Improvement in Tongue Pressure Precedes Improvement in Dysphagia in Dermatomyositis
Abstract
:1. Introduction
2. Case Presentations
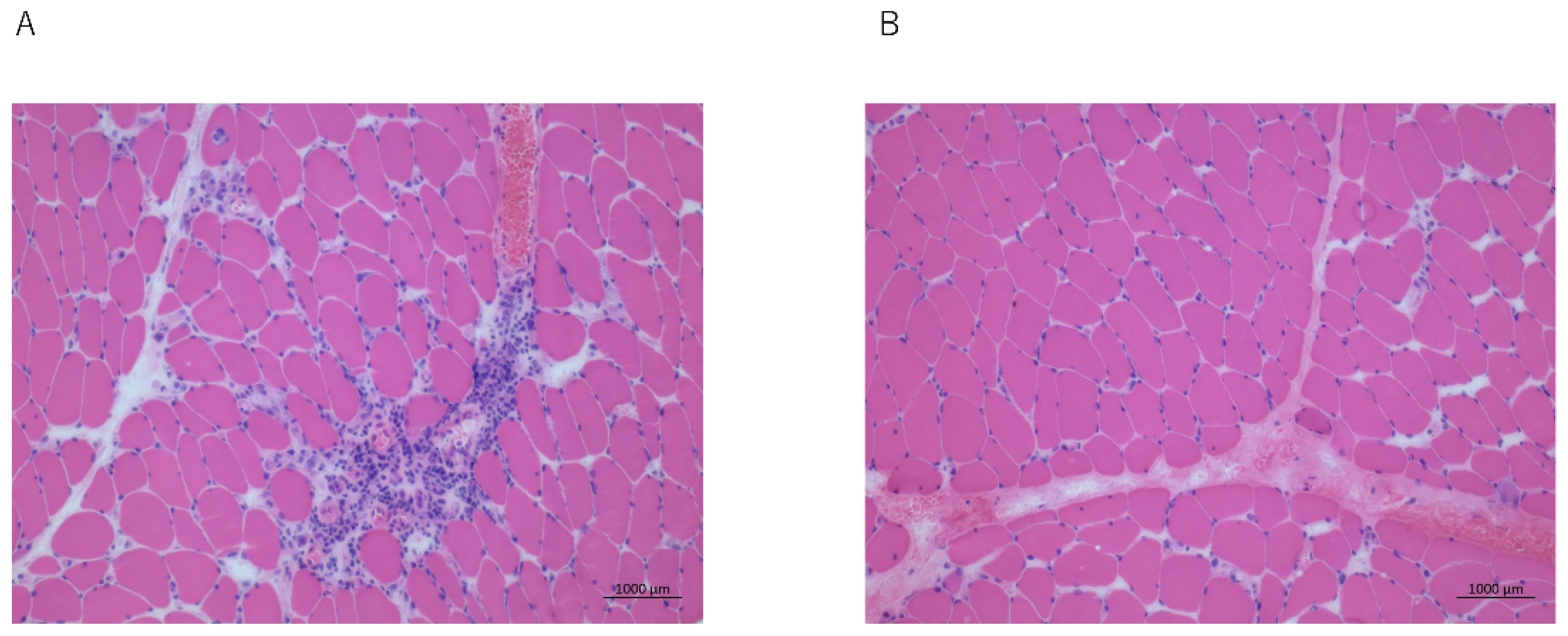
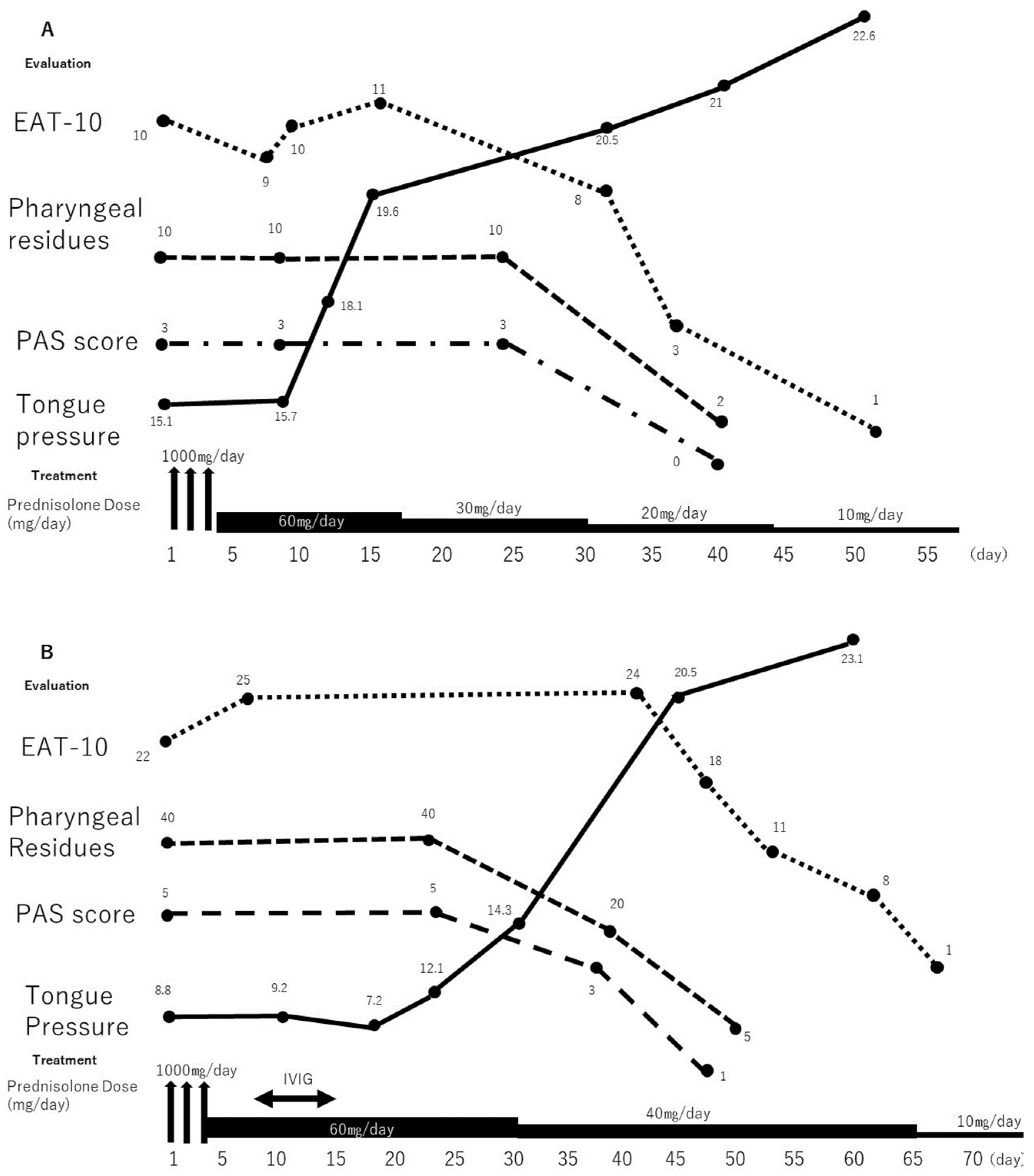
2.1. Case 1
2.2. Case 2
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gordon, P.A.; Winer, J.B.; Hoogendijk, J.E.; Choy, E.H.S. Immunosuppressant and immunomodulatory treatment for dermatomyositis and polymyositis. Cochrane Database Syst. Rev. 2012, 2012, CD003643. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.H.; Brumfield, K.A.; Hoskin, T.L.; Stolp, K.A.; Murray, J.A.; Bassford, J.R. Dysphagia in inflammatory myopathy: Clinical characteristics, treatment strategies, and outcome in 62 patients. Mayo. Clin. Proc. 2007, 82, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Langdon, P.C.; Mulcahy, K.; Shepherd, K.L.; Low, V.H.; Mastaglia, F.L. Pharyngeal dysphagia in inflammatory muscle diseases resulting from impaired suprahyoid musculature. Dysphagia 2012, 27, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Han, T.R.; Jeong, S.J.; Beom, J.W. Comparison between swallowing-related and limb muscle involvement in dermatomyositis patients. Scand. J. Rheumatol. 2010, 39, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.B.; Swash, M. Pharyngeal dysphagia in dermatomyositis: Responsive to cyclophosphamide. J. Clin. Neuromuscul. Dis. 2004, 5, 166–167. [Google Scholar] [CrossRef] [PubMed]
- Ebert, E.C. The gastrointestinal complications of myositis. Aliment. Pharmacol. Ther. 2010, 31, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Logemann, J.A.; Rademaker, A.W.; Pauloski, B.R.; Ohmae, Y.; Kahrilas, P.J. Interobserver agreement on normal swallowing physiology as viewed by videoendoscopy. Folia Phoniatr. Logop. 1999, 51, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Yoshida, M.; Tsuga, K.; Akagawa, Y.; Groher, M.E. Comparison of three types of tongue pressure measurement devices. Dysphagia 2011, 26, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Fukuoka, T.; Mori, T.; Hiraoka, A.; Higa, C.; Kuroki, A.; Takeda, C.; Maruyama, M.; Yoshida, M.; Tsuga, K. Comparison of the Iowa Oral Performance Instrument and JMS tongue pressure measurement device. J. Dent. Sci. 2021, 16, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Mano, T.; Katsuno, M.; Banno, H.; Suzuki, K.; Suga, N.; Hashizume, A.; Araki, A.; Watanabe, H.; Tanaka, S.; Yamamoto, M.; et al. Tongue pressure as a novel biomarker of spinal and bulbar muscular atrophy. Neurology 2014, 82, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, S.F.; Piersiala, K.; Hillel, A.T.; Akst, L.M.; Best, S.R. Dysphonia and dysphagia as early manifestations of autoimmune inflammatory myopathy. Am. J. Otolaryngol. 2021, 42, 102747. [Google Scholar] [CrossRef] [PubMed]
- Xiong, A.; Qiang, Y.; Cao, Y.; Shuai, Y.; Chen, H.; Xiang, Q.; Hu, Z.; Song, Z.; Zhou, S.; Zhang, Y.; et al. The therapeutic efficacy and safety of intravenous immunoglobulin in dermatomyositis and polymyositis: A systematic review and meta-analysis. Mod. Rheumatol. 2022, roac057. [Google Scholar] [CrossRef] [PubMed]
- Dalakas, M.C.; Illa, I.; Dambrosia, J.M.; Soueidan, S.A.; Stein, D.P.; Otero, C.; Dinsmore, S.T.; McCrosky, S. A controlled trial of high-dose intravenous immune globulin infusions as treatment for dermatomyositis. N. Engl. J. Med. 1993, 329, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Sandage, M.J.; Smith, A.G. Muscle Bioenergetic Considerations for Intrinsic Laryngeal Skeletal Muscle Physiology. J. Speech Lang. Hear. Res. 2017, 60, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Schmidt, J. Impact and Management of Dysphagia in Inflammatory Myopathies. Curr. Rheumatol. Rep. 2020, 22, 74. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, A.; Katsuno, M.; Banno, H.; Suzuki, K.; Suga, N.; Mano, T.; Atsuta, N.; Oe, H.; Watanabe, H.; Tanaka, F.; et al. Longitudinal changes of outcome measures in spinal and bulbar muscular atrophy. Brain 2012, 135, 2838–2848. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.; Keage, M.; Watanabe, M.; Schubiger, D.; Velakoulis, D.; Walterfang, M.; Vogel, A.P. Characterization of Dysphagia and Longitudinal Changes in Swallowing Function in Adults with Niemann-Pick Disease Type C Treated with Miglustat. Dysphagia 2021, 36, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Keage, M.; Delatycki, M.B.; Dyer, J.; Corben, L.A.; Vogel, A.P. Changes detected in swallowing function in Friedreich ataxia over 12 months. Neuromuscul. Disord. 2019, 29, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Oyama, G.; Umeda, M.; Funahara, M.; Soutome, S.; Nakamura, W.; Kojima, Y.; Iwai, H. Effect of decreased tongue pressure on dysphagia and survival rate in elderly people requiring long-term care. J. Dent. Sci. 2022, 17, 856–862. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mano, T.; Soyama, S.; Sugie, K. Improvement in Tongue Pressure Precedes Improvement in Dysphagia in Dermatomyositis. Clin. Pract. 2022, 12, 797-802. https://doi.org/10.3390/clinpract12050083
Mano T, Soyama S, Sugie K. Improvement in Tongue Pressure Precedes Improvement in Dysphagia in Dermatomyositis. Clinics and Practice. 2022; 12(5):797-802. https://doi.org/10.3390/clinpract12050083
Chicago/Turabian StyleMano, Tomoo, Shigeto Soyama, and Kazuma Sugie. 2022. "Improvement in Tongue Pressure Precedes Improvement in Dysphagia in Dermatomyositis" Clinics and Practice 12, no. 5: 797-802. https://doi.org/10.3390/clinpract12050083
APA StyleMano, T., Soyama, S., & Sugie, K. (2022). Improvement in Tongue Pressure Precedes Improvement in Dysphagia in Dermatomyositis. Clinics and Practice, 12(5), 797-802. https://doi.org/10.3390/clinpract12050083






