Uterine Artery Pseudoaneurysm after an Uncomplicated Vaginal Delivery: A Case Report
Abstract
1. Introduction
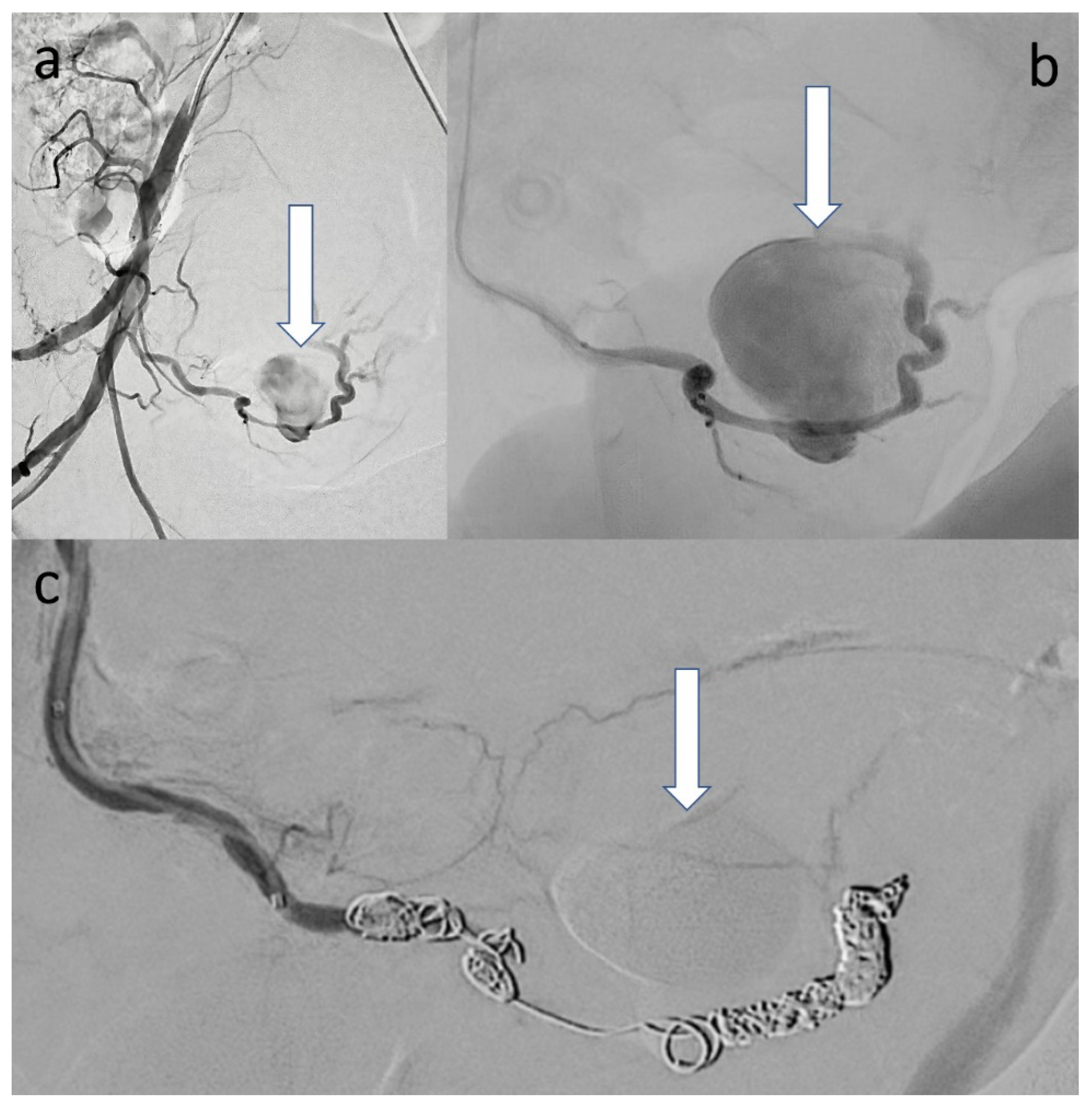
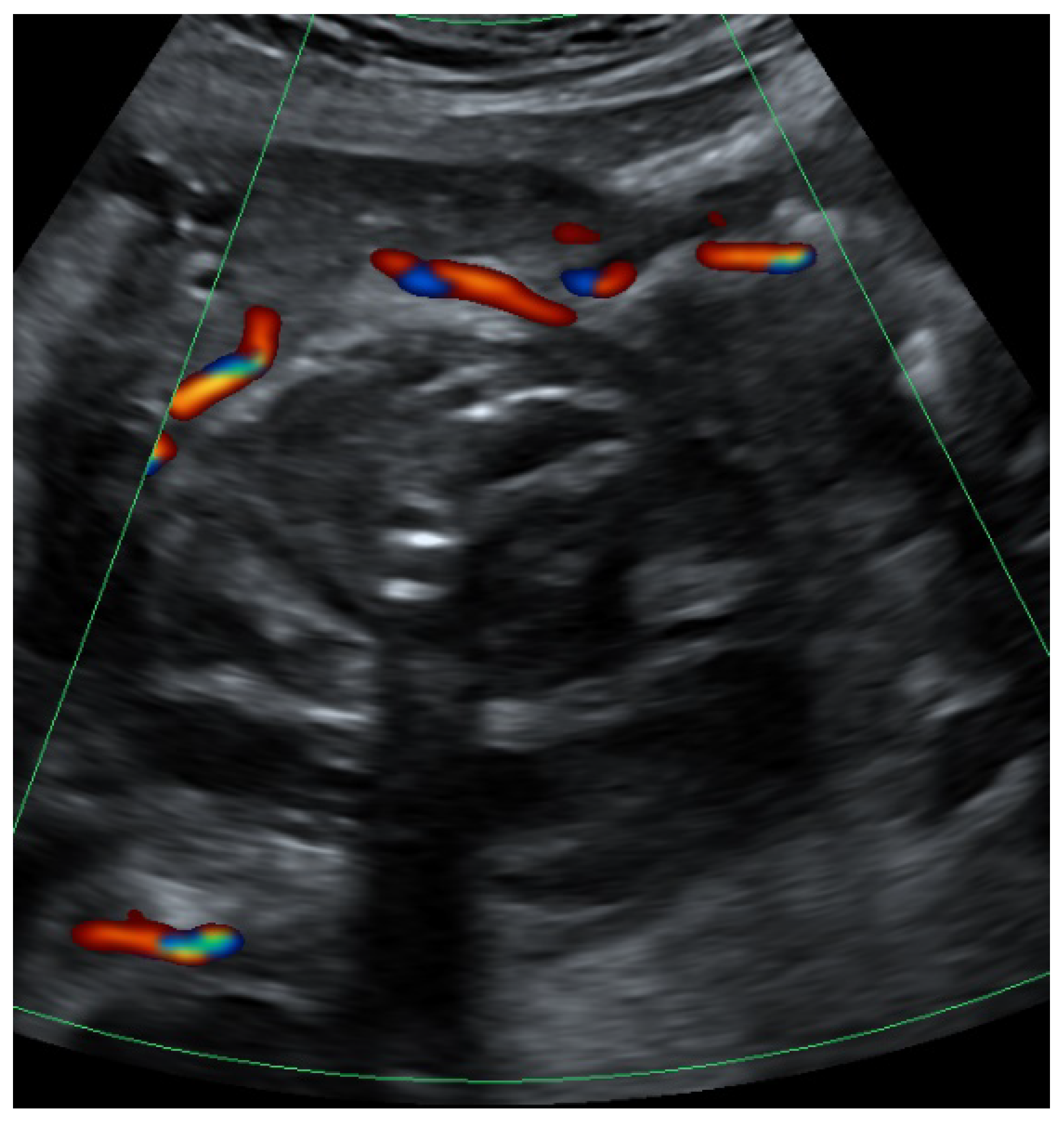
2. Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, C.Q.; Nayeemuddin, M.; Rattray, D. Uterine artery pseudoaneurysm with an anastomotic feeding vessel requiring repeat embolisation. BMJ Case Rep. 2018, 2018, bcr-2018. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Ganesh, D.; Devi, L.; Srinivasan, J.; Ranga, U. Prompt diagnosis and treatment of uterine arcuate artery pseudoaneurysm: A case report and review of literature. J. Clin. Diagn. Res. 2013, 7, 2303–2306. [Google Scholar] [CrossRef]
- Nanjundan, P.; Rohilla, M.; Raveendran, A.; Jain, V.; Khandelwal, N. Pseudoaneurysm of uterine artery: A rare cause of secondary postpartum hemorrhage, managed with uterine artery embolisation. J. Clin. Imaging Sci. 2011, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-P.; Su, X. Superficial temporal artery arteriovenous fistula and pseudoaneurysm: The Yin-Yang sign. Radiology 2020, 294, 508. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.Z.; Al-Saadi, M.; Abuderman, A.; Alzimami, K.S.; Alkhorayef, M.; Almagli, B.; Sulieman, A. “To-and-fro” waveform in the diagnosis of arterial pseudoaneurysms. World J. Radiol. 2015, 7, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Soe, L.; Thidar, S.; Myat, S.Y.; Mui, F.L.C.; Marie, C.K.P.S.; Tin, M.N. Uterine artery pseudoaneurysm: A case of late intra-abdominal haemorrhage after caesarean section. Med. J. Malays. 2020, 75, 298–300. [Google Scholar]
- Pohlan, J.; Hinkson, L.; Wickmann, U.; Henrich, W.; Althoff, C.E. Pseudo aneurysm of the uterine artery with arteriovenous fistula after cesarean section: A rare but sinister cause of delayed postpartum hemorrhage. J. Clin. Ultrasound 2021, 49, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Jain, J.; O’Leary, S.; Sarosi, M. Uterine artery pseudoaneurysm after uterine cervical conization. Obstet. Gynecol. 2014, 123 Pt 2 (Suppl. S2), 456–458. [Google Scholar] [CrossRef]
- Jennings, L.; Presley, B.; Krywko, D. Uterine artery pseudoaneurysm: A life-threatening cause of vaginal bleeding in the emergency department. J. Emerg. Med. 2019, 56, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Rossard, L.; Body, G.; Ouldamer, L. Uterine pseudoaneurysm after abdominal myomectomy: A case report. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101877. [Google Scholar] [CrossRef] [PubMed]
- McGonegle, S.J.; Dziedzic, T.S.; Thomas, J.; Hertzberg, B.S. Pseudoaneurysm of the uterine artery after an uncomplicated spontaneous vaginal delivery. J. Ultrasound Med. 2006, 25, 1593–1597. [Google Scholar] [CrossRef]
- Ota, H.; Fukushi, Y.; Wada, S.; Fujino, T.; Omori, Y.; Kushima, M. Successful treatment of uterine artery pseudoaneurysm with laparoscopic temporary clamping of bilateral uterine arteries, followed by hysteroscopic surgery. J. Obstet. Gynaecol. Res. 2017, 43, 1356–1359. [Google Scholar] [CrossRef] [PubMed]
- Das, C.J.; Rathinam, D.; Manchanda, S.; Srivastava, D.N. Endovascular uterine artery interventions. Indian J. Radiol. Imaging 2017, 27, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Ugwumadu, L.; Hayes, K.; Belli, A.-M.; Heenan, S.; Loftus, I. Uterine artery pseudoaneurysm requiring embolization in pregnancy: A case report and review of the literature. CVIR Endovasc. 2018, 1, 31. [Google Scholar] [CrossRef] [PubMed]
- Laubach, M.; Delahaye, T.; Van Tussenbroek, F.; Debing, E.; De Catte, L.; Foulon, W. Uterine artery pseudo-aneurysm: Diagnosis and therapy during pregnancy. J. Périnat. Med. 2000, 28, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Feld, Z.; Rowen, T.; Callen, A.; Goldstein, R.; Poder, L. Uterine artery pseudoaneurysm in the setting of deep endometriosis: An uncommon cause of hemoperitoneum in pregnancy. Emerg. Radiol. 2018, 25, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Leone Roberti Maggiore, U.; Ferrero, S.; Mangili, G.; Bergamini, A.; Inversetti, A.; Giorgione, V.; Vigano, P.; Candiani, M. A systematic review on endometriosis during pregnancy: Diagnosis, misdiagnosis, complications and outcomes. Hum. Reprod. Update 2016, 22, 70–103. [Google Scholar] [CrossRef] [PubMed]

| Before Birth | Day 7 after Birth (New Admission) | Day 8 after Birth (After Embolization) | |
|---|---|---|---|
| C-reactive protein (CRP) (mg/dL) | / | / | 168 |
| leuocytes (G/l) | 7.3 | 12.4 | 15.8 |
| hemoglobin (G/l) | 11.8 | 10.4 | 8.3 |
| fever (°C) | no | no | 38.1 |
| vaginal bleeding | birth: 500 mL | no | no |
| systolic blood pressure (mmHg) | 130 | 115 | 119 |
| diastolic blood pressure (mmHg) | 82 | 73 | 70 |
| pulse (bpm) | 79 | 72 | 70 |
| drugs | Sultamicillin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Böckenhoff, P.; Kupczyk, P.; Lindner, K.; Strizek, B.; Gembruch, U. Uterine Artery Pseudoaneurysm after an Uncomplicated Vaginal Delivery: A Case Report. Clin. Pract. 2022, 12, 826-831. https://doi.org/10.3390/clinpract12050087
Böckenhoff P, Kupczyk P, Lindner K, Strizek B, Gembruch U. Uterine Artery Pseudoaneurysm after an Uncomplicated Vaginal Delivery: A Case Report. Clinics and Practice. 2022; 12(5):826-831. https://doi.org/10.3390/clinpract12050087
Chicago/Turabian StyleBöckenhoff, Paul, Patrick Kupczyk, Kira Lindner, Brigitte Strizek, and Ulrich Gembruch. 2022. "Uterine Artery Pseudoaneurysm after an Uncomplicated Vaginal Delivery: A Case Report" Clinics and Practice 12, no. 5: 826-831. https://doi.org/10.3390/clinpract12050087
APA StyleBöckenhoff, P., Kupczyk, P., Lindner, K., Strizek, B., & Gembruch, U. (2022). Uterine Artery Pseudoaneurysm after an Uncomplicated Vaginal Delivery: A Case Report. Clinics and Practice, 12(5), 826-831. https://doi.org/10.3390/clinpract12050087






