Smoking Cessation after a Cancer Diagnosis: A Cross-Sectional Analysis in the Setting of a Developing Country
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Demographic Data
3.2. Smoking Cessation and Associated Factors
3.3. Number of Cigarettes Smoked before and after Cancer Diagnosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Includes the Original Questionnaire Translated to English
| The goal of this questionnaire is to evaluate habits regarding smoking, and exploring whether cancer diagnosis changes smoking habits. The research will be performed based on modern bioethical standards with respect on respecting your privacy and protecting the secrecy of your medical data. Your name and surname is only used to evaluate your initial disease status and WILL NEVER BE PUBLISHED. Your privacy is paramount to us, and when we publish the results, all the data WILL BE ANONYMOUS. The research was approved by the Ethical Committee of Clinical Hospital Center. |
- Did you regularly smoke cigarettes at ANY period before cancer diagnosis?
- 2.
- How long did you smoke in total?
- 3.
- Did you smoke cigarettes at the time of your cancer diagnosis?
- 4.
- How did cancer diagnosis change your smoking habits?
- 5.
- If you only REDUCED the number of cigarettes or DID NOT CHANGE smoking habits after cancer diagnosis, what is the reason that you did not quit completely? (more answers are accepted)
- (A)
- It is hard to stop
- (B)
- I do not believe smoking cessation has any effect on cancer treatment
- (C)
- I do not believe smoking cessation has any effect on prognosis
- (D)
- Other _____________________________________________________
- 6.
- How many cigarettes a day do you smoke NOW?
- 7.
- How many cigarettes a day did you smoke BEFORE cancer diagnosis?
References
- American Cancer Society. Cancer Facts and Figures; American Cancer Society: Atlanta, GA, USA, 2010; Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2010.html (accessed on 21 November 2018).
- Peppone, L.J.; Mustian, K.M.; Morrow, G.R.; Dozier, A.M.; Ossip, D.J.; Janelsins, M.C.; Sprod, L.K.; McIntosh, S. The Effect of Cigarette Smoking on Cancer Treatment–Related Side Effects. Oncologist 2011, 16, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Videtic, G.M.; Stitt, L.W.; Dar, A.R.; Kocha, W.I.; Tomiak, A.T.; Truong, P.T.; Vincent, M.D.; Yu, E.W. Continued Cigarette Smoking by Patients Receiving Concurrent Chemoradiotherapy for Limited-Stage Small-Cell Lung Cancer Is Associated with Decreased Survival. J. Clin. Oncol. 2003, 21, 1544–1549. [Google Scholar] [CrossRef] [PubMed]
- Parsons, A.; Daley, A.; Begh, R.; Aveyard, P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: Systematic review of observational studies with meta-analysis. BMJ 2010, 340, b5569. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, M.; Healy, P.; Daignault, S.; Ramnath, N. Cigarette Smoking and Gemcitabine-Induced Neutropenia in Advanced Solid Tumors. Oncologist 2013, 85, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.; Louzon, S.A.; Gritz, E.R. Why do cancer patients smoke and what can providers do about it? Community Oncol. 2012, 9, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.-S.; Lin, H.-Y.; Moody-Thomas, S.; Martin, M.; Chen, T. Who tended to continue smoking after cancer diagnosis: The national health and nutrition examination survey 1999–2008. BMC Public Health 2012, 12, 784. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, M.; Rowland, J.H.; Yancik, R. Cancer Survivors in the United States: Age, Health, and Disability. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2003, 58, M82–M91. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, N.A.; Smith, T.; Zhao, L.; Rodriguez, J.; Berkowitz, Z.; Stein, K.D. Health-related behavior change after cancer: Results of the American Cancer Society’s studies of cancer survivors (SCS). J. Cancer Surv. 2010, 4, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Demark-Wahnefried, W.; Peterson, B.; McBride, C.; Lipkus, I.; Clipp, E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer 2000, 88, 674–684. [Google Scholar] [CrossRef]
- Bellizzi, K.M.; Rowland, J.H.; Jeffery, D.D.; McNeel, T. Health Behaviors of Cancer Survivors: Examining Opportunities for Cancer Control Intervention. J. Clin. Oncol. 2005, 23, 8884–8893. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, C.M.; Cokkinides, V.; Courneya, K.S.; Nehl, E.J.; Stein, K.; Baker, F. A Comparison of Physical Activity of Posttreatment Breast Cancer Survivors and Noncancer Controls. Behav. Med. 2003, 28, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Coups, E.J.; Ostroff, J. A population-based estimate of the prevalence of behavioral risk factors among adult cancer survivors and noncancer controls. Prev. Med. 2005, 40, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Pinto, F.R.; Matos, L.L.; Gumz Segundo, W.; Vanni, C.M.; Rosa, D.S.; Kanda, J.L. Tobacco and alcohol use after head and neck cancer treatment: Influence of the type of oncological treatment employed. Rev. Assoc. Med. Bras. 2011, 57, 171–176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eakin, E.G.; Youlden, D.; Baade, P.; Lawler, S.; Reeves, M.M.; Heyworth, J.; Fritschi, L. Health behaviors of cancer survivors: Data from an Australian population-based survey. Cancer Causes Control. 2007, 18, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Croatian Institute of Public Health. A Survey about the Tobacco Use in Adult Population in the Republic of Croatia. 2015. Available online: https://www.hzjz.hr/wp-content/uploads/2016/02/Duhan_2015.pdf. (accessed on 21 November 2018).
- Asma, S.; Mackay, J.; Song, S.Y.; Zhao, L.; Morton, J.; Palipudi, K.M. The GATS Atlas; CDC Foundation: Atlanta, GA, USA, 2015; Available online: http://gatsatlas.org. (accessed on 21 November 2018).
- Persson, M.; Simonsson, M.; Markkula, A.; Rose, C.; Ingvar, C.; Jernström, H. Impacts of smoking on endocrine treatment response in a prospective breast cancer cohort. Br. J. Cancer 2016, 115, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, M.N.; Newcomb, P.A.; Hampton, J.M.; Trentham-Dietz, A.; Titus, L.J.; Egan, K.M.; Baron, J.A.; Willett, W.C. Cigarette Smoking Before and After Breast Cancer Diagnosis: Mortality From Breast Cancer and Smoking-Related Diseases. J. Clin. Oncol. 2016, 34, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Braithwaite, D.; Izano, M.; Moore, D.H.; Kwan, M.L.; Tammemagi, M.; Hiatt, R.A.; Kerlikowske, K.; Kroenke, C.H.; Sweeney, C.; Habel, L.; et al. Smoking and survival after breast cancer diagnosis: A prospective observational study and systematic review. Breast Cancer Res. Treat. 2012, 136, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Emmons, K.M.; Puleo, E.; Park, E.; Gritz, E.R.; Butterfield, R.M.; Weeks, J.C.; Mertens, A.; Li, F.P. Peer-Delivered Smoking Counseling for Childhood Cancer Survivors Increases Rate of Cessation: The Partnership for Health Study. J. Clin. Oncol. 2005, 23, 6516–6523. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.; Dalili, M.; Semwal, M.; Civljak, M.; Sheikh, A.; Car, J. Internet-based interventions for smoking cessation. Cochrane Database Syst. Rev. 2017, 9, CD007078. [Google Scholar] [CrossRef]
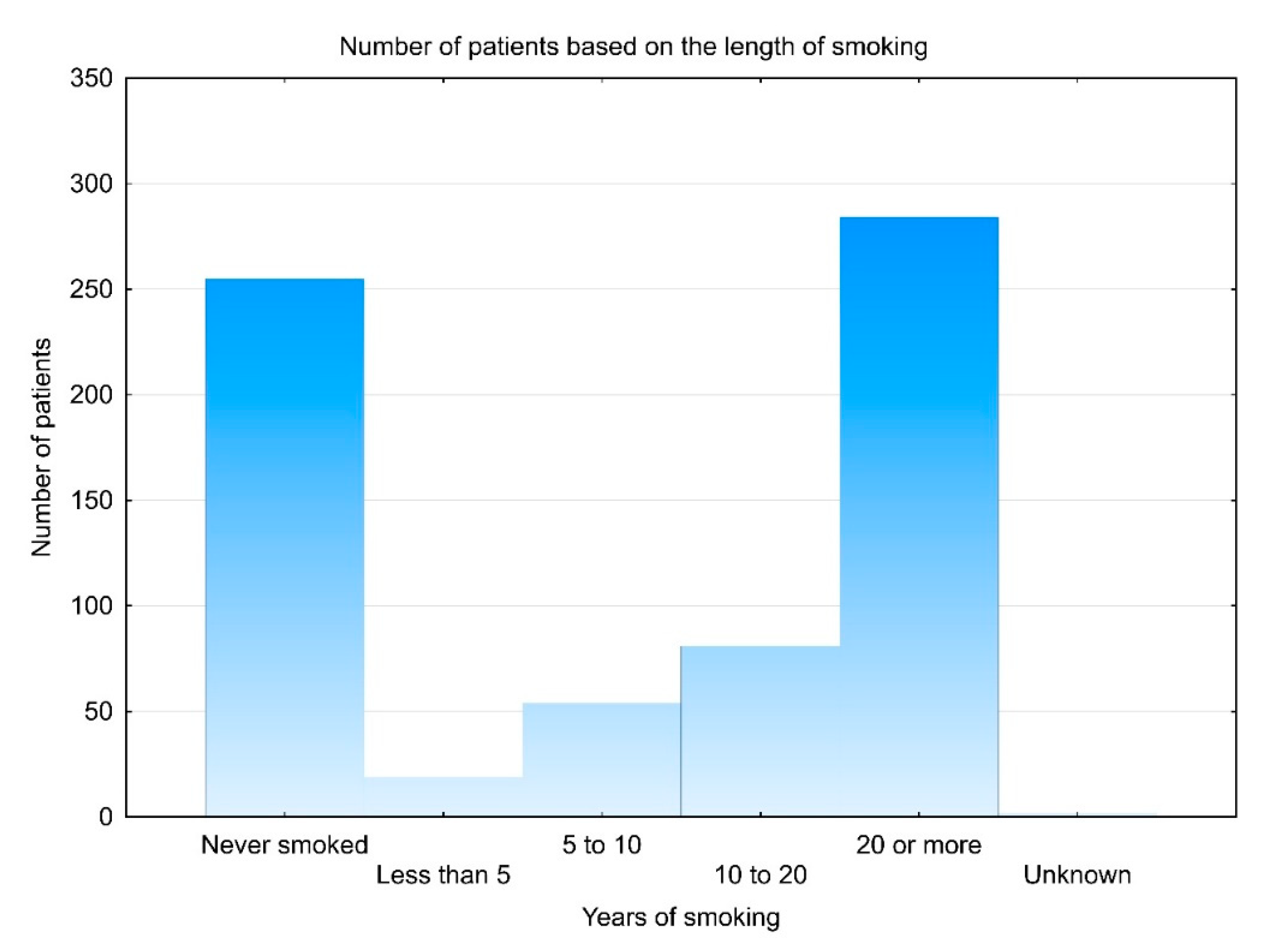
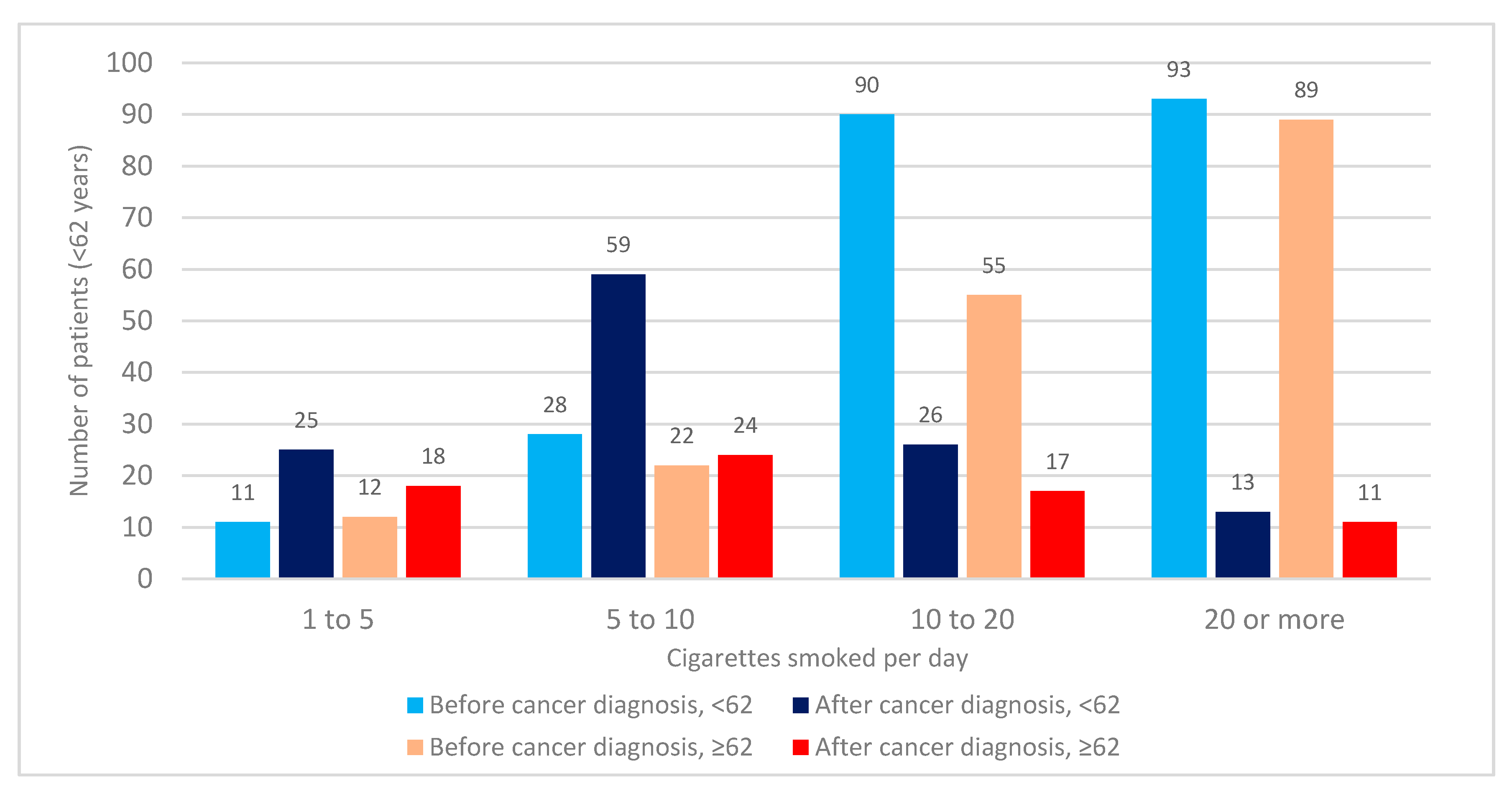
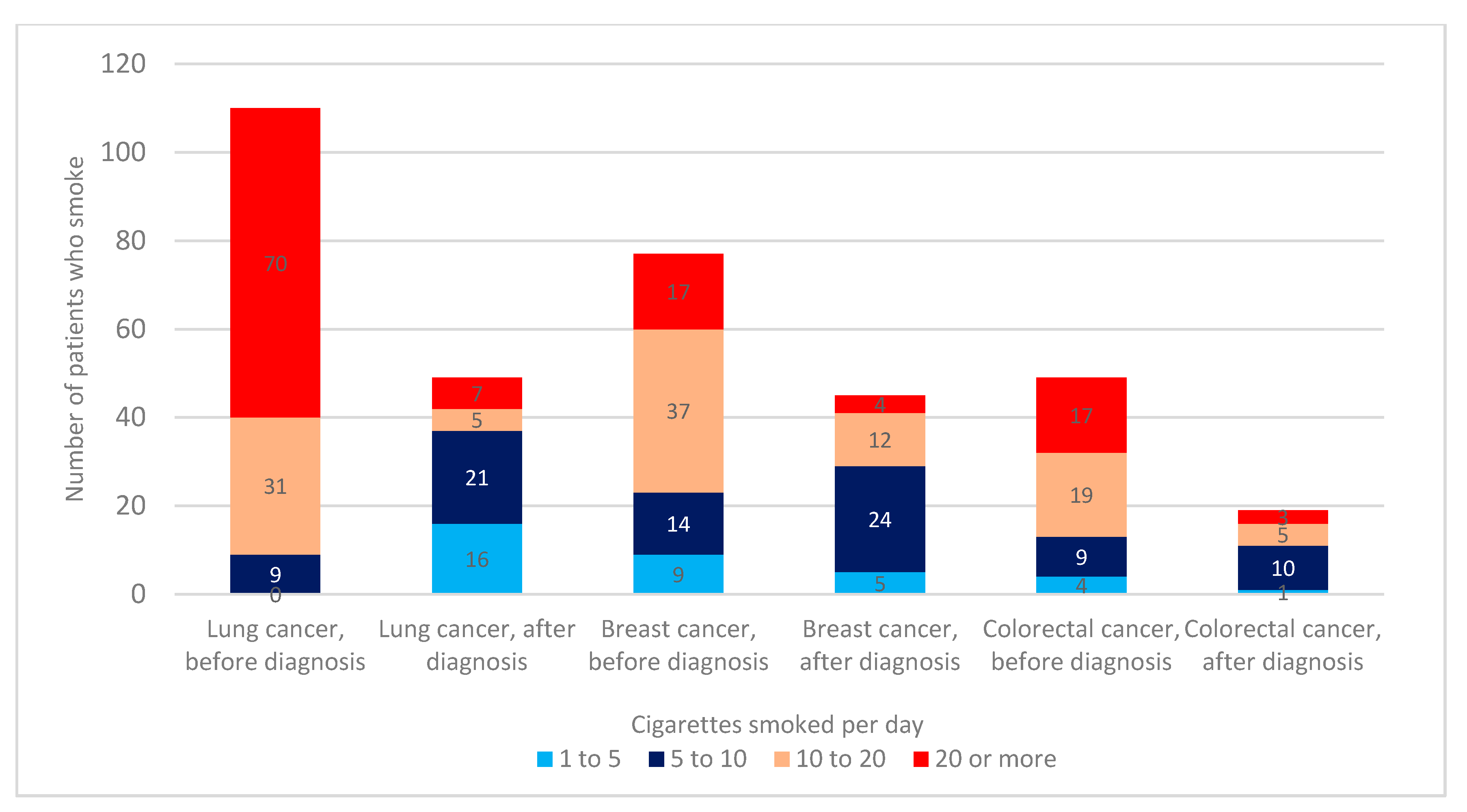
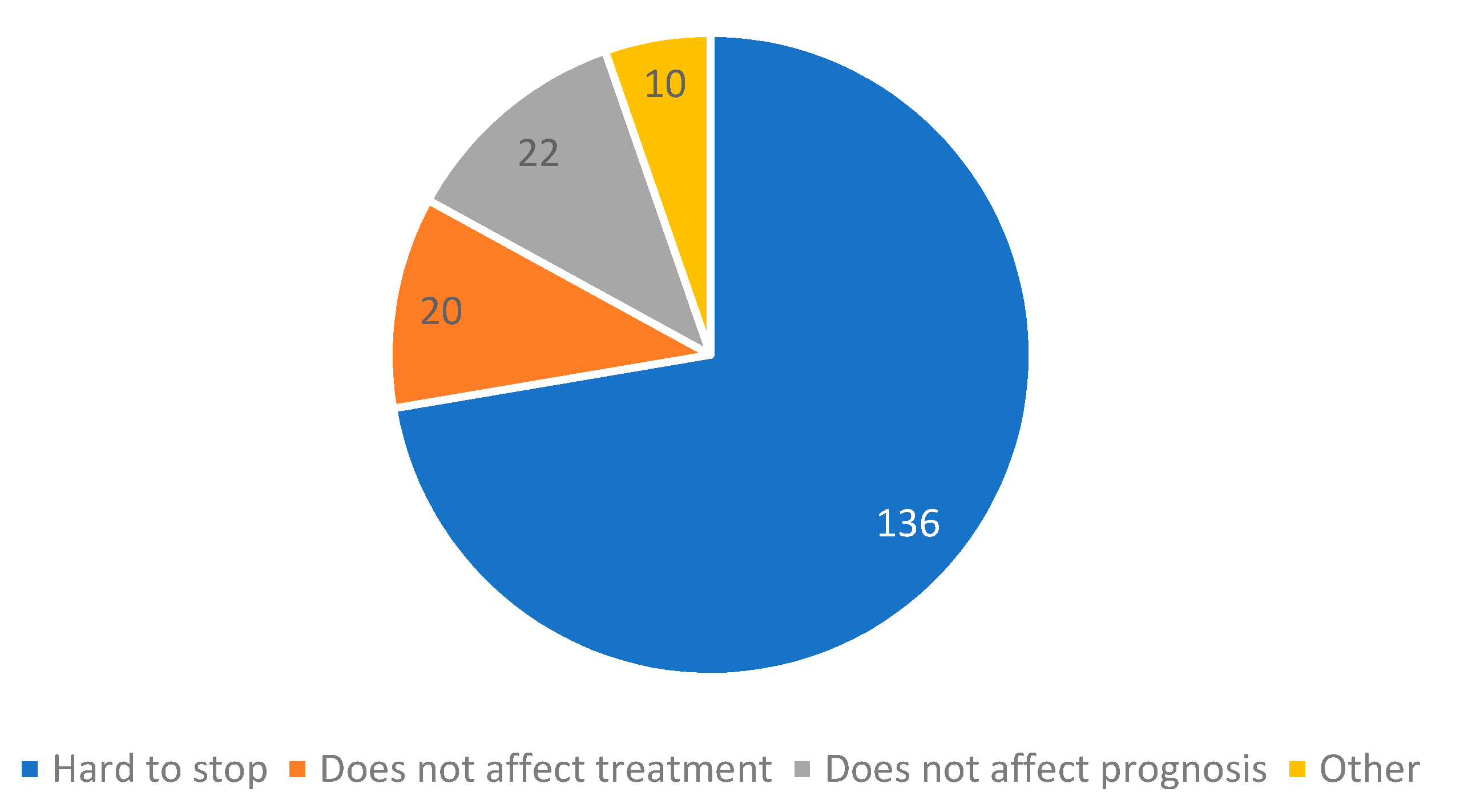
| Patient Characteristic | All Patients | Smoked at Any Period | % |
|---|---|---|---|
| N | 695 | 441 | 63 |
| The highest level of education | |||
| Elementary school | 125 | 79 | 63 |
| High school | 392 | 258 | 66 |
| College or higher | 109 | 56 | 51 |
| Gender | |||
| Male | 335 | 251 | 75 |
| Female | 359 | 189 | 53 |
| Age group (based on median age) | |||
| <62 | 322 | 232 | 72 |
| ≥62 and higher | 364 | 203 | 56 |
| Primary cancer | |||
| Lung | 127 | 117 | 92 |
| Breast | 168 | 82 | 49 |
| Colorectal | 103 | 60 | 58 |
| Prostate | 48 | 25 | 52 |
| Ovarian | 36 | 15 | 42 |
| Non-ovarian gynecological | 32 | 18 | 56 |
| Hepatobiliary | 25 | 15 | 60 |
| Upper gastrointestinal | 22 | 15 | 68 |
| Head and neck | 21 | 21 | 100 |
| NET & GIST | 19 | 11 | 58 |
| Mesothelioma | 18 | 9 | 50 |
| Brain | 16 | 10 | 63 |
| Kidney and bladder | 15 | 9 | 60 |
| Non-specified | 14 | 14 | 100 |
| Testicular | 11 | 9 | 82 |
| Melanoma | 7 | 3 | 43 |
| Sarcoma | 6 | 4 | 67 |
| Unknown origin | 5 | 3 | 60 |
| Mediastinal | 2 | 1 | 50 |
| Metastatic status | |||
| No metastases | 377 | 224 | 59 |
| Metastatic | 304 | 203 | 67 |
| Relation of cancer to smoking | |||
| Less smoking related | 500 | 276 | 55 |
| Smoking-related 2 | 195 | 165 | 85 |
| Patient Characteristic | Smoked at the Time of Canc. Dg. | % | Smokes During Treatment | % | Difference in Proportions (p) |
|---|---|---|---|---|---|
| Total number | 230 | 52 | 194 | 44 | |
| Highest level of education | |||||
| Elementary school | 44 | 56 | 41 | 52 | 0.453 |
| High school | 139 | 54 | 110 | 43 | <0.001 |
| College or higher | 23 | 41 | 18 | 32 | 0.125 |
| Chi-square | χ2 = 3.451 p = 0.178 | χ2 = 5.259 p = 0.072 | |||
| Gender | |||||
| Males | 130 | 52 | 100 | 40 | <0.001 |
| Females | 100 | 53 | 94 | 50 | 0.286 |
| Chi-square | χ2 = 0.054 p = 0.816 | χ2 = 4.282 p = 0.039 | |||
| Age group (based on median age) | |||||
| <62 | 147 | 63 | 123 | 53 | 0.001 |
| ≥62 and higher | 80 | 39 | 70 | 34 | 0.064 |
| Chi-square | χ2 = 24.489 p < 0.001 | χ2 = 12.09 p < 0.001 | |||
| Primary cancer site | |||||
| Lung | 65 | 56 | 50 | 43 | 0.001 |
| Breast | 47 | 57 | 45 | 55 | 0.754 |
| Colon and rectum | 21 | 35 | 19 | 32 | 0.754 |
| Chi-square | χ2 = 8.418 p = 0.015 | χ2 = 7.717 p = 0.021 | |||
| Metastatic status | |||||
| No metastases | 101 | 45 | 94 | 42 | 0.167 |
| Metastatic | 117 | 58 | 91 | 45 | <0.001 |
| Chi-square | χ2 = 6.708 p = 0.010 | χ2 = 0.356 p = 0.551 | |||
| Relation of cancer to smoking | |||||
| Less smoking related | 131 | 47 | 119 | 43 | 0.045 |
| Smoking-related | 99 | 60 | 75 | 46 | <0.001 |
| Chi-square | χ2 = 6.504 p = 0.011 | χ2 = 0.356 p = 0.551 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golčić, M.; Tomaš, I.; Stevanović, A.; Golčić, G.; Dobrila-Dintinjana, R.; Erić, S.; Šambić-Penc, M.; Baretić Marinac, M.; Gović-Golčić, L.; Majnarić, T. Smoking Cessation after a Cancer Diagnosis: A Cross-Sectional Analysis in the Setting of a Developing Country. Clin. Pract. 2021, 11, 509-519. https://doi.org/10.3390/clinpract11030067
Golčić M, Tomaš I, Stevanović A, Golčić G, Dobrila-Dintinjana R, Erić S, Šambić-Penc M, Baretić Marinac M, Gović-Golčić L, Majnarić T. Smoking Cessation after a Cancer Diagnosis: A Cross-Sectional Analysis in the Setting of a Developing Country. Clinics and Practice. 2021; 11(3):509-519. https://doi.org/10.3390/clinpract11030067
Chicago/Turabian StyleGolčić, Marin, Ilijan Tomaš, Aleksandra Stevanović, Goran Golčić, Renata Dobrila-Dintinjana, Suzana Erić, Mirela Šambić-Penc, Martina Baretić Marinac, Lidija Gović-Golčić, and Tea Majnarić. 2021. "Smoking Cessation after a Cancer Diagnosis: A Cross-Sectional Analysis in the Setting of a Developing Country" Clinics and Practice 11, no. 3: 509-519. https://doi.org/10.3390/clinpract11030067
APA StyleGolčić, M., Tomaš, I., Stevanović, A., Golčić, G., Dobrila-Dintinjana, R., Erić, S., Šambić-Penc, M., Baretić Marinac, M., Gović-Golčić, L., & Majnarić, T. (2021). Smoking Cessation after a Cancer Diagnosis: A Cross-Sectional Analysis in the Setting of a Developing Country. Clinics and Practice, 11(3), 509-519. https://doi.org/10.3390/clinpract11030067






