Prevalence, Trends, and Outcomes of Pulmonary Embolism Treated with Mechanical and Surgical Thrombectomy from a Nationwide Inpatient Sample
Abstract
:1. Introduction
2. Methods
2.1. Data Source
2.2. Study Population
2.3. Definition of Data Elements
2.4. Outcomes
2.5. Statistical Analysis
3. Results
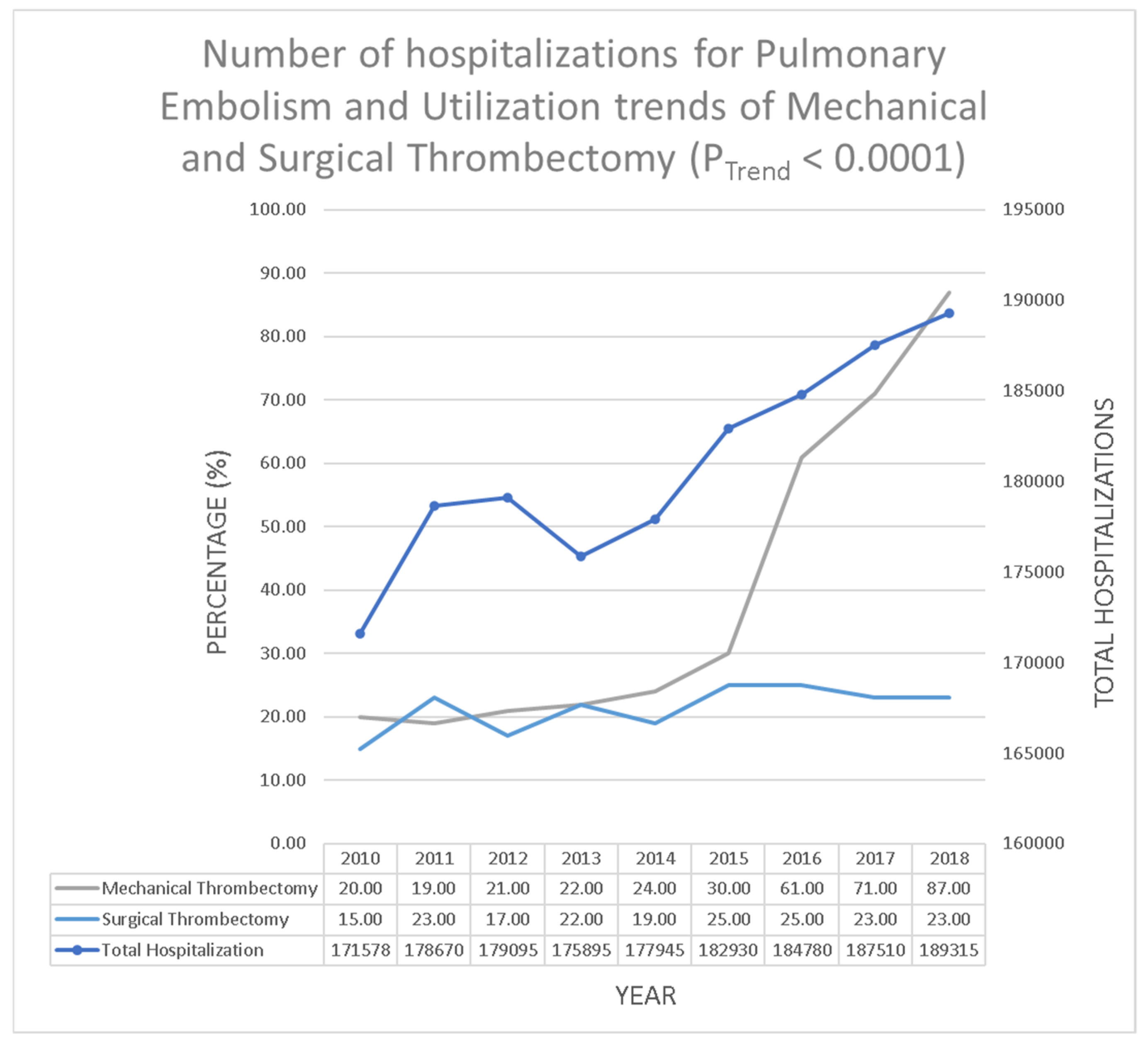
3.1. Utilization Trend of Mechanical and Surgical Thrombectomy during Hospitalization Due to Pulmonary Embolism
3.2. Baseline Characteristics of Pulmonary Embolism Patients Who Underwent Mechanical Thrombectomy
3.3. Outcomes and Peri-Procedural Complications of Pulmonary Embolism Patients Who Underwent Mechanical Thrombectomy
3.4. Baseline Characteristics of Pulmonary Embolism Patients Who Underwent Surgical Thrombectomy
3.5. Outcomes and Peri-Procedural Complications of Pulmonary Embolism Patients Who Underwent Surgical Thrombectomy
3.6. Predictors of In-Hospital Mortality of Pulmonary Embolism Patients Who Underwent Mechanical and Surgical Thrombectomy
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lavorini, F.; Di Bello, V.; De Rimini, M.L.; Lucignani, G.; Marconi, L.; Palareti, G.; Pesavento, R.; Prisco, D.; Santini, M.; Sverzellati, N.; et al. Diagnosis and treatment of pulmonary embolism: A multidisciplinary approach. Multidiscip. Respir. Med. 2013, 8, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudzinski, D.M.; Giri, J.; Rosenfield, K. Interventional Treatment of Pulmonary Embolism. Circ. Cardiovasc. Interv. 2017, 10, e004345. [Google Scholar] [CrossRef] [PubMed]
- Doherty, S. Pulmonary embolism: An update. Aust. Fam. Physician 2017, 11, 816–820. [Google Scholar]
- Nguyen, E.; Caranfa, J.T.; Lyman, G.H.; Kuderer, N.M.; Stirbis, C.; Wysocki, M.; Coleman, C.I.; Weeda, E.R.; Kohn, C.G. Clinical prediction rules for mortality in patients with pulmonary embolism and cancer to guide outpatient management: A meta-analysis. J. Thromb. Haemost. 2018, 16, 279–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, I.; Daitoku, K. Surgical Embolectomy for Acute Pulmonary Thromboembolism. Ann. Vasc. Dis. 2017, 10, 107–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayona Molano, M.D.P.; Salsamendi, J.; Mani, N. Emergent mechanical thrombectomy for right atrial clot and massive pulmonary embolism using flowtriever. Clin. Case Rep. 2021, 9, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Athappan, G.; Sengodan, P.; Chacko, P.; Gandhi, S. Comparative efficacy of different modalities for treatment of right heart thrombi in transit: A pooled analysis. Vasc. Med. 2015, 20, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kucher, N.; Boekstegers, P.; Müller, O.J.; Kupatt, C.; Beyer-Westendorf, J.; Heitzer, T.; Tebbe, U.; Horstkotte, J.; Müller, R.; Blessing, E.; et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014, 129, 479–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spies, C.; Khandelwal, A.; Smith, T.H.; Jolly, N.; Kavinsky, C.J. Percutaneous mechanical thrombectomy for massive pulmonary embolism using a conservative treatment strategy. J. Interv. Cardiol. 2008, 21, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Jolly, M.; Phillips, J. Pulmonary Embolism: Current Role of Catheter Treatment Options and Operative Thrombectomy. Surg. Clin. N. Am. 2018, 98, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.T.; Gould, M.K.; Louie, J.D.; Rosenberg, J.K.; Sze, D.Y.; Hofmann, L.V. Catheter-directed Therapy for the Treatment of Massive Pulmonary Embolism: Systematic Review and Meta-analysis of Modern Techniques. J. Vasc. Interv. Radiol. 2009, 20, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Kilic, A.; Shah, A.S.; Conte, J.V.; Yuh, D.D. Nationwide outcomes of surgical embolectomy for acute pulmonary embolism. J. Thorac. Cardiovasc. Surg. 2013, 145, 373–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keeling, W.B.; Sundt, T.; Leacche, M.; Okita, Y.; Binongo, J.; Lasajanak, Y.; Aklog, L.; Lattouf, O.M. Outcomes After Surgical Pulmonary Embolectomy for Acute Pulmonary Embolus: A Multi-Institutional Study. Ann. Thorac. Surg. 2016, 102, 1498–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loyalka, P.; Ansari, M.Z.; Cheema, F.H.; Miller, C.C.; Rajagopal, S.; Rajagopal, K. Surgical pulmonary embolectomy and catheter-based therapies for acute pulmonary embolism: A contemporary systematic review. J. Thorac. Cardiovasc. Surg. 2018, 156, 2155–2167. [Google Scholar] [CrossRef] [PubMed]
- Licha, C.R.M.; McCurdy, C.M.; Maldonado, S.M.; Lee, L.S. Current management of acute pulmonary embolism. Ann. Thorac. Cardiovasc. Surg. 2020, 26, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, X.; Sista, A.K. Catheter-Directed Thrombolysis for Pulmonary Embolism: The State of Practice. Tech. Vasc. Interv. Radiol. 2018, 21, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Gayou, E.L.; Makary, M.S.; Hughes, D.R.; Hemingway, J.; Elliott, E.D.; Spain, J.W.; Prevedello, L.M. Nationwide Trends in Use of Catheter-Directed Therapy for Treatment of Pulmonary Embolism in Medicare Beneficiaries from 2004 to 2016. J. Vasc. Interv. Radiol. 2019, 30, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, D.; De Miguel-Díez, J.; Guijarro, R.; Trujillo-Santos, J.; Otero, R.; Barba, R.; Muriel, A.; Meyer, G.; Yusen, R.D.; Monreal, M. Trends in the Management and Outcomes of Acute Pulmonary Embolism Analysis from the RIETE Registry. J. Am. Coll. Cardiol. 2016, 67, 162–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alqahtani, F.; Munir, M.B.; Aljohani, S.; Tarabishy, A.; Almustafa, A.; Alkhouli, M. Surgical thrombectomy for pulmonary embolism: Updated performance rates and outcomes. Tex. Hear. Inst. J. 2019, 46, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristics | Mechanical Thrombectomy | % | Surgical Thrombectomy | % |
|---|---|---|---|---|
| Overall | 6531 | 100 | 3465 | 100 |
| Age in years (%) | ||||
| 18–34 | 425 | 6.5 | 414 | 11.9 |
| 35–49 | 1093 | 16.7 | 722 | 20.8 |
| 50–64 | 2065 | 31.6 | 1204 | 34.8 |
| ≥65 | 2947 | 45.1 | 1126 | 32.5 |
| Sex (%) | ||||
| Male | 3320 | 50.8 | 1866 | 53.8 |
| Female | 3211 | 49.2 | 1599 | 46.2 |
| Race (%) | ||||
| White | 4343 | 66.5 | 2280 | 65.8 |
| Black | 1231 | 18.8 | 629 | 18.2 |
| Hispanic | 388 | 5.9 | 195 | 5.6 |
| Others | 253 | 3.9 | 173 | 5.0 |
| Missing | 317 | 4.9 | 187 | 5.4 |
| Comorbidities (%) | ||||
| Obesity | 1947 | 29.8 | 1097 | 31.7 |
| Hypertension | 3953 | 60.5 | 1761 | 50.8 |
| Diabetes mellitus with chronic complications | 628 | 9.6 | 218 | 6.3 |
| Diabetes mellitus without chronic complications | 1073 | 16.4 | 538 | 15.5 |
| Congestive heart failure | 1387 | 21.2 | 1042 | 30.1 |
| Valvular heart disease | 446 | 6.8 | 420 | 12.1 |
| History of chronic pulmonary disease | 1249 | 19.1 | 526 | 15.2 |
| Peripheral vascular disease | 621 | 9.5 | 213 | 6.2 |
| Coagulopathy | 1131 | 17.3 | 1015 | 29.3 |
| Solid tumor without metastasis | 324 | 5.0 | 112 | 3.2 |
| Metastatic cancer | 352 | 5.4 | 100 | 2.9 |
| Liver disorders | 239 | 3.7 | 89 | 2.6 |
| Weight loss | 364 | 5.6 | 364 | 10.5 |
| Alcoholism | 239 | 3.7 | 148 | 4.3 |
| Other neurological disorders | 482 | 7.4 | 304 | 8.8 |
| Renal failure | 924 | 14.1 | 263 | 7.6 |
| Hypothyroidism | 727 | 11.1 | 341 | 9.8 |
| Anemia | 1624 | 24.9 | 601 | 17.4 |
| Fluid and electrolyte disorders | 2407 | 36.9 | 1804 | 52.1 |
| Depression | 703 | 10.8 | 365 | 10.5 |
| Type of PE (%) | ||||
| Non-saddle | 4531 | 69.4 | 2068 | 59.7 |
| Saddle | 1999 | 30.6 | 1397 | 40.3 |
| Median household income (%) | ||||
| 1st quartile | 1919 | 29.4 | 931 | 26.9 |
| 2nd quartile | 1607 | 24.6 | 831 | 24.0 |
| 3rd quartile | 1604 | 24.6 | 852 | 24.6 |
| 4th quartile | 1301 | 19.9 | 767 | 22.1 |
| Primary insurance (%) | ||||
| Medicare/Medicaid | 3856 | 59.0 | 1749 | 50.5 |
| Private including HMO | 2233 | 34.2 | 1384 | 39.9 |
| Uninsured/self-pay | 427 | 6.5 | 333 | 9.6 |
| Hospital bed size (%) | ||||
| Small | 695 | 10.6 | 198 | 5.7 |
| Medium | 1567 | 24.0 | 598 | 17.3 |
| Large | 4261 | 65.2 | 2664 | 76.9 |
| Hospital type (%) | ||||
| Rural | 277 | 4.2 | 59 | 1.7 |
| Urban nonteaching | 1461 | 22.4 | 637 | 18.4 |
| Teaching | 4784 | 73.3 | 2764 | 79.8 |
| Hospital region (%) | ||||
| Northeast | 981 | 15.0 | 792 | 22.9 |
| Midwest | 1616 | 24.7 | 756 | 21.8 |
| South | 2553 | 39.1 | 1477 | 42.6 |
| West | 1382 | 21.2 | 440 | 12.7 |
| Day of admission | ||||
| Weekday | 5089 | 77.9 | 2621 | 75.6 |
| Weekend | 1442 | 22.1 | 844 | 24.4 |
| Source of admission (%) | ||||
| Transfer from other hospital or other health facility | 1659 | 25.4 | 1508 | 43.5 |
| Emergency department | 4872 | 74.6 | 1956 | 56.5 |
| Type of admission (%) | ||||
| Emergent or urgent | 6090 | 93.8 | 3208 | 92.9 |
| Elective | 405 | 6.2 | 247 | 7.1 |
| Complications | Mechanical Thrombectomy | % | Surgical Thrombectomy | % |
|---|---|---|---|---|
| In-hospital mortality | 592 | 9.1 | 482 | 13.9 |
| Discharge to facility | 1365 | 20.9 | 1108 | 32.0 |
| Length of stay (days) | 7 | 0.3 | 13 | 0.4 |
| Cardiopulmonary bypass | 217 | 3.3 | 2365 | 68.3 |
| ECMO | 35 | 0.5 | 130 | 3.7 |
| Converted to surgical thrombectomy | 113 | 1.7 | - | - |
| Invasive mechanical ventilation | 902 | 13.8 | 1108 | 32.0 |
| Intracerebral hemorrhage | 59 | 0.9 | 30 | 0.9 |
| Cardiac arrest | 152 | 2.3 | 333 | 9.6 |
| Myocardial infarction | 710 | 10.9 | 117 | 3.4 |
| Bleeding complications | 93 | 1.4 | 118 | 3.4 |
| Independent Variable/Characteristic | Odd Ratio | 95% CI (LL) | 95% CI (UL) | p-Value |
|---|---|---|---|---|
| Age (10 years increase) | 1.2 | 1.0 | 1.3 | 0.026 |
| Sex | ||||
| Female vs. male | 1.9 | 1.2 | 2.8 | 0.004 |
| Race | ||||
| Non-white vs. white | 0.9 | 0.6 | 1.4 | 0.570 |
| Elixhauser comorbidity score (10-point increment) | 1.0 | 0.9 | 1.1 | 0.792 |
| Hospital bed size | ||||
| Large vs. small/medium | 2.2 | 1.4 | 3.5 | 0.001 |
| Hospital type | ||||
| Teaching vs. rural/urban nonteaching | 1.8 | 1.1 | 3.1 | 0.023 |
| Hospital region | ||||
| Northeast vs. west | 1.0 | 0.5 | 1.9 | 0.964 |
| Midwest vs. west | 0.9 | 0.5 | 1.6 | 0.676 |
| South vs. west | 0.8 | 0.4 | 1.3 | 0.335 |
| Day of admission | ||||
| Weekend vs. weekday | 0.9 | 0.6 | 1.5 | 0.784 |
| Source of admission (%) | ||||
| Transfer from other hospital or other health facility vs. emergency department | 1.2 | 0.7 | 1.9 | 0.483 |
| Type of admission (%) | ||||
| Elective vs. emergent or urgent | 0.4 | 0.1 | 1.5 | 0.193 |
| Independent Variable/Characteristic | Odd Ratio | 95% CI (LL) | 95% CI (UL) | p-Value |
|---|---|---|---|---|
| Age (10 years increase) | 1.2 | 1.0 | 1.4 | 0.046 |
| Sex | ||||
| Female vs. male | 1.2 | 0.8 | 1.9 | 0.343 |
| Race | ||||
| Non-white vs. white | 0.8 | 0.5 | 1.3 | 0.373 |
| Elixhauser comorbidity score (10-point increment) | 1.0 | 0.9 | 1.0 | 0.577 |
| Hospital bed size | ||||
| Large vs. small/medium | 0.8 | 0.5 | 1.2 | 0.259 |
| Hospital type | ||||
| Teaching vs. rural/urban nonteaching | 0.9 | 0.6 | 1.5 | 0.686 |
| Hospital region | ||||
| Northeast vs. west | 1.0 | 0.4 | 2.2 | 0.987 |
| Midwest vs. west | 0.9 | 0.4 | 2.1 | 0.888 |
| South vs. west | 1.0 | 0.5 | 2.2 | 0.926 |
| Day of admission | ||||
| Weekend vs. weekday | 0.7 | 0.4 | 1.2 | 0.168 |
| Source of admission (%) | ||||
| Transfer from other hospital or other health facility vs. emergency department | 0.6 | 0.4 | 1.1 | 0.086 |
| Type of admission (%) | ||||
| Elective vs. emergent or urgent | 0.7 | 0.3 | 1.8 | 0.453 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raghupathy, S.; Barigidad, A.P.; Doorgen, R.; Adak, S.; Malik, R.R.; Parulekar, G.; Patel, J.J.; Lanka, S.P.; Varghese, G.M.; Rashid, M.; et al. Prevalence, Trends, and Outcomes of Pulmonary Embolism Treated with Mechanical and Surgical Thrombectomy from a Nationwide Inpatient Sample. Clin. Pract. 2022, 12, 204-214. https://doi.org/10.3390/clinpract12020024
Raghupathy S, Barigidad AP, Doorgen R, Adak S, Malik RR, Parulekar G, Patel JJ, Lanka SP, Varghese GM, Rashid M, et al. Prevalence, Trends, and Outcomes of Pulmonary Embolism Treated with Mechanical and Surgical Thrombectomy from a Nationwide Inpatient Sample. Clinics and Practice. 2022; 12(2):204-214. https://doi.org/10.3390/clinpract12020024
Chicago/Turabian StyleRaghupathy, Shalini, Achala Prashant Barigidad, Raydiene Doorgen, Shrestha Adak, Rohma Rafique Malik, Gaurav Parulekar, Jeet Janak Patel, Santh Prakash Lanka, George Mohan Varghese, Mohammed Rashid, and et al. 2022. "Prevalence, Trends, and Outcomes of Pulmonary Embolism Treated with Mechanical and Surgical Thrombectomy from a Nationwide Inpatient Sample" Clinics and Practice 12, no. 2: 204-214. https://doi.org/10.3390/clinpract12020024
APA StyleRaghupathy, S., Barigidad, A. P., Doorgen, R., Adak, S., Malik, R. R., Parulekar, G., Patel, J. J., Lanka, S. P., Varghese, G. M., Rashid, M., Patel, U., Patel, A., & Hsieh, Y.-C. (2022). Prevalence, Trends, and Outcomes of Pulmonary Embolism Treated with Mechanical and Surgical Thrombectomy from a Nationwide Inpatient Sample. Clinics and Practice, 12(2), 204-214. https://doi.org/10.3390/clinpract12020024







