Clin. Pract. 2023, 13, 715-730. https://doi.org/10.3390/clinpract13030065
Nguyen T, Tracy K, Ullah A, Karim NA. Effect of Exercise Training on Quality of Life, Symptoms, and Functional Status in Advanced-Stage Lung Cancer Patients: A Systematic Review. Clinics and Practice. 2023; 13(3):715-730. https://doi.org/10.3390/clinpract13030065
Chicago/Turabian StyleNguyen, Tena, Katharine Tracy, Asad Ullah, and Nagla Abdel Karim. 2023. "Effect of Exercise Training on Quality of Life, Symptoms, and Functional Status in Advanced-Stage Lung Cancer Patients: A Systematic Review" Clinics and Practice 13, no. 3: 715-730. https://doi.org/10.3390/clinpract13030065
APA StyleNguyen, T., Tracy, K., Ullah, A., & Karim, N. A. (2023). Effect of Exercise Training on Quality of Life, Symptoms, and Functional Status in Advanced-Stage Lung Cancer Patients: A Systematic Review. Clinics and Practice, 13(3), 715-730. https://doi.org/10.3390/clinpract13030065







