Decrease in Phagocytosis Capacity of Hemocyte During Spawning in Mytilus edulis: A Pilot Study †
Introduction
Materials and Methods
Animals
Gonads and hemolymph collection
Gonads integrity
Spawning biomarker
Cell viability and phagocytosis of hemocytes
Statistical analysis
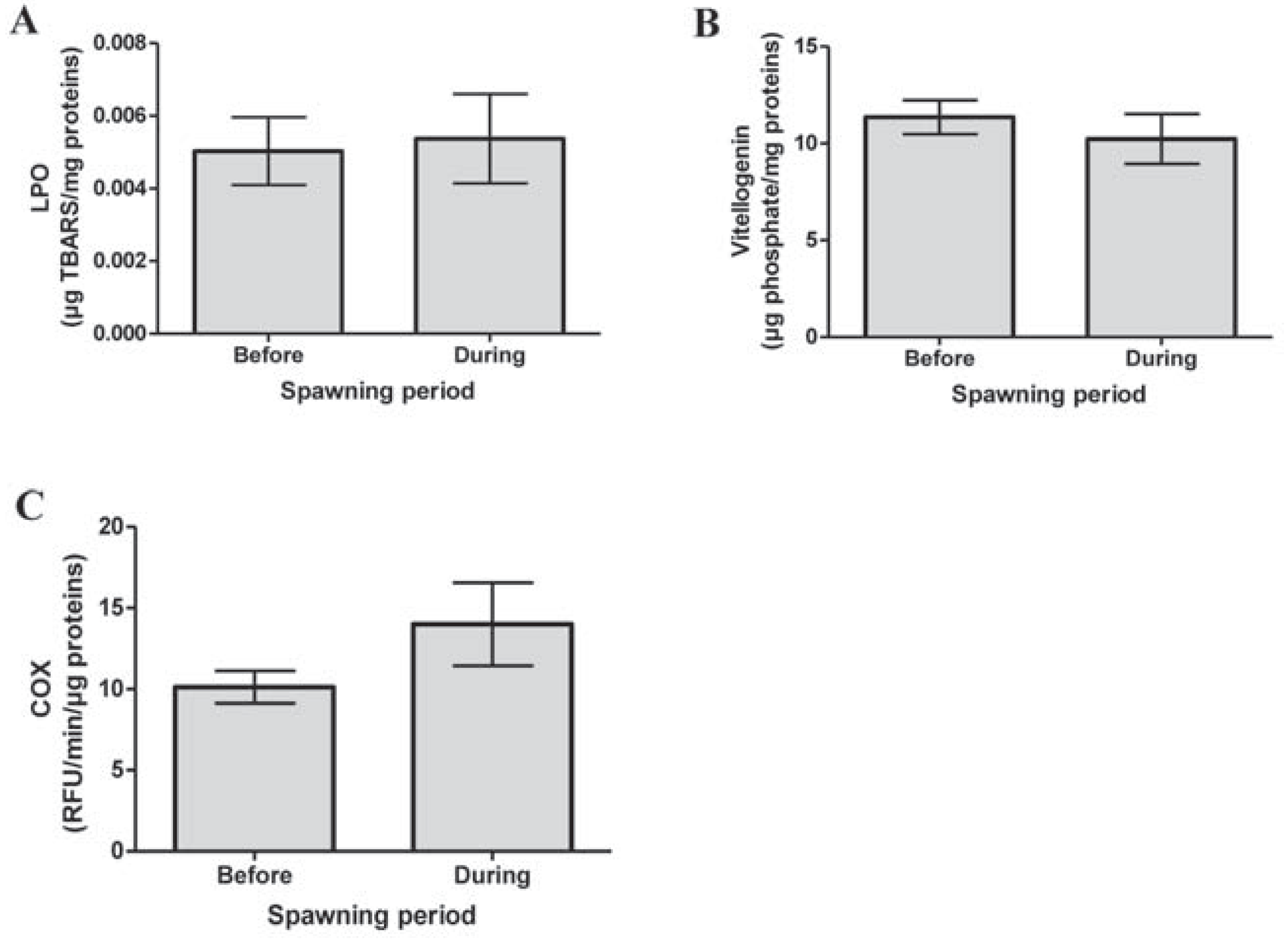
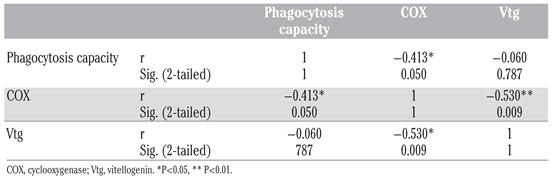
Results and Discussion
Author Contributions
References
- Morse, D.E.; Duncan, H.; Hooker, N.; Morse, A. Hydrogen peroxide induces spawning in mollusks, with activation of prostaglandin endoperoxide synthetase. Science 1977, 196, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Gagné, F.; Fournier, M.; Blaise, C. Serotonergic effects of municipal effluents: Induced spawning activity in freshwater mussels. Fresenius Environ Bull 2004, 13, 1099–1103. [Google Scholar]
- Matsutani, T.; Nomura, T. In vitro effects of serotonin and prostaglandins on release of eggs from the ovary of the scallop, Patinopecten yessoensis. General Comparat Endocrinol 1987, 67, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.; Mettifogo, L.; Lenoir, R.; Olivares, A. Prostaglandins and reproduction of the scallop Argopecten purpuratus: II. Relationship with gamete release. J Exp Zool 2000, 287, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Fong, P.P.; Noordhuis, R.; Ram, J.L. Dopamine reduces intensity of serotonin-induced spawning in the zebra mussel Dreissenapolymorpha (pallas). J Exp Zool 1993, 266, 79–83. [Google Scholar] [CrossRef]
- Gagné, F.; André, C.; Cejka, P.; Hausler, R.; Fournier, M. Evidence of neuroendocrine disruption in freshwater mussels exposed to municipal wastewaters. Sci Total Environ 2011, 409, 3711–3718. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qin, J.G.; Li, X.; Benkendorff, K. Assessment of metabolic and immune changes in postspawning Pacific oyster Crassostrea gigas: Identification of a critical period of vulnerability after spawning. Aquacult Res 2010, 41, e155–e165. [Google Scholar] [CrossRef]
- Li, Y.; Qin, J.G.; Abbott, C.A.; Li, X.; Benkendorff, K. Synergistic impacts of heat shock and spawning on the physiology and immune health of Crassostrea gigas: An explanation for summer mortality in Pacific oysters. Am J Physiol Regulat Integrat Comparat Physiol 2007, 293, R2353–R2362. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qin, J.G.; Li, X.; Benkendorff, K. Spawning-dependent stress responses in pacific oysters Crassostrea gigas: A simulated bacterial challenge in oysters. Aquaculture 2009, 293, 164–171. [Google Scholar] [CrossRef]
- Rasmussen, L.P.D.; Hage, E.; Karlog, O. An electron microscope study of the circulating leucocytes of the marine mussel, Mytilus edulis. J Inverteb Pathol 1985, 45, 158–167. [Google Scholar] [CrossRef]
- Lemaire, N.; Pellerin, J.; Fournier, M.; Girault, L.; Tamigneaux, E.; Cartier, S.; et al. Seasonal variations of physiological parameters in the blue mussel mytilus spp. from farm sites of eastern Quebec. Aquaculture 2006, 261, 729–751. [Google Scholar]
- Cartier, S.; Pellerin, J.; Fournier, M.; Tamigneaux, E.; Girault, L.; Lemaire, N. Use of an index based on the blue mussel (Mytilus edulis and Mytilus trossulus) digestive gland weight to assess the nutritional quality of mussel farm sites. Aquaculture 2004, 241, 633–654. [Google Scholar]
- Gagne, F.; Bouchard, B.; André, C.; Farcy, E.; Fournier, M. Evidence of feminization in wild Elliptio complanata mussels in the receiving waters downstream of a municipal effluent outfall. Comp Biochem Physiol C Toxicol Pharmacol 2011, 153, 99–106. [Google Scholar] [PubMed]
- Bradford, M.M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Wills, E. Evaluation of lipid peroxidation in lipids and biological membranes. In Biochemical toxicology, a pratical approaches; Snell, K., Mullok, B., Eds.; IRL Press: Oxford, UK, 1987. [Google Scholar]
- Gagné, F.; Blaise, C.; Andre, C.; Gagnon, C.; Salazar, M. Neuroendocrine disruption and health effects in Elliptio complanata mussels exposed to aeration lagoons for waste-water treatment. Chemosphere 2007, 68, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Sakuma, S.; Inoue, T.; Uno, E.; Fujita, T. The endocrine disruptor nonylphenol preferentially blocks cyclooxygenase-1. Life Sci 2002, 70, 2209–2214. [Google Scholar] [CrossRef] [PubMed]
- Gagné, F.; André, C.; Bouchard, B.; Fournier, M.; Gagnon, C. Neurotoxicological effects of aeration lagoon effluents for the treatment of domestic and hospital wastewaters on Elliptio complanata. In Ecotoxicology around the globe; Visser, J.E., Ed.; Nova Science Publishers: New York, NY, USA, 2010; pp. 1–20. [Google Scholar]
- Brousseau, P.; Payette, Y.; Tryphonas, H.; Blakley, B.; Boernaus, H.; Flipo, D.; et al. Manual of immunological methods; CRS Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Reis-Henriques, M.A.; Le Guellec, D.; Remy-Martin, J.P.; Adessi, G.L. Studies of endogenous steroids from the marine mollusc Mytilus edulis L. By gas chromatography and mass spectrometry. Comp Biochem Physiol B Comp Biochem 1990, 95, 303–309. [Google Scholar] [CrossRef]
- Watanuki, H.; Yamaguchi, T.; Sakai, M. Suppression in function of phagocytic cells in common carp Cyprinus carpio L. injected with estradiol, progesterone or 11-ketotestosterone. Comp Biochem Physiol C Toxicol Pharmacol 2002, 132, 407–413. [Google Scholar] [CrossRef] [PubMed]

© Copyright M. Fraser et al., 2013 Licensee PAGEPress, Italy. This work is licensed under a Creative Commons Attribution NonCommercial 3.0 License (CC BYNC 3.0).
Share and Cite
Fraser, M.; Rault, P.; Roumier, P.-H.; Fortier, M.; André, C.; Brousseau, P.; Gagné, F.; Fournier, M.; Surette, C.; Vaillancourt, C. Decrease in Phagocytosis Capacity of Hemocyte During Spawning in Mytilus edulis: A Pilot Study. J. Xenobiot. 2013, 3, e12. https://doi.org/10.4081/xeno.2013.s1.e12
Fraser M, Rault P, Roumier P-H, Fortier M, André C, Brousseau P, Gagné F, Fournier M, Surette C, Vaillancourt C. Decrease in Phagocytosis Capacity of Hemocyte During Spawning in Mytilus edulis: A Pilot Study. Journal of Xenobiotics. 2013; 3(s1):e12. https://doi.org/10.4081/xeno.2013.s1.e12
Chicago/Turabian StyleFraser, M., P. Rault, P-H. Roumier, M. Fortier, C. André, P. Brousseau, F. Gagné, M. Fournier, C. Surette, and C. Vaillancourt. 2013. "Decrease in Phagocytosis Capacity of Hemocyte During Spawning in Mytilus edulis: A Pilot Study" Journal of Xenobiotics 3, no. s1: e12. https://doi.org/10.4081/xeno.2013.s1.e12
APA StyleFraser, M., Rault, P., Roumier, P.-H., Fortier, M., André, C., Brousseau, P., Gagné, F., Fournier, M., Surette, C., & Vaillancourt, C. (2013). Decrease in Phagocytosis Capacity of Hemocyte During Spawning in Mytilus edulis: A Pilot Study. Journal of Xenobiotics, 3(s1), e12. https://doi.org/10.4081/xeno.2013.s1.e12





