Impact of Heat Stress on Mytilus edulis: Contaminants Affect Survival and HSP 70 Proteoform Expression
Introduction
Materials and Methods
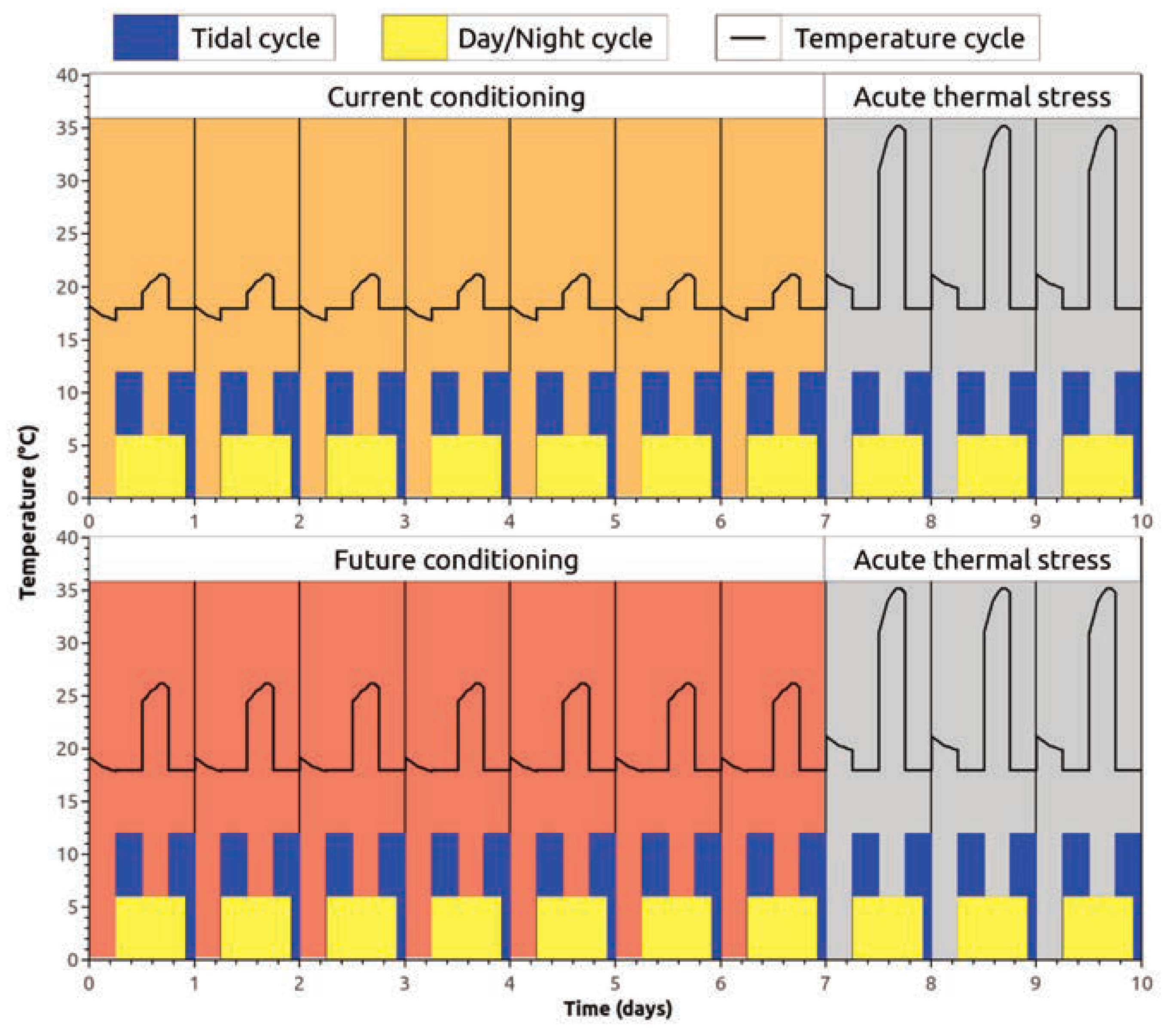
Sample collection and microcosm exposition
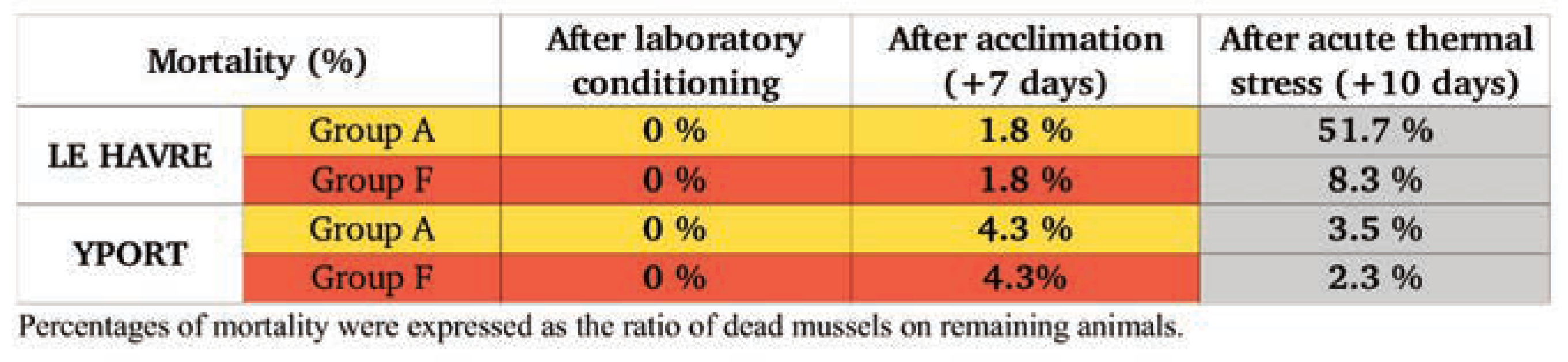
Mortality monitoring
Proteomic analysis
Results and Discussion
Mortality
Heat Shock Protein 70 expression
Conclusions
Acknowledgments
References
- Pachauri, R.K.; Reisinger, A. Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press; 2007.
- Stocker, T.F.; Qin, D.; Plattner, G.K.; Tignor, M.; Allen, S.K.; Boschung, J.; et al. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press; 2013.
- Tomanek, L. Proteomics to study adaptations on marine organisms to environmental stress. J Proteomics 2014, 105, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Letendre, J.; Dupont-Rouzeyrol, M.; Hanquet, A.C.; Durand, F.; Budzinski, H.; Chan, P.; et al. Impact of toxicant exposure on the proteomic response to intertidal condition in Mytilus edulis. Comparat Biochem Physiol Part D Genom Proteom 2011, 6, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Chapple, J.P.; Smerdon, G.R.; Berry, R.J.; Hawkins, A.J.S. Seasonal changes in stress-70 protein levels reflect thermal tolerance in the marine bivalve Mytilus edulis L. J Exp Marine Biol Ecol 1998, 229, 53–68. [Google Scholar] [CrossRef]
- Buckley, B.A.; Owen, M.E.; Hofmann, G.E. Adjusting the thermostat: the threshold induction temperature for the heat-shock response in intertidal mussels (genus Mytilus) changes as a function of thermal history. J Exp Biol 2001, 204, 3571–3579. [Google Scholar] [CrossRef] [PubMed]
- Spiró, Z.; Arslan, M.A.; Somogyvári, M.; Nguyen, M.T.; Smolders, A.; Dancsó, B.; et al. RNA Interference Links Oxidative Stress to the Inhibition of Heat Stress Adaptation. Antioxid Redox Signal 2012, 17, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, A.J.S. Relationships between the synthesis and breakdown of protein, dietary absorption and turnovers of nitrogen and carbon in the blue mussel, Mytilus edulis L. Oecologia 1985, 66, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Anestis, A.; Lazou, A.; Pörtner, H.O.; Michaelidis, B. Behavioral, metabolic, and molecular stress responses of marine bivalve Mytilus galloprovincialis during long-term acclimation at increasing ambient temperature. Am J Physiol Regul Integr Comparat Physiol 2007, 293, 911–921. [Google Scholar] [CrossRef] [PubMed]

© Copyright R. Péden et al., 2014 Licensee PAGEPress, Italy. This work is licensed under a Creative Commons Attribution NonCommercial 3.0 License (CC BY-NC 3.0).
Share and Cite
Péden, R.; Letendre, J.; Rocher, B.; Durand, F.; Le Foll, F.; Chan, P.; Vaudry, D.; Bultelle, F. Impact of Heat Stress on Mytilus edulis: Contaminants Affect Survival and HSP 70 Proteoform Expression. J. Xenobiot. 2014, 4, 4892. https://doi.org/10.4081/xeno.2014.4892
Péden R, Letendre J, Rocher B, Durand F, Le Foll F, Chan P, Vaudry D, Bultelle F. Impact of Heat Stress on Mytilus edulis: Contaminants Affect Survival and HSP 70 Proteoform Expression. Journal of Xenobiotics. 2014; 4(2):4892. https://doi.org/10.4081/xeno.2014.4892
Chicago/Turabian StylePéden, R., J. Letendre, B. Rocher, F. Durand, F. Le Foll, P. Chan, D. Vaudry, and F. Bultelle. 2014. "Impact of Heat Stress on Mytilus edulis: Contaminants Affect Survival and HSP 70 Proteoform Expression" Journal of Xenobiotics 4, no. 2: 4892. https://doi.org/10.4081/xeno.2014.4892
APA StylePéden, R., Letendre, J., Rocher, B., Durand, F., Le Foll, F., Chan, P., Vaudry, D., & Bultelle, F. (2014). Impact of Heat Stress on Mytilus edulis: Contaminants Affect Survival and HSP 70 Proteoform Expression. Journal of Xenobiotics, 4(2), 4892. https://doi.org/10.4081/xeno.2014.4892



