Introduction
Polycyclic aromatic hydrocarbons (PAHs) are major group of contaminants for sediment monitoring and are included in most of the priority pollutants lists of environmental regulatory agencies. PAHs are naturally produced by incomplete combustion and pyrolysis of organic matter and are largely present in fossil fuel. Their analysis in biological tissues can be useful for monitoring of anthropogenic activities related with combustion and heavy industries, and for the characterization of fossil fuel such as petroleum hydrocarbons spill [1]. PAHs are highly hydrophobic compounds and are preferably monitored in biota matrix or sediment than water samples (US-EPA [2], EU Directive 2008/105/EC [3]) since they are readily bioavailable towards biota.
Mussels have been extensively used as sentinel species, especially for marine waters since they are filter feeding organisms that bioaccumulate pollutant mainly by the ingestion of particulate matter [4,5,6,7,8]. The interaction between pollution and climatic changes program (IPOC), proposed to use mussels as bioindicator species for freshwater and marine water quality. The species selected are dressenids (Dreissena bugensis and Dreissena polymorpha) and blue mussels (Mytilus edulis) for freshwaters and marine waters respectively.
Instrumental analysis by mass spectrometry allows low-level (pg/g) quantification of PAHs in different matrices such as tissues but lipids and other matrix interferences must be removed to assure quality of results. Acid digestion usually used to remove the lipids cause decomposition of PAHs and saponification may cause degradation of deuterated internal standards. Lipids can also be removed by silica or alumina columns requiring large amount of solvent and efficiency is variable. Traditional size-exclusion with biobeads is proposed by the US EPA 3640A for such purpose, but it’s however time-consuming and also requires large amount of organic solvent [9]. Nowadays, commercial gel permeation columns (GPC) are coupled with conventional HPLC systems offer good alternative to easily remove lipids, with lower solvent consumption and overnight automation. This paper presents a method to remove lipids and macromolecules efficiently from mussels and salmon samples by GPC for PAHs analysis.
Materials and Methods
Material and standards
Methylene chloride and isooctane are pesticide grade. A mix of forty-nine native PAHs, 13C- labeled and deuterated internal standards (Accustandard). The GPC column and pre-column are the Phenogel 5u 100A 21.2x300 mm and 21.2x50 mm respectively (Phenomenex, USA). The HPLC system used is an HP1100 with a collection fraction Agilent 1260. The GC- HRMS system used is a Waters system with an Agilent GC computed with Masslynx 4.1. Reference standard solution (no. 32042) for GPC performance contains corn oil (250 mg/mL), bis(2-ethylhexyl)phthalate (5 mg/mL), methoxychlor (1 mg/mL), perylene (0.2 mg/mL) and sulfur (0.8 mg/mL) (Restek). The solution was diluted 50:1 in methylene chloride.
Stability of the gel permeation columns system with a performance standard
The stability of the GPC system for chromatography separation and the injection of large volume (1 mL) were evaluated with the commercial GPC performance standard by several injections (n=6). The reference standard solution used is the same as proposed in the EPA method 3640A for GPC system. The standard was kept in the freezer (T=-18°C) until the instrumental analysis. One ml is injected in the GPC system with a flow of 5 mL/min of methylene chloride.
Determination of the collected fractions for the recuperation of poly- cyclic aromatic hydrocarbons
Injection of the 49 highly concentrated native PAHs targeted was done in the GPC system and monitored through UV absorbance at 254 nm. The estimated fraction collection was then evaluated with 100 ng/mL of native 49 PAHs spiked and the 13C-labeled and deuterated internal standard. The eluate was then concentrated to 0.5 mL in isooctane and injected on column in a GC-HRMS instrument equipped with a 30 m DB35-MS column.
Removal efficiency of the lipids by the gel permeation columns system for collected fractions
The removal efficiency of lipids for fish and mussel samples was evaluated by injection of extracts in the GPC system and the recording of the UV absorbance at 254 nm. Collection of the fractions was performed to selectively separate lipids from PAHs. A gravimetric analysis was performed for both fractions by concentration of the solvent to dryness under nitrogen stream.
Results and Discussion
Stability of the gel permeation columns system for a reference standard
The stability of the GPC system was evaluated for chromatography separation and the injection of large volume (1 mL) with the reference standard solution for GPC performance. The chromatogram obtained shows 5 major compounds as the expected chromatograms by the US EPA 3640A but with less solvent consumption and faster chromatography. The compounds are corn oil (11.56±0.01 min), bis(2-ethylhexyl)phthalate (13.44±0.01 min), methoxychlor (14.46±0.01 min), perylene (18.88±0.03 min) and sulfur (21.12±0.02 min).The results show that for all the compounds the repeatability was elevated with less than 0.2% of RSD for the retention time and less than 2% RSD for the calculated area that corresponds to the injected volume. The elevated % RSD for the calculated area is due to a little tailing of for the corn oil, the perylene and the sulphurpresent in the chromatogram.
Recovery and accuracy for polycyclic aromatic hydrocarbons in the targeted fraction
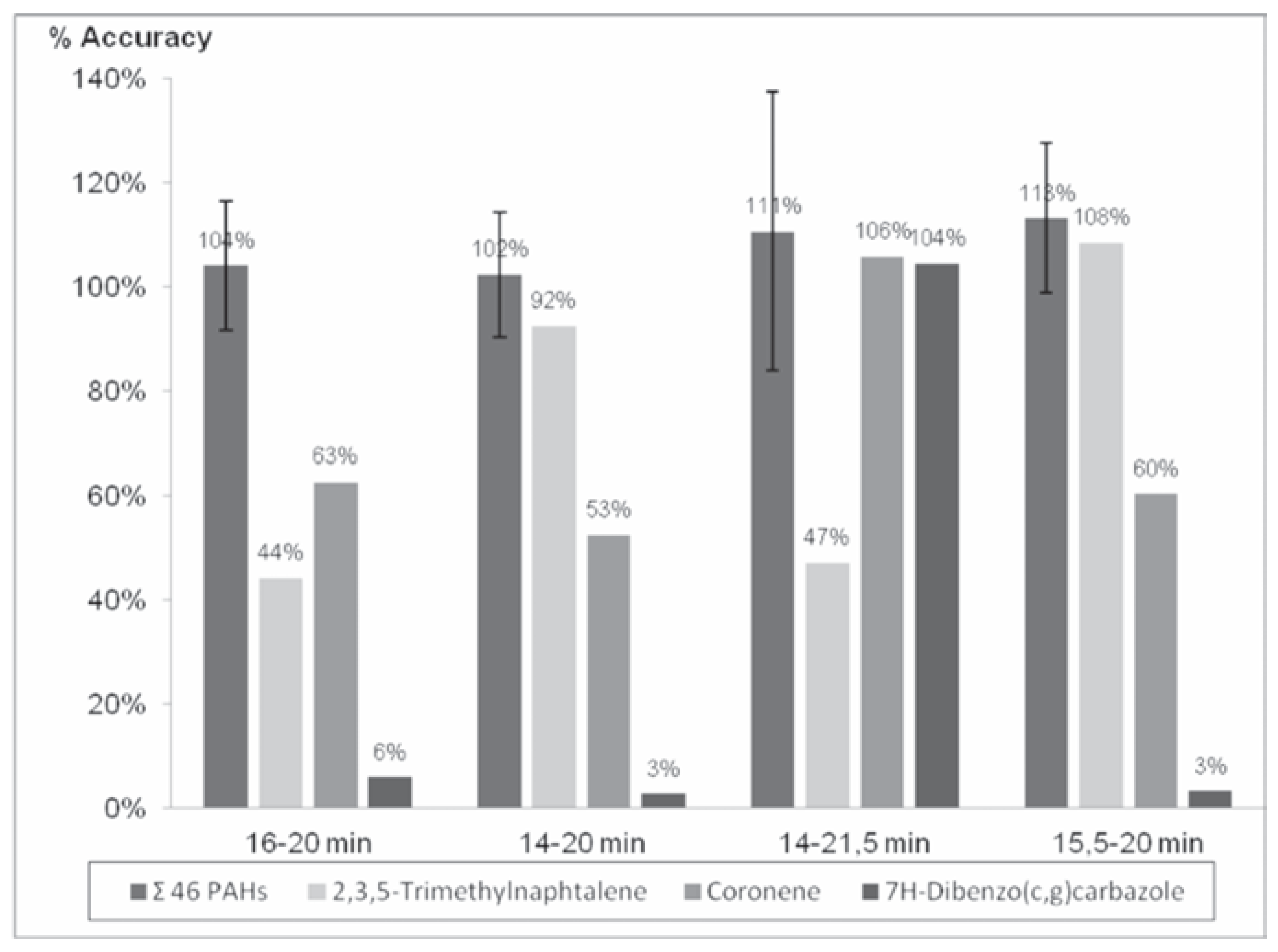
The injection of a 1.4 mL of a 1.25 µg/mL solution of PAHs in the GPC system and the reading at 254 nm shows an important peak corresponding to the PAHs centered at 17.329 min that starts at 15.5 min and finish at 20 min.
Four different fractions (16-20 min, 14-20 min, 14-21.5 min and 15.5-20 min) were collected and confirmed by GC-HRMS analysis to ensure an adequate recovery of all the PAHs targeted in the proposed method (Table 1).

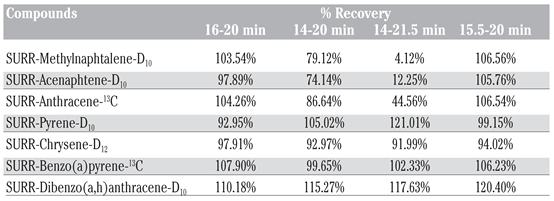
Table 1.
Recovery percentage of the 13C-labeled and deuterated internal standards for the four collections zones targeted for the polycyclic aromatic hydrocarbons.
The recovery of the deuterated and 13C- labeled standards was acceptable except for the 14-21.5 min was accidently evaporated to dryness, causing the loss of the volatile compounds (SURR-Methylnaphtalene-D10, SURR- Acenaphtene-D10, SURR-Anthracene-13C).
There is no significant difference between the recovery of the 16-20 min and 15.5-20 min for the 13C labeled and deuterated internal standards (Table 1).
Although the 14-21.5 min fraction was accidentally evaporated to dryness that causes loss of the volatiles PAHs compounds, the surrogates correct the accuracy adequately for the less volatile compounds (Figure 1). Results are better with the 15.5-20 min than the 16-20 min for 2,3,5-Trimethylnaphtalene (108% and 44% respectively). The fraction collection should start at 15.5 min to ensure a correct recovery of this compound. The fraction collection of the 21.5 min test shows a better recovery for 7H-Dibenzo(c,g)carbazole and Coronene (104% and 106% respectively) than the test until 20 min (around 3% and 60% respectively). For these reasons, the targeted fraction collection that would ensure adequate recovery of all the PAHs should be by 15.5 to 21.5 min.
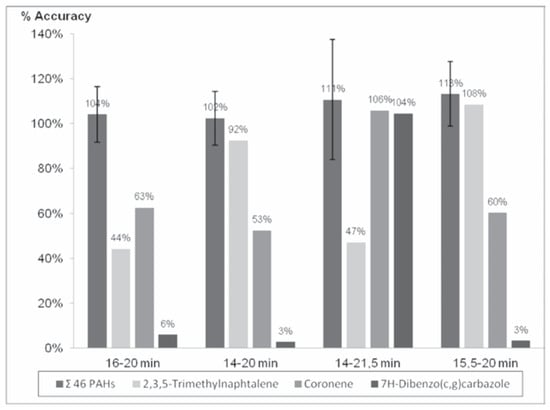
Figure 1.
Accuracy percentage of the targeted polycyclic aromatic hydrocarbons (PAHs) for the four fractions.
Removal efficiency of the lipids by the gel permeation columns system for the collected fractions
Gravimetric analysis of the residual lipids for the PAHs newly determinated collected zone allows an efficient confirmation of the removal almost complete of the lipids (91±1% for the mussels extracts and 97±1% for the salmon extracts). Less than 10 mg of lipids are still present for mussels (≈1.5% of lipid) after the GPC purification and less than 15 mg (7-8% of lipid). Theses residual lipids are small and near of the error of the measurements for lipids. For that reasons, this method allows almost complete removal of lipids in biological tissues such as fish and mussels.
Conclusions
This method allows efficient removal of lipids in biological samples and high recuperation of the targeted 49 PAHs with a significant repeatability. The procedure can easily be automated for overnight injection saving considerable time. Other test will be performed to propose an integrated method for the complete extraction and purification of different com- plex biological matrices. PCBs and PBDEs have also shown preliminary interesting results and further development will be performed to analyze all persistent organic pollutants and PAHs simultaneously.
Acknowledgments
this study is part of a larger collaborative program funded by a Strategic NSERC program, with the support of the Centre d’expertise en analyse environnementale du Québec (CEAEQ) for chemical analysis. The authors would like to address special thanks to the member of the Division des contaminants industriels organiques of the CEAEQ for their technical assistance.
References
- Poster, D.L.; Schantz, M.M.; Sander, L.C.; Wise, S.A. Analysis of polycyclic aromatic hydrocarbons (PAHs) in environmental samples: a critical review of gas chromatography (GC) methods. Analyt. Bioanalyt. Chem. 2006, 386, 859–881. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Appendix A to 40 CFR, Part 423—126 Priority Pollutants. 2013. [Google Scholar]
- Besse, J.P.; Geffard, O.; Coquery, M. Relevance and applicability of active biomonitoring in continental waters under the Water Framework Directive. TrAC-Trends Anal. Chem. 2012, 36, 113–127. [Google Scholar] [CrossRef]
- Binelli, A.; Ricciardi, F.; Riva, C.; Provini, A. Integrated use of biomarkers and bioaccumulation data in Zebra mussel (Dreissena polymorpha) for site-specific quality assessment. Biomarkers 2006, 11, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.D.; Slone, E. Long-term monitoring using resident and caged mussels in Boston Harbor yield similar spatial and temporal trends in chemical contamination. Marine Environ. Res. 2010, 70, 343–357. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Minier, C.; Abarnou, A.; Jaouen-Madoulet, A.; Le Guellec, A.M. A pollution-monitoring pilot study involving contaminant and biomarker measurements in the Seine Estuary, France, using zebra mussels (Dreissena polymorpha). Environ. Toxicol. Chem. 2006, 25, 112–119. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, T.P. Mussel Watch results from 1986 to 1996. Mar. Pollut. Bull. 1998, 37, 14–19. [Google Scholar] [CrossRef]
- Roper, J.M.; Cherry, D.S.; Simmers, J.W.; Tatem, H.E. Bioaccumulation of toxicants in the zebra mussel, dreissena polymorpha, at the times beach confined disposal facility, Buffalo, New York. Environ. Pollut. 1996, 94, 117–129. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Method 3640A Gel-Permeation Cleanup. 1994; p 24. [Google Scholar]
© Copyright A. Robert et al., 2014. Licensee PAGEPress, Italy. This work is licensed under a Creative Commons Attribution NonCommercial 3.0 License (CC BY-NC 3.0).