Severe Stenosis of Mitral Bioprosthetic Valve Thrombosis in a Patient with HCV-Related Cirrhosis and Duodenal Variceal Bleeding: The Deadly Triad
Abstract
:1. Introduction
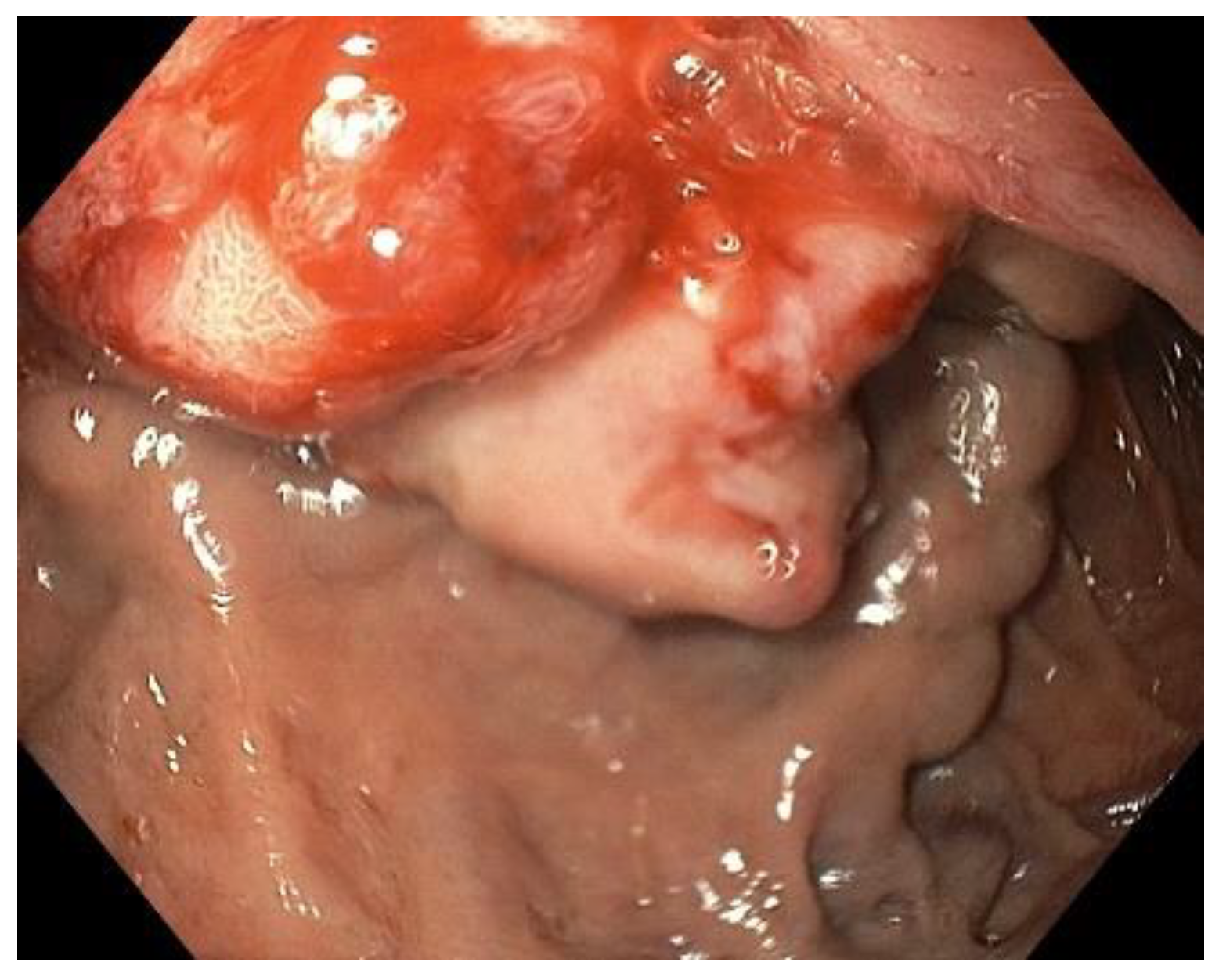
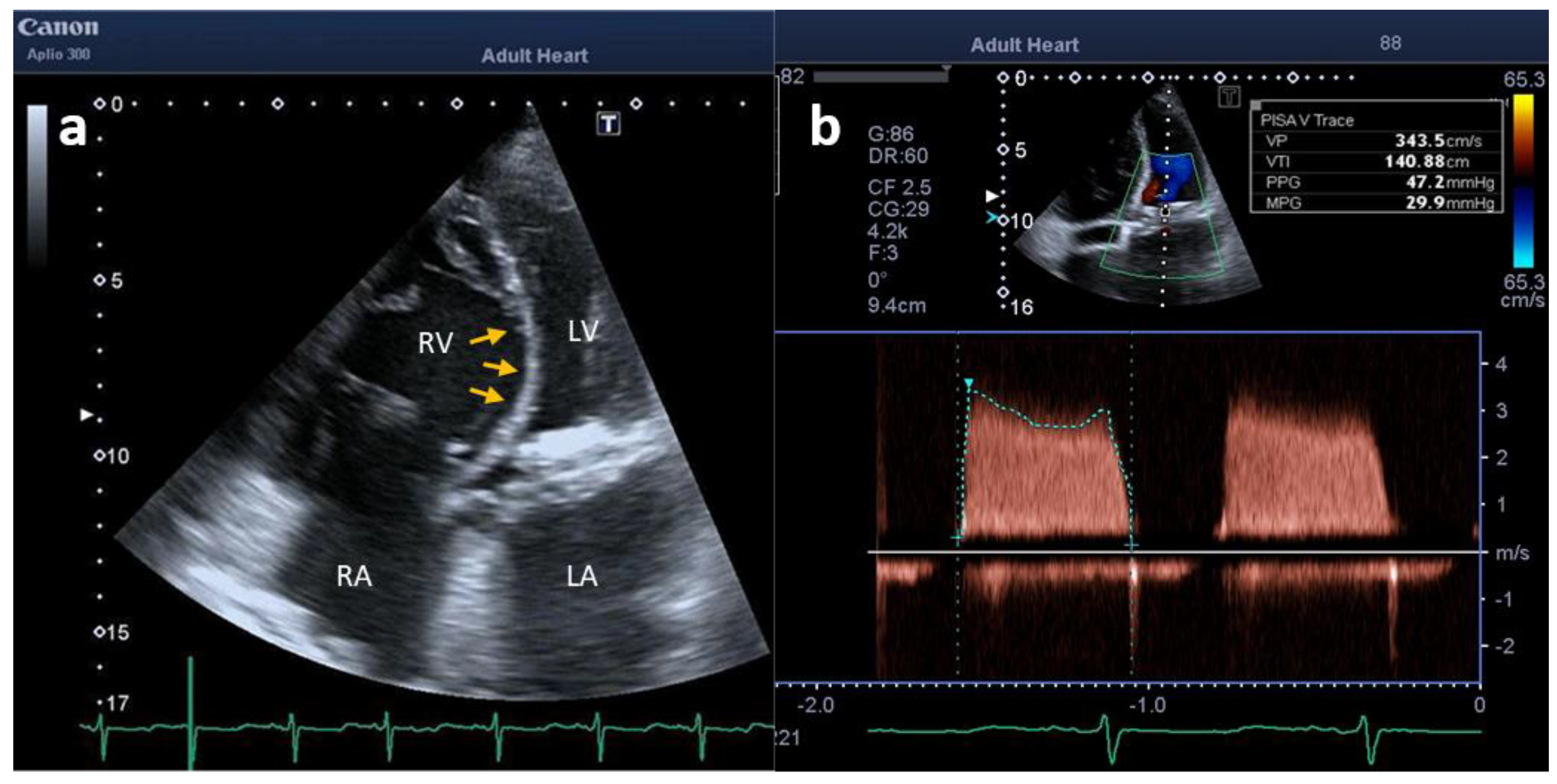
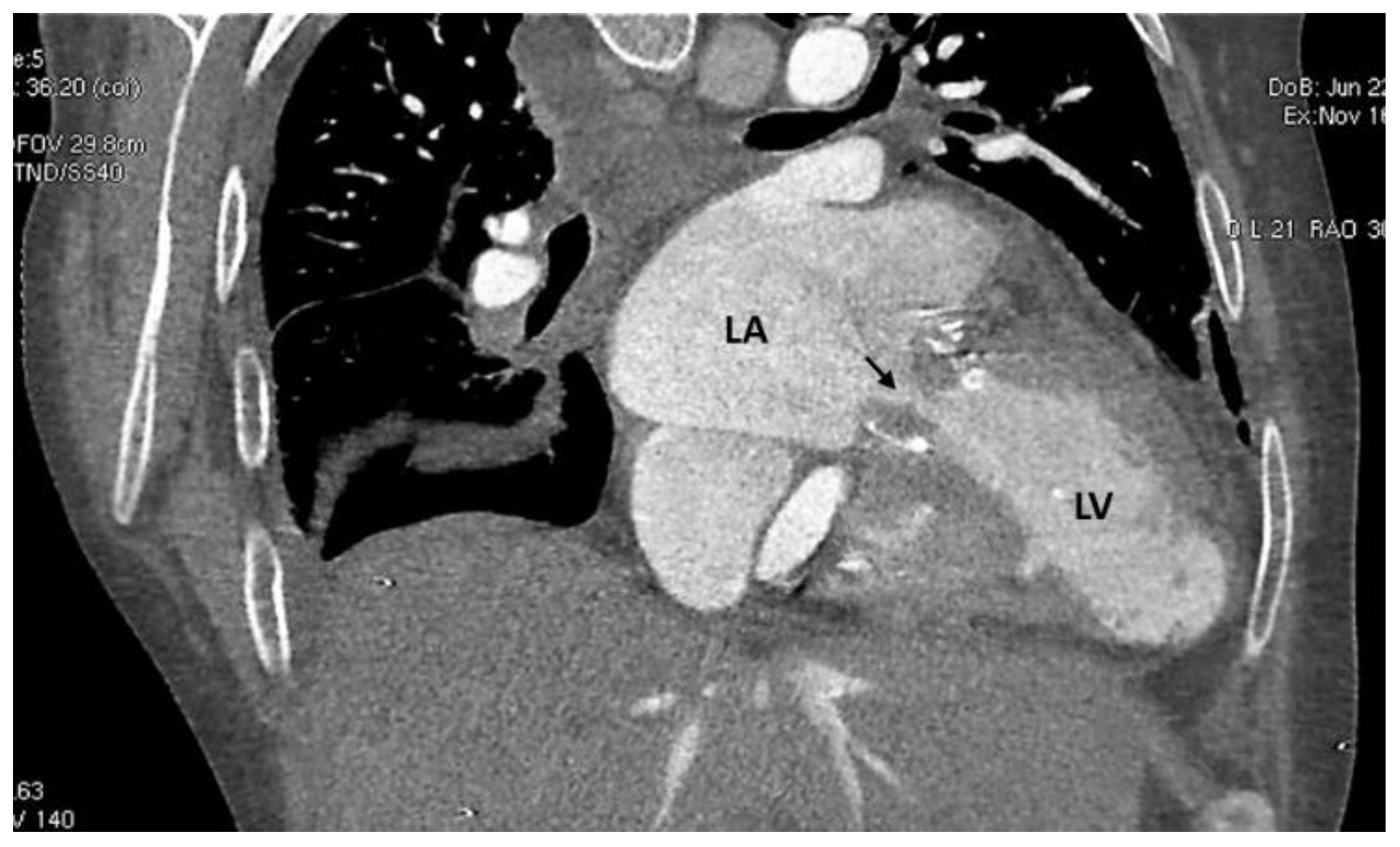
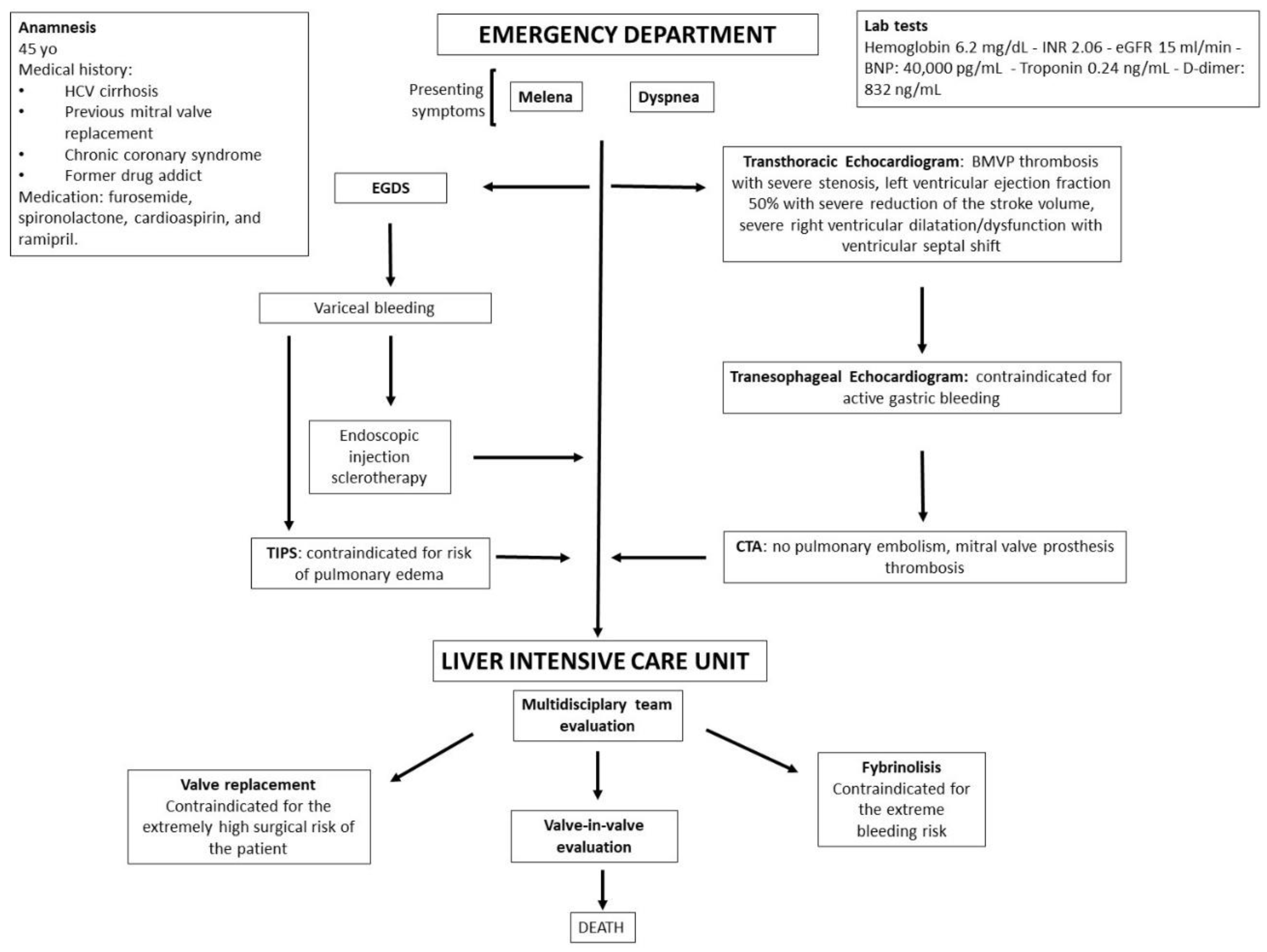
2. Case Description
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zipes, D.P.; Libby, P.; Bonow, R.; Mann, L.D.; Tomaselli, G.F.; Braunwald, E. Braunwald’s Heart Disease a Textbook of Cardiovascular Medicine, 11th ed.; Elsevier: Philadelphia, PA, USA, 2019; pp. 1455–1463. [Google Scholar]
- Puri, R.; Auffret, V.; Rodés-Cabau, J. Bioprosthetic Valve Thrombosis. J. Am. Coll. Cardiol. 2017, 69, 2193–2211. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.L.; Fauci, A.S.; Kasper, D.L.; Hauser, S.L.; Longo, D.L.; Loscalzo, J. Harrison’s Principles of Internal Medicine, 20th ed.; McGraw Hill Higher Education: New York, NY, USA, 2018; pp. 2405–2414. [Google Scholar]
- GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef]
- Tsochatzis, E.A.; Bosch, J.; Burroughs, A.K. Liver cirrhosis. Lancet 2014, 383, 1749–1761. [Google Scholar] [CrossRef]
- Weishaupt, D.; Pfammatter, T.; Hilfiker, P.R.; Wolfensberger, U.; Marincek, B. Detecting bleeding duodenal varices with multislice helical CT. AJR Am. J. Roentgenol. 2002, 178, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Saad, W.E.; Lippert, A.; Saad, N.E.; Caldwell, S. Ectopic varices: Anatomical classification, hemodynamic classification, and hemodynamic-based management. Tech. Vasc. Interv. Radiol. 2013, 16, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Henry, Z.; Uppal, D.; Saad, W.; Caldwell, S. Gastric and ectopic varices. Clin. Liver Dis. 2014, 18, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Lotterer, E.; Wengert, A.; Fleig, W.E. Transjugular intrahepatic portosystemic shunt: Short-term and long-term effects on hepatic and systemic hemodynamics in patients with cirrhosis. Hepatology 1999, 29, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, 2440–2492. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; Edvardsen, T.; Delgado, V.; Dulgheru, R.; Pepi, M.; Cosyns, B.; Dweck, M.R.; Garbi, M.; et al. Recommendations for the imaging assessment of prosthetic heart valves: A report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 589–590. [Google Scholar]
- Mauro, C.; Vriz, O.; Romano, L.; Citro, R.; Russo, V.; Ranieri, B.; Alamro, B.; Aladmawi, M.; Granata, R.; Galzerano, D.; et al. Imaging Cardiovascular Emergencies: Real World Clinical Cases. Heart Fail. Clin. 2020, 16, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Roudaut, R.; Serri, K.; Lafitte, S. Thrombosis of prosthetic heart valves: Diagnosis and therapeutic considerations. Heart 2007, 93, 137–142. [Google Scholar] [CrossRef]
- Karamitsos, T.D.; Karvounis, H. Magnetic resonance imaging is a safe technique in patients with prosthetic heart valves and coronary stents. Hellenic J. Cardiol. 2019, 60, 38–39. [Google Scholar] [CrossRef] [PubMed]
- Boyer, T.D.; Haskal, Z.J. American Association. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology 2005, 41, 386–400. [Google Scholar] [CrossRef] [PubMed]
- Copelan, A.; Chehab, M.; Dixit, P.; Cappell, M.S. Safety and efficacy of angiographic occlusion of duodenal varices as an alternative to TIPS: Review of 32 cases. Ann. Hepatol. 2015, 14, 369–379. [Google Scholar] [CrossRef]
- Lee, E.W.; Saab, S.; Gomes, A.S.; Busuttil, R.; McWilliams, J.; Durazo, F.; Han, S.H.; Goldstein, L.; Tafti, B.A.; Moriarty, J.; et al. Coil-assisted retrograde transvenous obliteration (CARTO) for the treatment of portal hypertensive variceal bleeding: Preliminary results. Clin. Transl. Gastroenterol. 2014, 5, e61. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Jeon, G.S. Coil-assisted retrograde transvenous obliteration for the treatment of duodenal varix. Diagn. Interv. Radiol. 2018, 24, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Darcy, M.D.; Mani, N.B.; Park, A.W.; Akinwande, O.; Ramaswamy, R.S.; Kim, S.K. Modified balloon-occluded retrograde transvenous obliteration (BRTO) techniques for the treatment of gastric varices: Vascular plug-assisted retrograde transvenous obliteration (PARTO)/coil-assisted retrograde transvenous obliteration (CARTO)/balloon-occluded antegrade transvenous obliteration (BATO). Cardiovasc. Interv. Radiol. 2018, 41, 835–847. [Google Scholar]
| Mechanical Prosthetic Valve Thrombosis | Bioprosthetic Valve Thrombosis | |
|---|---|---|
| Incidence in developed countries | 0.3% to 1.3% | 0.03% to 0.5% |
| Etiology |
|
|
| Clinical presentation |
|
|
| Imaging |
If inconclusive echocardiography and/or additional information needed:
|
If inconclusive echocardiography and/or additional information needed:
|
| Main treatment | Obstructive Thrombosis
| Anticoagulation using a VKA and/or UFH is recommended in BPVT s before considering re-intervention. Obstructive Thrombosis
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocchia, R.; Chianese, S.; Lombardi, G.; Romano, L.; Capone, V.; Amitrano, L.; Bennato, R.; Ranieri, B.; Russo, G.; Mauro, C.; et al. Severe Stenosis of Mitral Bioprosthetic Valve Thrombosis in a Patient with HCV-Related Cirrhosis and Duodenal Variceal Bleeding: The Deadly Triad. Clin. Pract. 2022, 12, 686-691. https://doi.org/10.3390/clinpract12050071
Cocchia R, Chianese S, Lombardi G, Romano L, Capone V, Amitrano L, Bennato R, Ranieri B, Russo G, Mauro C, et al. Severe Stenosis of Mitral Bioprosthetic Valve Thrombosis in a Patient with HCV-Related Cirrhosis and Duodenal Variceal Bleeding: The Deadly Triad. Clinics and Practice. 2022; 12(5):686-691. https://doi.org/10.3390/clinpract12050071
Chicago/Turabian StyleCocchia, Rosangela, Salvatore Chianese, Giovanni Lombardi, Luigia Romano, Valentina Capone, Lucio Amitrano, Raffaele Bennato, Brigida Ranieri, Giuseppe Russo, Ciro Mauro, and et al. 2022. "Severe Stenosis of Mitral Bioprosthetic Valve Thrombosis in a Patient with HCV-Related Cirrhosis and Duodenal Variceal Bleeding: The Deadly Triad" Clinics and Practice 12, no. 5: 686-691. https://doi.org/10.3390/clinpract12050071
APA StyleCocchia, R., Chianese, S., Lombardi, G., Romano, L., Capone, V., Amitrano, L., Bennato, R., Ranieri, B., Russo, G., Mauro, C., & Bossone, E. (2022). Severe Stenosis of Mitral Bioprosthetic Valve Thrombosis in a Patient with HCV-Related Cirrhosis and Duodenal Variceal Bleeding: The Deadly Triad. Clinics and Practice, 12(5), 686-691. https://doi.org/10.3390/clinpract12050071







