Role of Lactobacillus reuteri DSM 17938 on Crying Time Reduction in Infantile Colic and Its Impact on Maternal Depression: A Real-Life Clinic-Based Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
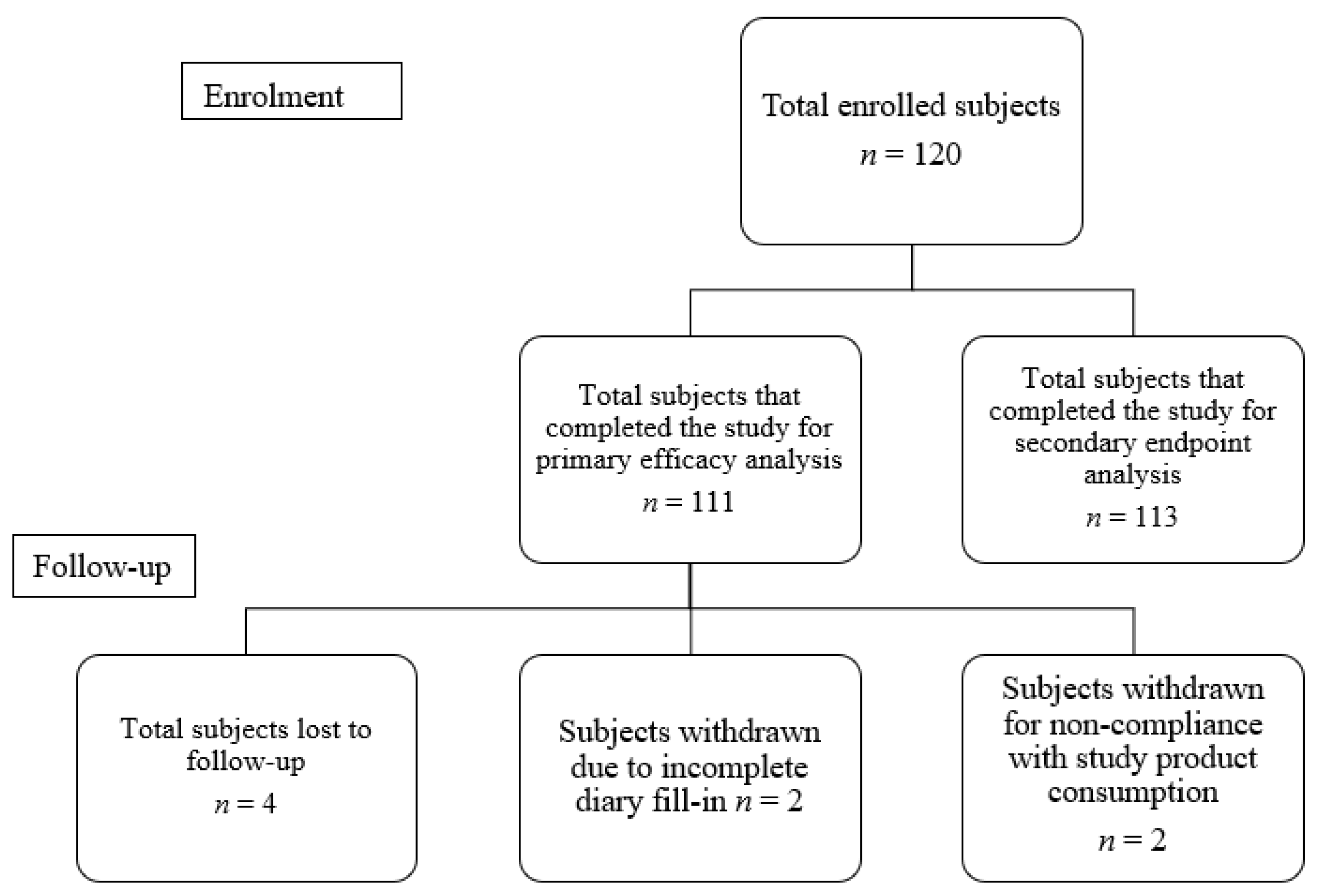
2.2. Study Participants
2.3. Study Outcomes and Assessments
2.4. Adverse Events
2.5. Statistical Analysis
Exploratory Statistical Analysis Method
3. Results
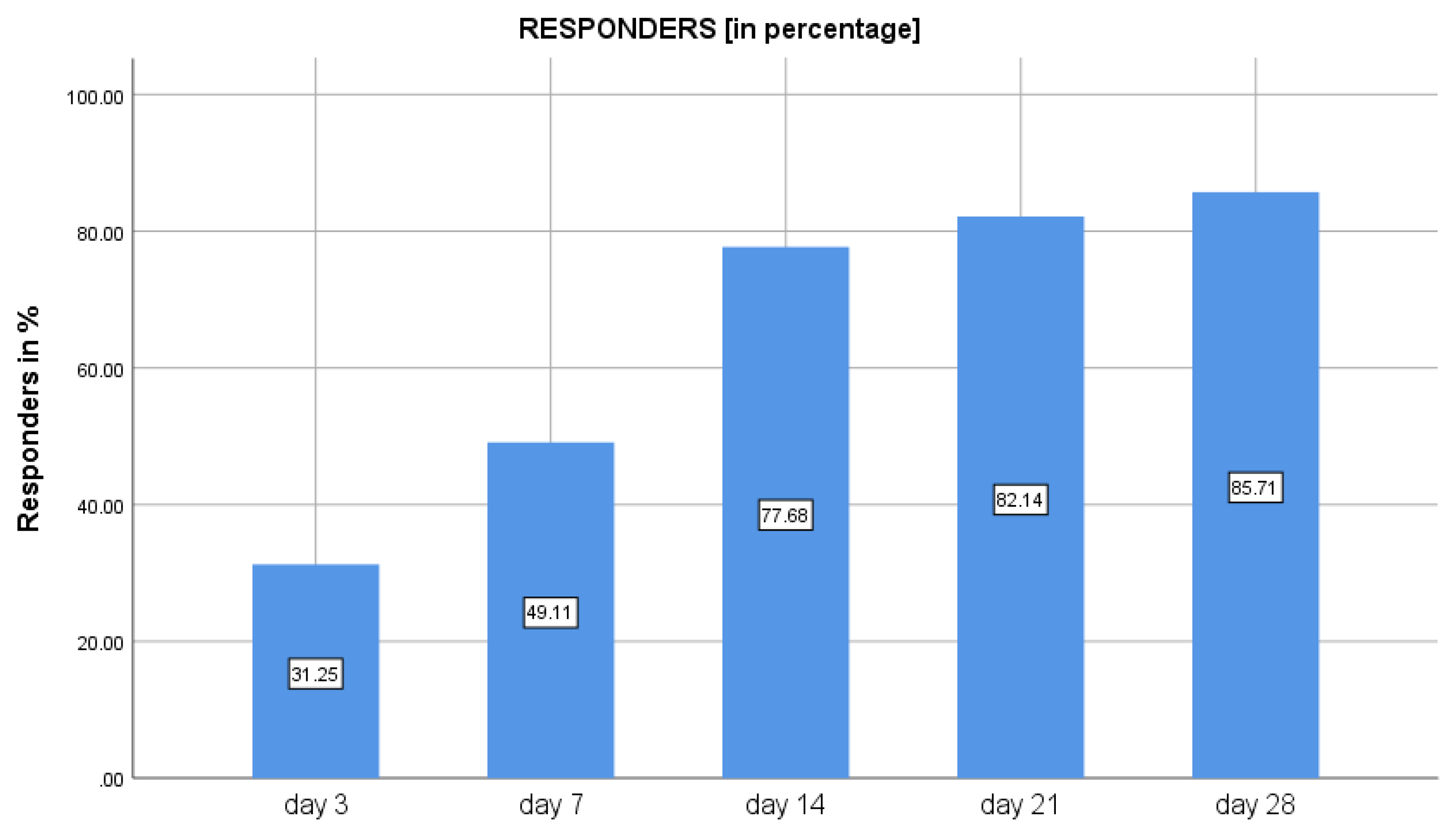
3.1. Primary Outcomes
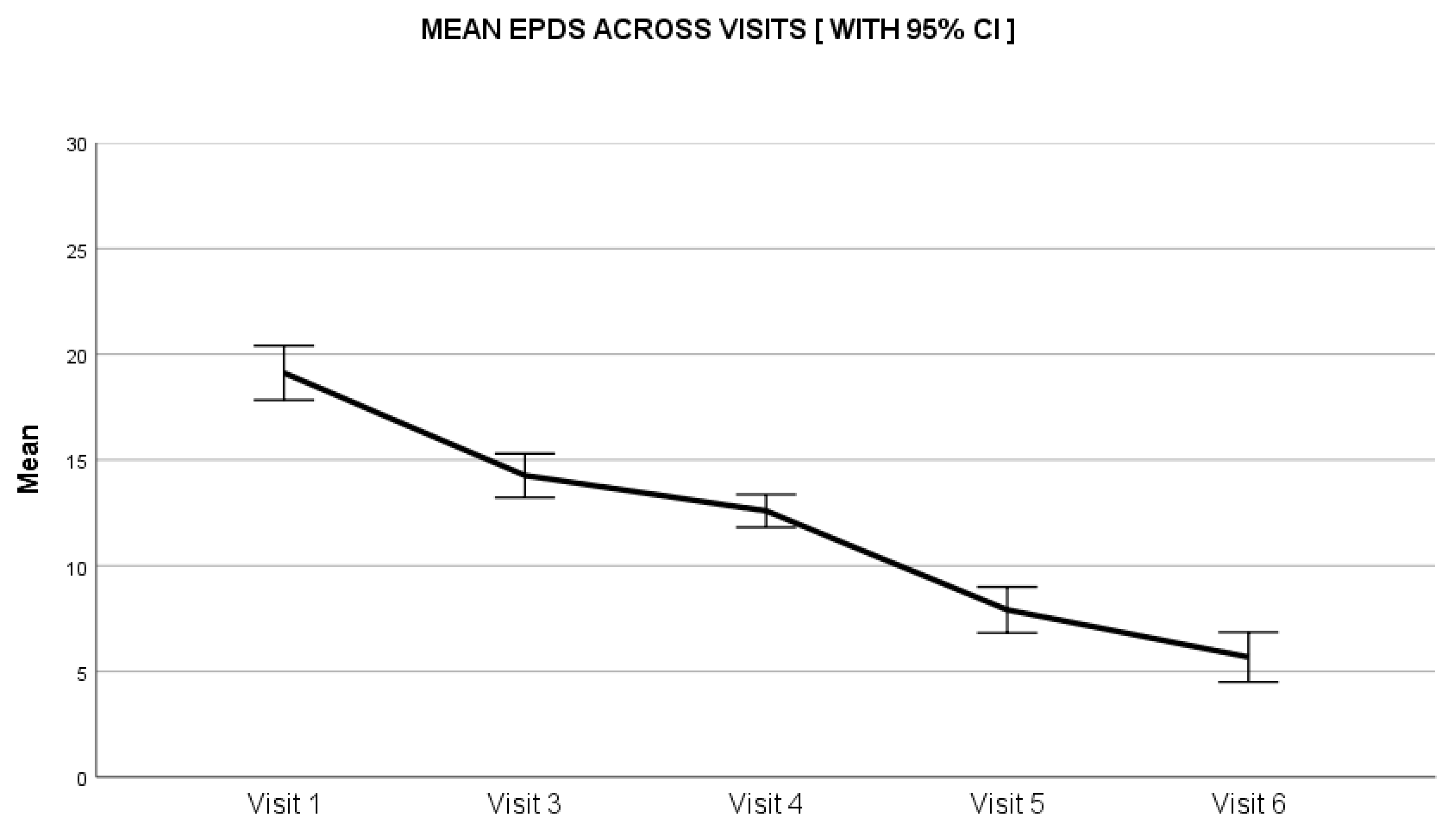
3.2. Secondary Outcomes
Results of Exploratory Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koppen, I.J.; Nurko, S.; Saps, M.; Di Lorenzo, C.; Benninga, M.A. The pediatric Rome IV criteria: What’s new? Expert Rev. Gastroenterol. Hepatol. 2017, 11, 193–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benninga, M.A.; Nurko, S.; Faure, C.; Hyman, P.E.; Roberts, I.S.J.; Schechter, N.L. Childhood functional gastrointestinal disorders: Neonate/toddler. Gastroenterology 2016, 150, 1443–1455.e2. [Google Scholar] [CrossRef]
- Savino, F.; Pelle, E.; Palumeri, E.; Oggero, R.; Miniero, R. Lactobacillus reuteri (American Type Culture Collection Strain 55730) versus simethicone in the treatment of infantile colic: A prospective randomized study. Pediatrics 2007, 119, e124–e130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szajewska, H.; Dryl, R. Probiotics for the management of infantile colic. J. Pediatr. Gastroenterol. Nutr. 2016, 63, S22–S24. [Google Scholar] [CrossRef]
- Savino, F.; Cordisco, L.; Tarasco, V.; Palumeri, E.; Calabrese, R.; Oggero, R.; Roos, S.; Matteuzzi, D. Lactobacillus reuteri DSM 17938 in infantile colic: A randomized, double-blind, placebo-controlled trial. Pediatrics 2010, 126, e526–e533. [Google Scholar] [CrossRef] [PubMed]
- Sung, V.; Hiscock, H.; Tang, M.; Mensah, F.; Nation, M.; Satzke, C.; Heine, R.G.; Stock, A.; Barr, R.G.; Wake, M. Treating infant colic with the probiotic Lactobacillus reuteri: Double blind, placebo controlled randomised trial. BMJ 2014, 348, g2107. [Google Scholar] [CrossRef] [Green Version]
- Sung, V.; D’Amico, F.; Cabana, M.D.; Chau, K.; Koren, G.; Savino, F.; Szajewska, H.; Deshpande, G.; Dupont, C.; Indrio, F.; et al. Lactobacillus reuteri to treat infant colic: A meta-analysis. Pediatrics 2018, 141, e20171811. [Google Scholar] [CrossRef] [Green Version]
- Sung, V. Infantile colic. Aust. Prescr. 2018, 41, 105–110. [Google Scholar] [CrossRef]
- Mi, G.L.; Zhao, L.; Qiao, D.D.; Kang, W.Q.; Tang, M.Q.; Xu, J.K. Effectiveness of Lactobacillus reuteri in infantile colic and colicky induced maternal depression: A prospective single blind randomized trial. Antonie Van Leeuwenhoek 2015, 107, 1547–1553. [Google Scholar] [CrossRef]
- Cox, J.L.; Chapman, G.; Murray, D.; Jones, P. Validation of the Edinburgh Postnatal Depression Scale (EPDS) in non-postnatal women. J. Affect. Disord. 1996, 39, 185–189. [Google Scholar] [CrossRef]
- Keefe, M.R.; Froese-Fretz, A.; Kotzer, A.M. Newborn predictors of infant irritability. J. Obstet. Gynecol. Neonatal Nurs. 1998, 27, 513–520. [Google Scholar] [CrossRef]
- McFarland, L.V. Use of probiotics to correct dysbiosis of normal microbiota following disease or disruptive events: A systematic review. BMJ Open 2014, 4, 005047. [Google Scholar] [CrossRef] [Green Version]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in Human Health and Diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef] [PubMed]
- Blum, S.; Haller, D.; Pfeifer, A.; Schiffrin, E. Probiotics and immune response. Clin. Rev. Allergy Immunol. 2002, 22, 287–309. [Google Scholar] [CrossRef] [PubMed]
- Pärtty, A.; Kalliomäki, M.; Salminen, S.; Isolauri, E. Infantile Colic Is Associated with Low-grade Systemic Inflammation. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Gyrczuk, E.; Horvath, A. Lactobacillus reuteri DSM 17938 for the management of infantile colic in breastfed infants: A randomized, double-blind, placebo-controlled trial. J. Pediatr. 2013, 162, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Indrio, F.; Riezzo, G.; Raimondi, F.; Bisceglia, M.; Filannino, A.; Cavallo, L.; Francavilla, R. Lactobacillus reuteri accelerates gastric emptying and improves regurgitation in infants. Eur. J. Clin. Investig. 2011, 41, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Zermiani, A.P.D.R.B.; de Paula, A.L.P.P.; Miguel, E.R.A.; Lopes, L.D.G.; Santana, N.D.C.S.; da Silva Santos, T.; Demarchi, I.G.; Teixeira, J.J. Evidence of Lactobacillus reuteri to reduce colic in breastfed babies: Systematic review and meta-analysis. Complement Ther. Med. 2021, 63, 102781. [Google Scholar] [CrossRef]
- Räihä, H.; Lehtonen, L.; Huhtala, V.; Saleva, K.; Korvenranta, H. Excessively crying infant in the family: Mother–infant, father–infant and mother–father interaction. Child Care Health Dev. 2002, 28, 419–429. [Google Scholar] [CrossRef]
- Wolke, D.; Bilgin, A.; Samara, M. Systematic Review and Meta-Analysis: Fussing and Crying Durations and Prevalence of Colic in Infants. J. Pediatr. 2017, 185, 55–61.e4. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, S.; Mane, R.; Shaikh, I.; Kadhe, G. A cross sectional survey in paediatricians pertaining to management of functional gastrointestinal disorders in infants: Indian perspectives. Int. J. Contemp. Pediatr. 2020, 7, 2015–2020. [Google Scholar] [CrossRef]

| Days | Crying Time Reduction in Mins | Percentage Reduction | 95% Confidence Interval |
|---|---|---|---|
| −1 to 3 | −76.6 (113.7) | −25.2 (43.8) | 0.9, 1.0 |
| −1 to 7 | −108.9 (108.4) | −41.2 (44.0) | 1.4, 2.1 |
| −1 to 14 | −157.3 (109.8) | −60.8 (42.5) | 2.2, 2.9 |
| −1 to 21 | −185.8 (116.0) | −72.4 (47.2) | 2.7, 3.4 |
| −1 to 28 | −202.5 (122.3) | −78.9 (40.3) | 2.9, 3.7 |
| 21 to 28 | −16.6 (45.6) | −37.2 (48.9) | 0.1, 0.4 |
| S. No. | Visit | Mean in Min (Standard Deviation) |
|---|---|---|
| Average crying/fussiness per day | ||
| 1 | 1 | 270.9 (97.0) |
| 2 | 3 | 175.4 (87.0) |
| 3 | 4 | 139.9 (96.2) |
| 4 | 5 | 102.0 (88.4) |
| 5 | 6 | 63.0 (63.8) |
| Visit-wise amount of fussiness | ||
| 1 | 1 | 4.5 (1.0) |
| 2 | 3 | 3.4 (1.2) |
| 3 | 4 | 2.8 (1.4) |
| 4 | 5 | 2.1 (1.0) |
| 5 | 6 | 1.4 (1.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wadhwa, A.; Kesavelu, D.; Kumar, K.; Chatterjee, P.; Jog, P.; Gopalan, S.; Paul, R.; Veligandla, K.C.; Mehta, S.; Mane, A.; et al. Role of Lactobacillus reuteri DSM 17938 on Crying Time Reduction in Infantile Colic and Its Impact on Maternal Depression: A Real-Life Clinic-Based Study. Clin. Pract. 2022, 12, 37-45. https://doi.org/10.3390/clinpract12010005
Wadhwa A, Kesavelu D, Kumar K, Chatterjee P, Jog P, Gopalan S, Paul R, Veligandla KC, Mehta S, Mane A, et al. Role of Lactobacillus reuteri DSM 17938 on Crying Time Reduction in Infantile Colic and Its Impact on Maternal Depression: A Real-Life Clinic-Based Study. Clinics and Practice. 2022; 12(1):37-45. https://doi.org/10.3390/clinpract12010005
Chicago/Turabian StyleWadhwa, Arun, Dhanasekhar Kesavelu, Kishore Kumar, Pallab Chatterjee, Pramod Jog, Sarath Gopalan, Rudra Paul, Krishna Chaitanya Veligandla, Suyog Mehta, Amey Mane, and et al. 2022. "Role of Lactobacillus reuteri DSM 17938 on Crying Time Reduction in Infantile Colic and Its Impact on Maternal Depression: A Real-Life Clinic-Based Study" Clinics and Practice 12, no. 1: 37-45. https://doi.org/10.3390/clinpract12010005
APA StyleWadhwa, A., Kesavelu, D., Kumar, K., Chatterjee, P., Jog, P., Gopalan, S., Paul, R., Veligandla, K. C., Mehta, S., Mane, A., Pandit, S., Rathod, R., Jayan, S., & Mitra, M. (2022). Role of Lactobacillus reuteri DSM 17938 on Crying Time Reduction in Infantile Colic and Its Impact on Maternal Depression: A Real-Life Clinic-Based Study. Clinics and Practice, 12(1), 37-45. https://doi.org/10.3390/clinpract12010005






