Aggressiveness of Grade 4 Gliomas of Adults
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Clinical Characterization
3.2. Imaging Features
3.3. Morphogenetic Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Forjaz, G.; Barnholtz-Sloan, J.S.; Kruchko, C.; Siegel, R.; Negoita, S.; Ostrom, Q.T.; Dickie, L.; Ruhl, J.; van Dyke, A.; Patil, N.; et al. An updated histology recode for the analysis of primary malignant and nonmalignant brain and other central nervous system tumors in the Surveillance, Epidemiology, and End Results Program. Neurooncol. Adv. 2020, 3, vdaa175. [Google Scholar] [CrossRef] [PubMed]
- Biserova, K.; Jakovlevs, A.; Uljanovs, R.; Strumfa, I. Cancer Stem Cells: Significance in Origin, Pathogenesis and Treatment of Glioblastoma. Cells 2021, 10, 621. [Google Scholar] [CrossRef] [PubMed]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef]
- Georgescu, M.M.; Olar, A. Genetic and histologic spatiotemporal evolution of recurrent, multifocal, multicentric and metastatic glioblastoma. Acta Neuropathol. Commun. 2020, 8, 10. [Google Scholar] [CrossRef]
- Gonzalez Castro, L.N.; Wesseling, P. The cIMPACT-NOW updates and their significance to current neuro-oncology practice. Neurooncol. Pract. 2020, 8, 4–10. [Google Scholar] [CrossRef]
- Jiang, T.; Nam, D.H.; Ram, Z.; Poon, W.S.; Wang, J.; Boldbaatar, D.; Mao, Y.; Ma, W.; Mao, Q.; You, Y.; et al. Clinical practice guidelines for the management of adult diffuse gliomas. Cancer Lett. 2021, 499, 60–72. [Google Scholar] [CrossRef]
- Kayabolen, A.; Yilmaz, E.; Bagci-Onder, T. IDH Mutations in Glioma: Double-Edged Sword in Clinical Applications? Biomedicines 2021, 9, 799. [Google Scholar] [CrossRef]
- Sun, X.; Turcan, S. From Laboratory Studies to Clinical Trials: Temozolomide Use in IDH-Mutant Gliomas. Cells 2021, 10, 1225. [Google Scholar] [CrossRef]
- Lukas, R.V.; Wainwright, D.A.; Ladomersky, E.; Sachdev, S.; Sonabend, A.M.; Stupp, R. Newly Diagnosed Glioblastoma: A Review on Clinical Management. Oncology 2019, 33, 91–100. [Google Scholar]
- Philips, A.; Henshaw, D.L.; Lamburn, G.; O’Carroll, M.J. Brain Tumours: Rise in Glioblastoma Multiforme Incidence in England 1995–2015 Suggests an Adverse Environmental or Lifestyle Factor. J. Environ. Public Health 2018, 2018, 7910754. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.M.; Cloughesy, T.F. Adult Glioblastoma. J. Clin. Oncol 2017, 35, 2402–2409. [Google Scholar] [CrossRef]
- Gilard, V.; Tebani, A.; Dabaj, I.; Laquerrière, A.; Fontanilles, M.; Derrey, S.; Marret, S.; Bekri, S. Diagnosis and Management of Glioblastoma: A Comprehensive Perspective. J. Pers Med. 2021, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Kerkhof, M.; Vecht, C.J. Seizure characteristics and prognostic factors of gliomas. Epilepsia 2013, 54 (Suppl. 9), 12–17. [Google Scholar] [CrossRef]
- Johnson, D.R.; Wefel, J.S. Relationship between cognitive function and prognosis in glioblastoma. CNS Oncol. 2013, 2, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Pierscianek, D.; Ahmadipour, Y.; Kaier, K.; Darkwah Oppong, M.; Michel, A.; Kebir, S.; Stuschke, M.; Glas, M.; Sure, U.; Jabbarli, R. The SHORT Score for Preoperative Assessment of the Risk for Short-Term Survival in Glioblastoma. World Neurosurg. 2020, 138, 370–380. [Google Scholar] [CrossRef]
- Houben, M.P.; Louwman, W.J.; Tijssen, C.C.; Teepen, J.L.; van Duijn, C.M.; Coebergh, J.W. Hypertension as a risk factor for glioma? Evidence from a population-based study of comorbidity in glioma patients. Ann. Oncol. 2004, 15, 1256–1260. [Google Scholar] [CrossRef]
- Montemurro, N.; Perrini, P.; Rapone, B. Clinical Risk and Overall Survival in Patients with Diabetes Mellitus, Hyperglycemia and Glioblastoma Multiforme. A Review of the Current Literature. Int. J. Environ. Res. Public Health 2020, 17, 8501. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Central Nervous System Tumours, 5th ed.; IARC: Lyon, France, 2021; pp. 23–43. [Google Scholar]
- De Vocht, F. Inferring the 1985-2014 impact of mobile phone use on selected brain cancer subtypes using Bayesian structural time series and synthetic controls. Environ. Int. 2016, 97, 100–107. [Google Scholar] [CrossRef]
- Youland, R.S.; Brown, P.D.; Giannini, C.; Parney, I.F.; Uhm, J.H.; Laack, N.N. Adult low-grade glioma: 19-year experience at a single institution. Am. J. Clin. Oncol. 2013, 36, 612–619. [Google Scholar] [CrossRef]
- Bette, S.; Barz, M.; Wiestler, B.; Huber, T.; Gerhardt, J.; Buchmann, N.; Combs, S.E.; Schmidt-Graf, F.; Delbridge, C.; Zimmer, C.; et al. Prognostic Value of Tumor Volume in Glioblastoma Patients: Size Also Matters for Patients with Incomplete Resection. Ann. Surg. Oncol. 2018, 25, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Leu, S.; Boulay, J.L.; Thommen, S.; Bucher, H.C.; Stippich, C.; Mariani, L.; Bink, A. Preoperative Two-Dimensional Size of Glioblastoma is Associated with Patient Survival. World Neurosurg. 2018, 115, 448–463. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Seppä, K.; Luostarinen, T.; Malila, N.; Seppälä, M.; Pitkäniemi, J.; Korja, M. Disparities in glioblastoma survival by case volume: A nationwide observational study. J. Neurooncol. 2020, 147, 361–370. [Google Scholar] [CrossRef]
- Bizu, I.; Trifanescu, O.G.; Georgescu, M.T.; Gruia, M.I.; Anghel, R. Clinical prognostic factors in newly diagnosed glioblastoma. Rom. J. Neurol. 2019, 18, 71–77. [Google Scholar] [CrossRef]
- Śledzińska, P.; Bebyn, M.G.; Furtak, J.; Kowalewski, J.; Lewandowska, M.A. Prognostic and Predictive Biomarkers in Gliomas. Int. J. Mol. Sci. 2021, 22, 10373. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Wu, F.; Li, J.J.; Li, Y.F.; Liu, X.; Wang, Z.; Chai, R.C. Gene Expression Profiling Stratifies IDH-Wildtype Glioblastoma with Distinct Prognoses. Front. Oncol. 2019, 9, 1433. [Google Scholar] [CrossRef]
- Brown, N.F.; Ottaviani, D.; Tazare, J.; Gregson, J.; Kitchen, N.; Brandner, S.; Fersht, N.; Mulholland, P. Survival Outcomes and Prognostic Factors in Glioblastoma. Cancers 2022, 14, 3161. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Raj, K.V.; Atla, B. Clinicohistopathological study of astrocytomas along with Ki-67 proliferative index. Int. J. Res. Med. Sci. 2018, 6, 665–670. [Google Scholar] [CrossRef][Green Version]
- Armocida, D.; Frati, A.; Salvati, M.; Santoro, A.; Pesce, A. Is Ki-67 index overexpression in IDH wild type glioblastoma a predictor of shorter Progression Free survival? A clinical and Molecular analytic investigation. Clin. Neurol. Neurosurg. 2020, 198, 106126. [Google Scholar] [CrossRef]
- Dahlrot, R.H.; Bangsø, J.A.; Petersen, J.K.; Rosager, A.M.; Sørensen, M.D.; Reifenberger, G.; Hansen, S.; Kristensen, B.W. Prognostic role of Ki-67 in glioblastomas excluding contribution from non-neoplastic cells. Sci. Rep. 2021, 11, 17918. [Google Scholar] [CrossRef]
- Hu, W.; Lu, H.; Wang, S.; Yin, W.; Liu, X.; Dong, L.; Chiu, R.; Shen, L.; Lu, W.J.; Lan, F. Suppression of Nestin reveals a critical role for p38-EGFR pathway in neural progenitor cell proliferation. Oncotarget 2016, 7, 87052–87063. [Google Scholar] [CrossRef] [PubMed]
- Wiese, C.; Rolletschek, A.; Kania, G.; Blyszczuk, P.; Tarasov, K.V.; Tarasova, Y.; Wersto, R.P.; Boheler, K.R.; Wobus, A.M. Nestin expression--a property of multi-lineage progenitor cells? Cell Mol. Life Sci. 2004, 61, 2510–2522. [Google Scholar] [CrossRef] [PubMed]
- Abdelkareem, R.M.; Elnashar, A.T.; Fadle, K.N.; Muhammad, E.M.S. Immunohistochemical expression of Nestin as Cancer Stem Cell Marker in gliomas. J. Neurosci. Neurol. Disord. 2019, 3, 162–166. [Google Scholar] [CrossRef]
- Chinnaiyan, P.; Wang, M.; Rojiani, A.M.; Tofilon, P.J.; Chakravarti, A.; Ang, K.K.; Zhang, H.Z.; Hammond, E.; Curran, W.; Mehta, M.P. The prognostic value of nestin expression in newly diagnosed glioblastoma: Report from the Radiation Therapy Oncology Group. Radiat. Oncol. 2008, 3, 32. [Google Scholar] [CrossRef]
- Kim, K.J.; Lee, K.H.; Kim, H.S.; Moon, K.S.; Jung, T.Y.; Jung, S.; Lee, M.C. The presence of stem cell marker-expressing cells is not prognostically significant in glioblastomas. Neuropathology 2011, 31, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Behling, F.; Barrantes-Freer, A.; Behnes, C.L.; Stockhammer, F.; Rohde, V.; Adel-Horowski, A.; Rodríguez-Villagra, O.A.; Barboza, M.A.; Brück, W.; Lehmann, U.; et al. Expression of Olig2, Nestin, NogoA and AQP4 have no impact on overall survival in IDH-wildtype glioblastoma. PLoS ONE 2020, 15, 0229274. [Google Scholar] [CrossRef]
- Huang, L.E. Impact of CDKN2A/B Homozygous Deletion on the Prognosis and Biology of IDH-Mutant Glioma. Biomedicines 2022, 10, 246. [Google Scholar] [CrossRef]
- Khani, P.; Nasri, F.; Khani Chamani, F.; Saeidi, F.; Sadri Nahand, J.; Tabibkhooei, A.; Mirzaei, H. Genetic and epigenetic contribution to astrocytic gliomas pathogenesis. J. Neurochem. 2019, 148, 188–203. [Google Scholar] [CrossRef]
- Santosh, V.; Rao, S. A review of adult-type diffuse gliomas in the WHO CNS5 classification with special reference to Astrocytoma, IDH-mutant and Oligodendroglioma, IDH-mutant and 1p/19q codeleted. Indian J. Pathol. Microbiol. 2022, 65, S14–S23. [Google Scholar] [CrossRef]
- Ghasimi, S.; Wibom, C.; Dahlin, A.M.; Brännström, T.; Golovleva, I.; Andersson, U.; Melin, B. Genetic risk variants in the CDKN2A/B, RTEL1 and EGFR genes are associated with somatic biomarkers in glioma. J. Neurooncol. 2016, 127, 483–492. [Google Scholar] [CrossRef]
- Marker, D.F.; Pearce, T.M. Homozygous deletion of CDKN2A by fluorescence in situ hybridization is prognostic in grade 4, but not grade 2 or 3, IDH-mutant astrocytomas. Acta Neuropathol. Commun. 2020, 8, 169. [Google Scholar] [CrossRef] [PubMed]
- Appay, R.; Dehais, C.; Maurage, C.A.; Alentorn, A.; Carpentier, C.; Colin, C.; Ducray, F.; Escande, F.; Idbaih, A.; Kamoun, A.; et al. CDKN2A homozygous deletion is a strong adverse prognosis factor in diffuse malignant IDH-mutant gliomas. Neuro. Oncol. 2019, 21, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.J.; Keblusek, P.; Robanus-Maandag, E.; Kristel, P.; Lingbeek, M.; Nederlof, P.M.; van Welsem, T.; van de Vijver, M.J.; Koh, E.Y.; Daley, G.Q.; et al. Senescence bypass screen identifies TBX2, which represses Cdkn2a (p19(ARF)) and is amplified in a subset of human breast cancers. Nat. Genet. 2000, 26, 291–299. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | IDH-Mutant Astrocytoma * (n = 46) | IDH-Wildtype Glioblastoma (n = 39) | p-Value ** |
|---|---|---|---|
Age:
| 57.93 (37–82) 32.61% | 60.36 (20–81) 12.82% | p = 0.041 |
Gender:
| 56.52% 43.48% | 51.28% 48.72% | p = 0.667 |
The onset of symptoms:
| 41.30% 45.65% 13.04% | 38.46% 43.59% 17.95% | p = 0.876 |
Symptoms:
| 60.87% 39.13% 63.04% 21.74% 19.57% 28.26% 65.22% | 59.87% 30.77% 56.41% 30.77% 12.82% 46.15% 58.97% | p = 0.859 p = 0.498 p = 0.657 p = 0.457 p = 0.559 p = 0.115 p = 0.655 |
Comorbidities:
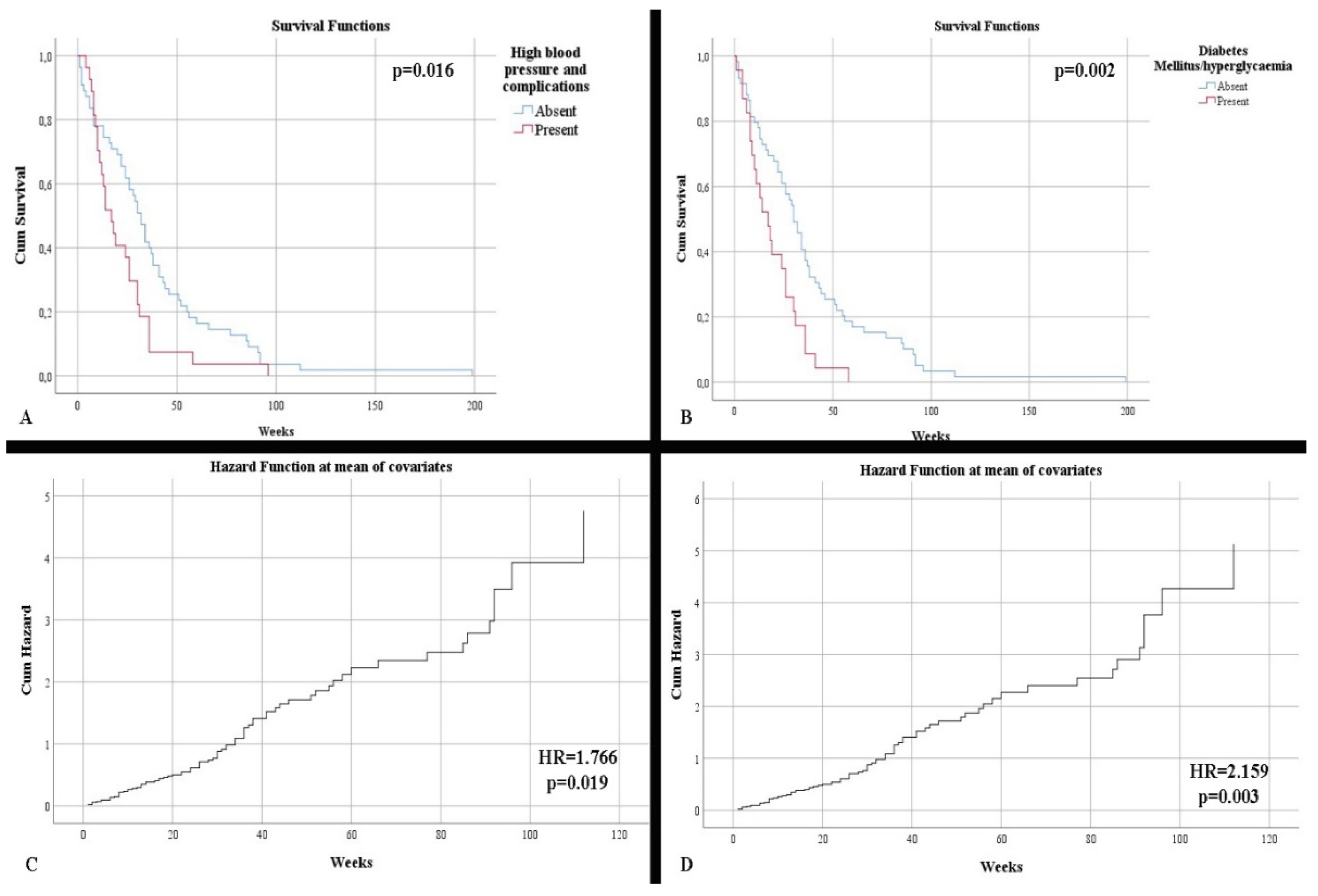
| 26.09% 28.26% 8.70% | 41.03% 25.64% 7.69% | p = 0.169 p = 0.812 p = 0.867 |
| Complete treatment | 69.57% | 82.05 | p = 0.215 |
| Imaging Characteristics | IDH-Mutant Astrocytoma * (n = 46) | IDH-Wildtype Glioblastoma (n = 39) | p-Value ** |
|---|---|---|---|
Accuracy:
| 92.85% 59.47% | 87.50% 92% | p = 0.389 p = 0.883 |
Location:
| 95.65% 4.35% | 94.87% 5.13% | p = 0.627 |
Cerebral/cerebellar hemisphere
| 50% 50% | 53.85% 46.15% | p = 0.828 |
Lobe
| 17.39% 4.35% 17.39% 2.17% 8.70% 8.70% 8.70% 23.91% 0% 2.17% 2.17% 4.35% | 7.69% 0% 5.13% 7.69% 17.95% 12.82% 10.26% 20.51% 5.13% 7.69% 0% 5.13% | p = 0.264 |
Maximum diameter
| 50.13 (10–80) 10.87% 41.30% 47.83% | 52.10 (20–87) 2.56% 51.28% 46.15% | p = 0.704 p = 0.306 |
Volume
| 85 (0.9–388.94) | 91.69 (1.22–324.72) | p = 0.672 |
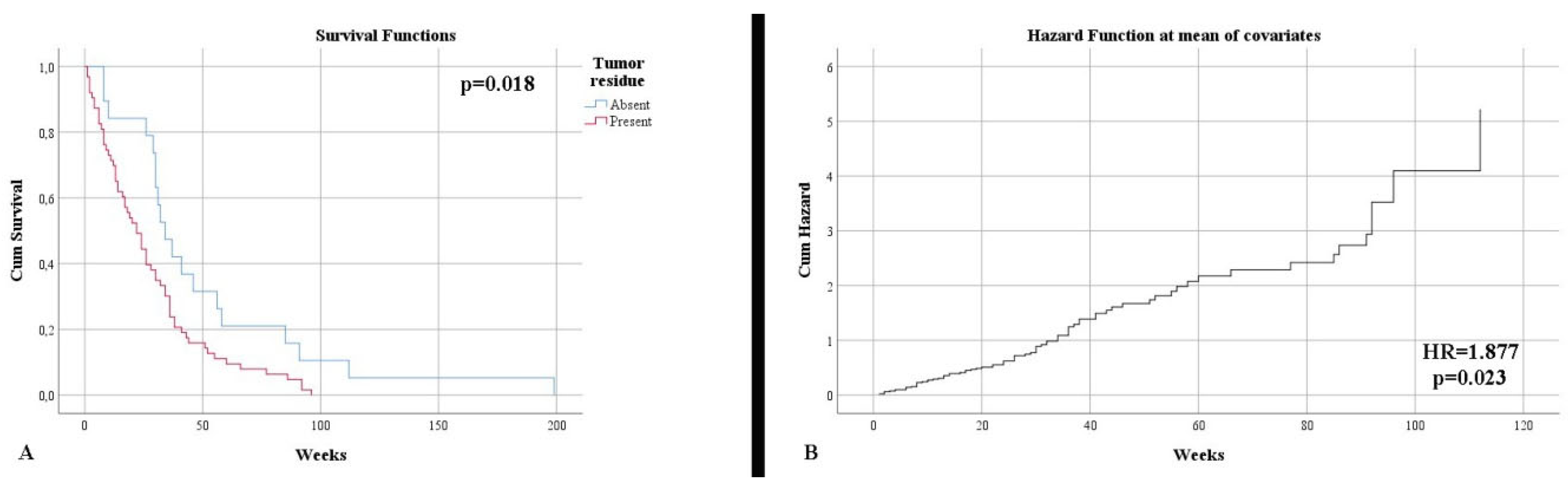
Resection type
| 23.91% 76.09% | 23.08% 76.92% | p = 0.567 |
| Morphogenetic Characteristics | IDH-Mutant Astrocytoma * (n = 46) | IDH-Wildtype Glioblastoma (n = 39) | p-Value ** |
|---|---|---|---|
Proliferative index (Ki-67)
| 49.78 (15–95) | 38.15 (4–90) | p = 0.030 |
Nestin:
| 82.16% 17.39% | 94.87% 5.13% | p = 0.100 |
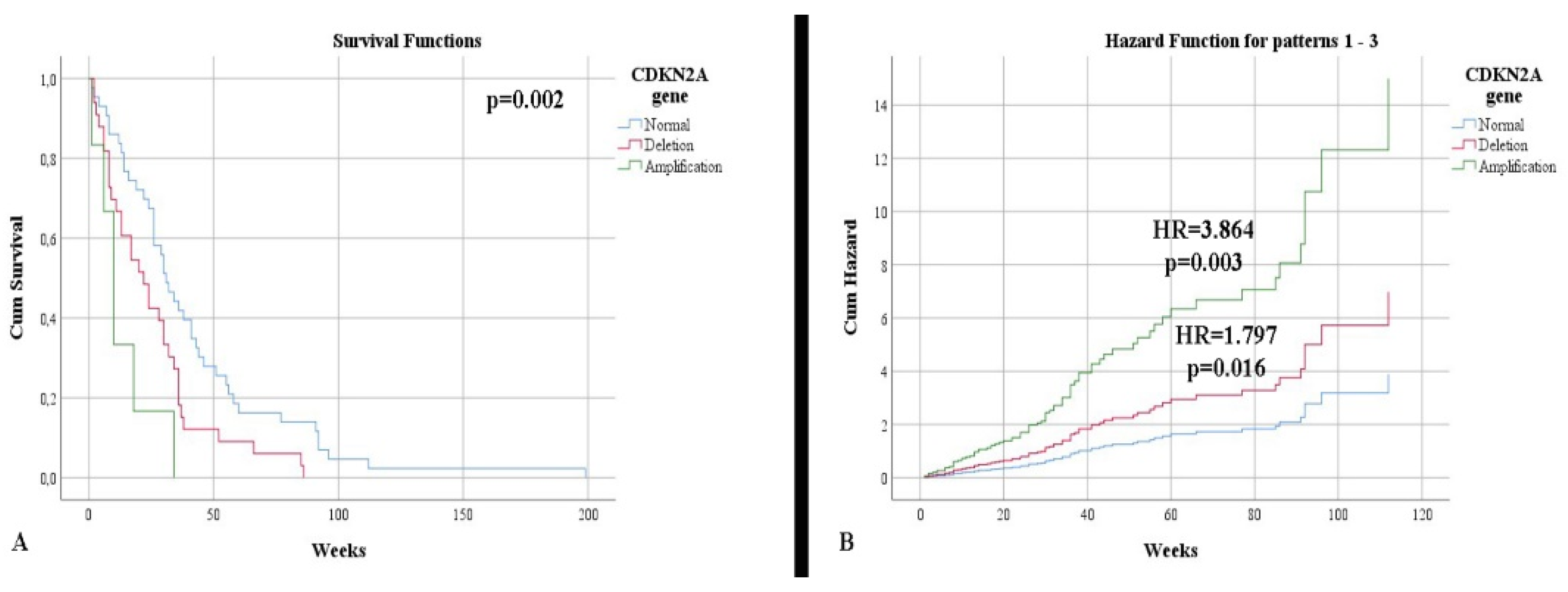
CDKN2A
| 45.65% 47.83% 6.52% | 64.10% 28.21% 7.69% | p = 0.173 |
Survival according to CDKN2A (weeks)
| 50.74 23.96 9.67 | 34.08 28.73 16.67 | p = 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deacu, M.; Docu Axelerad, A.; Popescu, S.; Topliceanu, T.S.; Aschie, M.; Bosoteanu, M.; Cozaru, G.C.; Cretu, A.M.; Voda, R.I.; Orasanu, C.I. Aggressiveness of Grade 4 Gliomas of Adults. Clin. Pract. 2022, 12, 701-713. https://doi.org/10.3390/clinpract12050073
Deacu M, Docu Axelerad A, Popescu S, Topliceanu TS, Aschie M, Bosoteanu M, Cozaru GC, Cretu AM, Voda RI, Orasanu CI. Aggressiveness of Grade 4 Gliomas of Adults. Clinics and Practice. 2022; 12(5):701-713. https://doi.org/10.3390/clinpract12050073
Chicago/Turabian StyleDeacu, Mariana, Any Docu Axelerad, Steliana Popescu, Theodor Sebastian Topliceanu, Mariana Aschie, Madalina Bosoteanu, Georgeta Camelia Cozaru, Ana Maria Cretu, Raluca Ioana Voda, and Cristian Ionut Orasanu. 2022. "Aggressiveness of Grade 4 Gliomas of Adults" Clinics and Practice 12, no. 5: 701-713. https://doi.org/10.3390/clinpract12050073
APA StyleDeacu, M., Docu Axelerad, A., Popescu, S., Topliceanu, T. S., Aschie, M., Bosoteanu, M., Cozaru, G. C., Cretu, A. M., Voda, R. I., & Orasanu, C. I. (2022). Aggressiveness of Grade 4 Gliomas of Adults. Clinics and Practice, 12(5), 701-713. https://doi.org/10.3390/clinpract12050073







