An Exploration of Heart Failure Risk in Breast Cancer Patients Receiving Anthracyclines with or without Trastuzumab in Thailand: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Patients and Treatments
2.3. Outcomes
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Incidence of HF after Breast Cancer Treatments
3.3. Factor Associated with HF on Univariable and Multivariable Analysis
4. Discussion
4.1. Incidence of HF after Initiating Chemotherapy
4.2. Factors Associated with HF in Breast Cancer Patients
4.3. Strengths and Limitations
4.4. Study Implications and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Health Statistics 2018: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- World Health Organization. World Health Organization Cancer Fact. All Cancer Fact Sheet; International Agency for Research on Cancer: Lyon, France, 2019.
- Cardoso, F.; Costa, A.; Senkus, E.; Aapro, M.; Andre, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.; Biganzoli, L.; Cardoso, M.J.; et al. 3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3). Ann. Oncol. 2017, 28, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Telli, M.L.; Gradishar, W.J.; Ward, J.H. NCCN guidelines updates: Breast cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 552–555. [Google Scholar]
- Peto, R.; Davies, C.; Godwin, J. Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012, 379, 432–444. [Google Scholar]
- Sledge, G.W.; Neuberg, D.; Bernardo, P.; Ingle, J.N.; Martino, S.; Rowinsky, E.K.; Wood, W.C. Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: An intergroup trial (E1193). J. Clin. Oncol. 2003, 21, 588–592. [Google Scholar] [CrossRef]
- Cameron, D.; Piccart-Gebhart, M.J.; Gelber, R.D.; Procter, M.; Goldhirsch, A.; de Azambuja, E.; Castro, G.; Untch, M.; Smith, I.; Gianni, L.; et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: Final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 2017, 389, 1195–1205. [Google Scholar] [CrossRef] [Green Version]
- Perez, E.A.; Romond, E.H.; Suman, V.J.; Jeong, J.; Sledge, G.; Geyer, C.E., Jr.; Martino, S.; Rastogi, P.; Gralow, J.; Swain, S.M.; et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: Planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J. Clin. Oncol. 2014, 32, 3744–3752. [Google Scholar] [CrossRef]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef] [Green Version]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Armenian, S.H.; Lacchetti, C.; Barac, A.; Carver, J.; Constine, L.S.; Denduluri, N.; Dent, S.; Douglas, P.S.; Durand, J.-B.; Ewer, M.; et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2017, 35, 893–911. [Google Scholar] [CrossRef]
- Tan, T.C.; Neilan, T.G.; Francis, S.; Plana, J.C.; Scherrer-Crosbie, M. Anthracycline-Induced Cardiomyopathy in Adults. Compr. Physiol. 2015, 5, 1517–1540. [Google Scholar] [PubMed]
- Telli, M.L.; Hunt, S.A.; Carlson, R.W.; Guardino, A.E. Trastuzumab-related cardiotoxicity: Calling into question the concept of reversibility. J. Clin. Oncol. 2007, 25, 3525–3533. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef] [Green Version]
- Bowles, E.J.A.; Wellman, R.; Feigelson, H.S.; Onitilo, A.A.; Freedman, A.N.; Delate, T.; Allen, L.A.; Nekhlyudov, L.; Goddard, K.A.B.; Davis, R.L.; et al. Risk of Heart Failure in Breast Cancer Patients After Anthracycline and Trastuzumab Treatment: A Retrospective Cohort Study. J. Natl. Cancer Inst. 2012, 104, 1293–1305. [Google Scholar] [CrossRef]
- de Azambuja, E.; Procter, M.J.; van Veldhuisen, D.J.; Agbor-Tarh, D.; Metzger-Filho, O.; Steinseifer, J.; Untch, M.; Smith, I.E.; Gianni, L.; Baselga, J.; et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in the Herceptin Adjuvant trial (BIG 1–01). J. Clin. Oncol. 2014, 32, 2159–2165. [Google Scholar] [CrossRef]
- Perez, E.A.; Suman, V.J.; Davidson, N.E.; Gralow, J.R.; Kaufman, P.A.; Visscher, D.W.; Chen, B.; Ingle, J.N.; Dakhil, S.R.; Zujewski, J.; et al. Sequential Versus Concurrent Trastuzumab in Adjuvant Chemotherapy for Breast Cancer. J. Clin. Oncol. 2011, 29, 4491–4497. [Google Scholar] [CrossRef] [PubMed]
- Banke, A.; Fosbøl, E.; Ewertz, M.; Videbæk, L.; Dahl, J.; Poulsen, M.K.; Cold, S.; Jensen, M.-B.; Gislason, G.; Schou, M.; et al. Long-Term Risk of Heart Failure in Breast Cancer Patients After Adjuvant Chemotherapy with or Without Trastuzumab. JACC Heart Fail. 2019, 7, 217–224. [Google Scholar] [CrossRef]
- Chien, H.C.; Yang, K.Y.H.; Bai, J.P. Trastuzumab-related cardiotoxic effects in Taiwanese women: A nationwide cohort study. JAMA Oncol. 2016, 2, 317–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Cancer Institute. Common Terminology Criteria for Adverse Events v5.0 (CTCAE); National Institutes of Health: Baltimore, ML, USA, 2018.
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart failure society of america. J. Am. Coll. Cardiol. 2017, 70, 776–803. [Google Scholar]
- Vittinghoff, E.; Glidden, D.V.; Shiboski, S.C.; McCulloch, C.E. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models; Springer Science & Business Media: New York, NY, USA, 2005. [Google Scholar]
- Prachuabaree, L.; Ket-aim, S. A retrospective study of cardiotoxicity of breast cancer patients treated with doxorubicin at Phrachomklao Hospital. Isan J. Pharm. Sci. 2021, 17, 68–79. [Google Scholar]
- Advani, P.P.; Ballman, K.V.; Dockter, T.J.; Colon-Otero, G.; Perez, E.A. Long-Term Cardiac Safety Analysis of NCCTG N9831 (Alliance) Adjuvant Trastuzumab Trial. J. Clin. Oncol. 2016, 34, 581–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romond, E.H.; Jeong, J.; Rastogi, P.; Swain, S.; Greyer, C.E., Jr.; Ewer, M.S.; Rathi, V.; Fehrenbacher, L.; Brufsky, A.; Azar, C.A.; et al. Seven-Year Follow-Up Assessment of Cardiac Function in NSABP B-31, a Randomized Trial Comparing Doxorubicin and Cyclophosphamide Followed by Paclitaxel (ACP) With ACP Plus Trastuzumab as Adjuvant Therapy for Patients with Node-Positive, Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer. J. Clin. Oncol. 2012, 30, 3792–3799. [Google Scholar]
- Boerman, L.M.; Berendsen, A.J.; Van Der Meer, P.; Maduro, J.H.; Berger, M.Y.; De Bock, G.H.; De Bock, G. Long-term follow-up for cardiovascular disease after chemotherapy and/or radiotherapy for breast cancer in an unselected population. Support. Care Cancer 2014, 22, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Meinardi, M.T.; van Veldhuisen, D.J.; Gietema, J.A.; Dolsma, W.V.; Boomsma, F.; van den Berg, M.P.; Volkers, C.; Haaksma, J.; de Vries, E.G.; Sleijfer, D.T.; et al. Prospective evaluation of early cardiac damage induced by epirubicin-containing adjuvant chemotherapy and locoregional radiotherapy in breast cancer patients. J. Clin. Oncol. 2001, 19, 2746–2753. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Hu, W.; Kirova, Y.; Yang, Z.; Cai, G.; Yu, X.; Ma, J.; Guo, X.; Shao, Z.; Chen, J. Potential impact of cardiac dose–volume on acute cardiac toxicity following concurrent trastuzumab and radiotherapy. Cancer Radiothérapie 2014, 18, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2014, 27, 911–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kittiwarawut, A.; Vorasettakarnkij, Y.; Tanasanvimon, S.; Manasnayakorn, S.; Sriuranpong, V. Serum NT-proBNP in the early detection of doxorubicin-induced cardiac dysfunction. Asia Pac. J. Clin. Oncol. 2013, 9, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Mehta, L.S.; Watson, K.E.; Barac, A.; Beckie, T.M.; Bittner, V.; Cruz-Flores, S.; Dent, S.; Kondapalli, L.; Ky, B.; Okwuosa, T.; et al. Cardiovascular Disease and Breast Cancer: Where These Entities Intersect: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e30–e66. [Google Scholar] [CrossRef]
| Characteristic | Total (n = 475) | HF Event (n = 15) | Non-HF Event (n = 460) | p-Value |
|---|---|---|---|---|
| Gender (female), n (%) | 473 (99.6) | 15 (100.0) | 458 (99.6) | 1.000 |
| Age at diagnosis (years), mean ± SD (95% CI) | 52.4 ± 10.7 (51.4–53.3) | 56.7 ± 11.2 (50.5–62.8) | 52.3 ± 10.8 (51.3–52.3) | 0.115 |
| Age (years), n (%) | ||||
| <65 | 411 (86.5) | 11 (73.3) | 400 (87.0) | 0.130 |
| ≥65 | 64 (13.5) | 4 (26.7) | 60 (13.0) | |
| BMI (kg/m2), mean ± SD (95% CI) | 24.5 ± 4.1 (24.1–24.8) | 25.8 ± 3.8 (23.7–27.9) | 24.4 ± 4.1 (24.1–24.8) | 0.190 |
| Disease stage, n (%) | ||||
| I | 43 (9.0) | 2 (13.3) | 41 (8.9) | 0.379 |
| II | 225 (47.4) | 6 (40.0) | 219 (47.6) | |
| III | 160 (33.7) | 4 (26.7) | 156 (33.9) | |
| IV | 47 (9.9) | 3 (20.0) | 44 (9.6) | |
| Postmenopausal status, n (%) | 305 (64.2) | 10 (66.7) | 295 (64.1) | 1.000 |
| Hormone receptor status, n (%) | ||||
| ER-positive | 301 (63.4) | 8 (55.3) | 293 (63.7) | 0.425 |
| PR-positive | 231 (48.6) | 6 (40.0) | 225 (48.9) | 0.603 |
| HER2-positive, n (%) | 177 (37.3) | 11 (73.3) | 166 (39.1) | 0.005 |
| LVEF before chemotherapy (%), mean ± SD (95% CI) | 68.6 ± 6.5 (68.0–69.1) | 65.3 ± 6.1 (61.8–68.7) | 68.7 ± 6.5 (68.1–69.3) | 0.046 |
| LVEDD before chemotherapy (mm), mean ± SD (95% CI) | 44.0 ± 4.7 (43.5–44.4) | 48.7 ± 4.9 (45.5–51.8) | 43.8 ± 4.6 (43.3–44.3) | 0.003 |
| LA size before chemotherapy (mm), mean ± SD (95% CI) | 31.1 ± 5.3 (30.1–31.7) | 36.8 ± 4.6 (33.8–39.9) | 30.9 ± 5.2 (30.4–31.5) | 0.002 |
| Cardiovascular-related comorbidities, n (%) a | 107 (22.5) | 5 (33.3) | 102 (22.2) | 0.345 |
| Medication for pre-existing cardiovascular comorbidities, n (%) b | 89 (18.7) | 4 (26.7) | 85 (18.5) | 0.497 |
| Radiation therapy, n (%) | 260 (54.7) | 12 (80.0) | 248 (53.9) | 0.063 |
| Chemotherapy, n (%) | 0.177 | |||
| Curative-intent c | 428 (90.1) | 12 (80.0) | 416 (90.4) | |
| Palliative-intent | 47 (9.9) | 3 (20.0) | 44 (9.6) | |
| Hormone therapy use, n (%) d | 288 (60.6) | 7 (46.7) | 281 (61.1) | 0.290 |
| Cumulative anthracycline dose (mg/m2), mean ± SD (95% CI) | 247.5 ± 39.7 (243.8–251.1) | 240.2 ± 37.8 (219.1–261.0) | 247.7 ± 39.8 (244.0–251.4) | 0.466 |
| Trastuzumab use, n (%) | 90 (19.0) | 9 (60) | 81 (17.6) | <0.001 |
| Incidence of Heart Failure | Overall (n = 475) | Anthracycline Alone (n = 385) | With Trastuzumab (n = 90) | p-Value |
|---|---|---|---|---|
| Clinical HF a | 15 (3.2) | 6 (1.6) | 9 (10.0) | <0.001 |
| Curative-intent treatment b | 12 | 3 | 9 | |
| Palliative-intent treatment | 3 | 3 | 0 | |
| Incidence rate (per 1000 person-years) | 11.1 | 5.3 | 39.7 | <0.001 |
| Median (IQR) time between treatment initiation and HF (days) | 392 (238–681) | 613 (140–1478) | 380 (273–437) | 0.020 |
| Clinical characteristics of HF | ||||
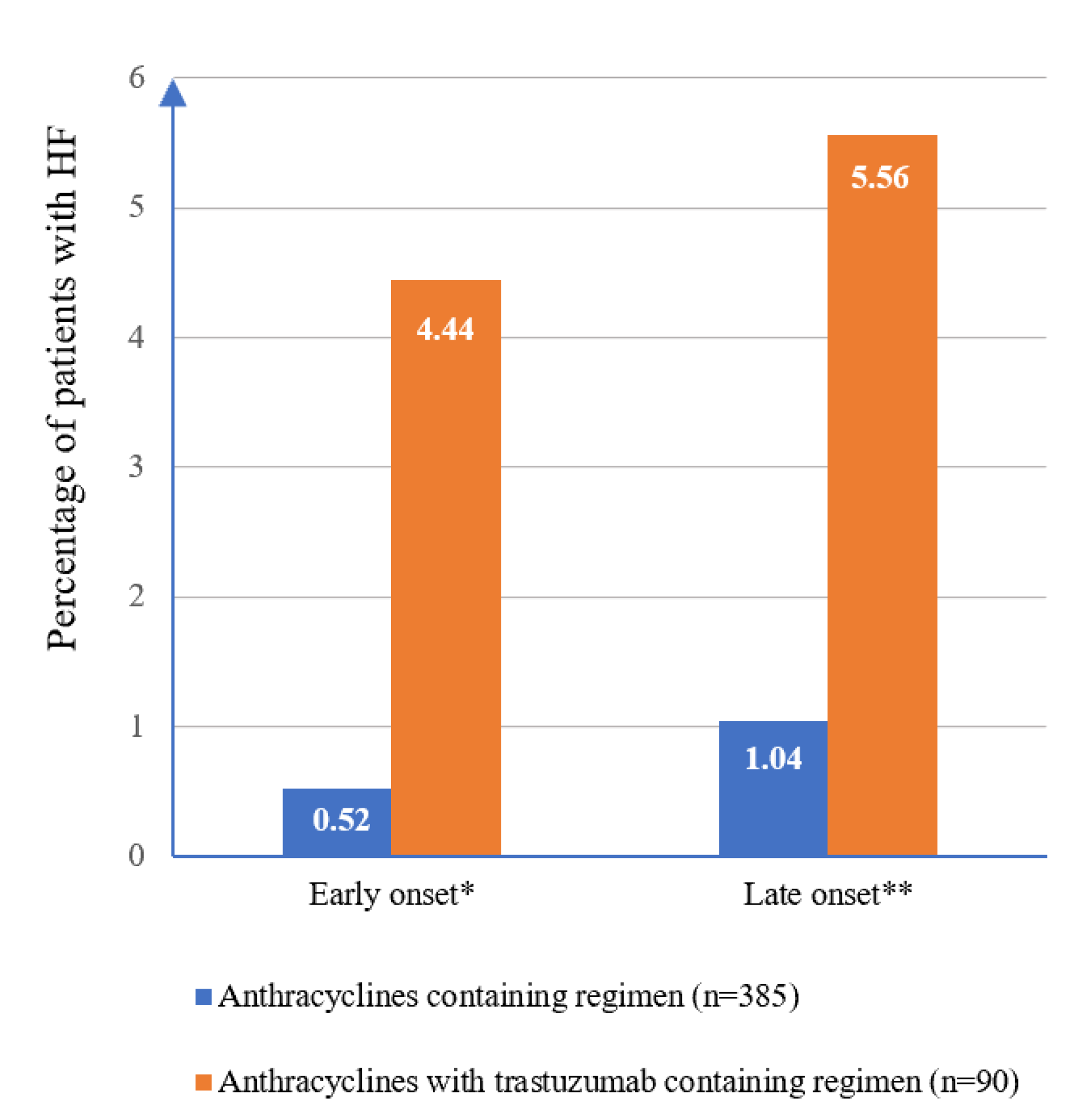
| Significant decrease in LVEF c and symptomatic HF d | 8 (1.7) | 3 (0.8) | 5 (5.6) | 0.622 |
| Symptomatic HF only d | 7 (1.5) | 3 (0.8) | 4 (4.4) | |
| Risk Factor | Univariable Analysis | Multivariable Analysis * | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-Value | HR | 95%CI | p-Value | |
| Age | ||||||
| <65 years | 1.00 | 1.00 | ||||
| ≥65 years | 2.68 | 0.85–8.43 | 0.093 | 3.55 | 0.95–13.21 | 0.059 |
| Baseline LVEF | ||||||
| ≥65% | 1.00 | 1.00 | ||||
| <65% | 3.48 | 1.26–9.59 | 0.016 | 3.89 | 1.36–11.10 | 0.011 |
| Cardiovascular comorbidities a | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 2.03 | 0.69–5.97 | 0.198 | 1.33 | 0.39–4.58 | 0.65 |
| Radiotherapy | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 3.11 | 0.88–11.03 | 0.079 | 5.03 | 1.26–22.46 | 0.034 |
| Treatment in palliative setting | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 3.62 | 0.99–13.18 | 0.051 | 7.06 | 1.53–32.23 | 0.012 |
| Cumulative doxorubicin | ||||||
| <250 mg/m2 | 1.00 | 1.00 | ||||
| ≥250 mg/m2 | 0.61 | 0.19–1.93 | 0.401 | 1.21 | 0.33–4.50 | 0.772 |
| Trastuzumab use | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 7.36 | 2.62–20.69 | <0.001 | 5.46 | 1.67–17.83 | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoodee, J.; Sookprasert, A.; Sanguanboonyaphong, P.; Chanthawong, S.; Seateaw, M.; Subongkot, S. An Exploration of Heart Failure Risk in Breast Cancer Patients Receiving Anthracyclines with or without Trastuzumab in Thailand: A Retrospective Study. Clin. Pract. 2021, 11, 484-493. https://doi.org/10.3390/clinpract11030064
Yoodee J, Sookprasert A, Sanguanboonyaphong P, Chanthawong S, Seateaw M, Subongkot S. An Exploration of Heart Failure Risk in Breast Cancer Patients Receiving Anthracyclines with or without Trastuzumab in Thailand: A Retrospective Study. Clinics and Practice. 2021; 11(3):484-493. https://doi.org/10.3390/clinpract11030064
Chicago/Turabian StyleYoodee, Jukapun, Aumkhae Sookprasert, Phitjira Sanguanboonyaphong, Suthan Chanthawong, Manit Seateaw, and Suphat Subongkot. 2021. "An Exploration of Heart Failure Risk in Breast Cancer Patients Receiving Anthracyclines with or without Trastuzumab in Thailand: A Retrospective Study" Clinics and Practice 11, no. 3: 484-493. https://doi.org/10.3390/clinpract11030064
APA StyleYoodee, J., Sookprasert, A., Sanguanboonyaphong, P., Chanthawong, S., Seateaw, M., & Subongkot, S. (2021). An Exploration of Heart Failure Risk in Breast Cancer Patients Receiving Anthracyclines with or without Trastuzumab in Thailand: A Retrospective Study. Clinics and Practice, 11(3), 484-493. https://doi.org/10.3390/clinpract11030064






