In Vitro and In Vivo Genotoxicity of Polystyrene Microplastics: Evaluation of a Possible Synergistic Action with Bisphenol A
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Media
2.2. Subjects for In Vitro Experiments
2.3. Blood Sample Collection, Lymphocyte Cultures and Cytokinesis-Block Micronucleus Assay
2.4. Lymnaea stagnalis
2.5. Micronuclei Assay on Hemocytes from Lymnaea stagnalis
2.6. Statistical Analysis
3. Results
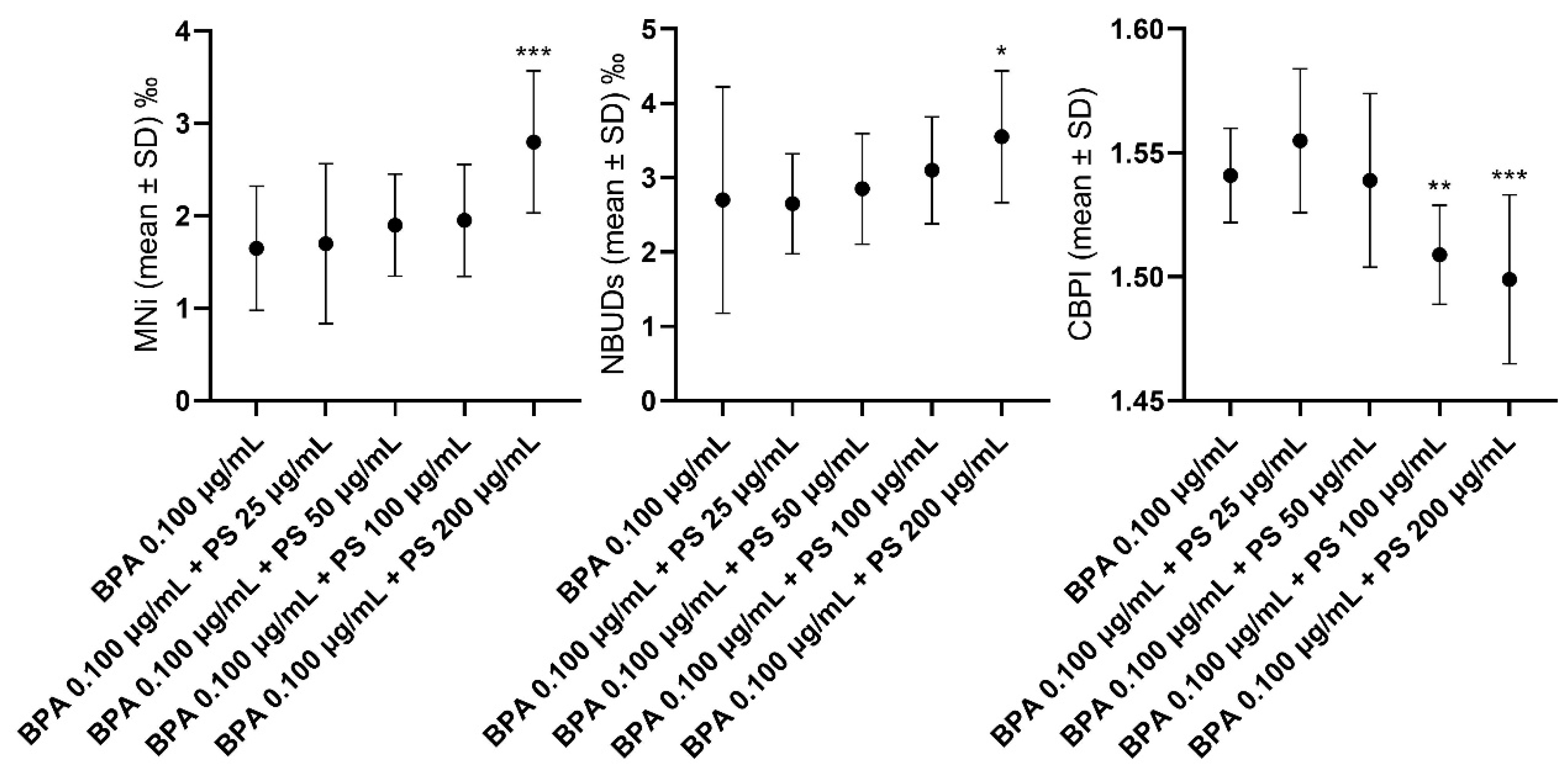
3.1. Lymphocytes
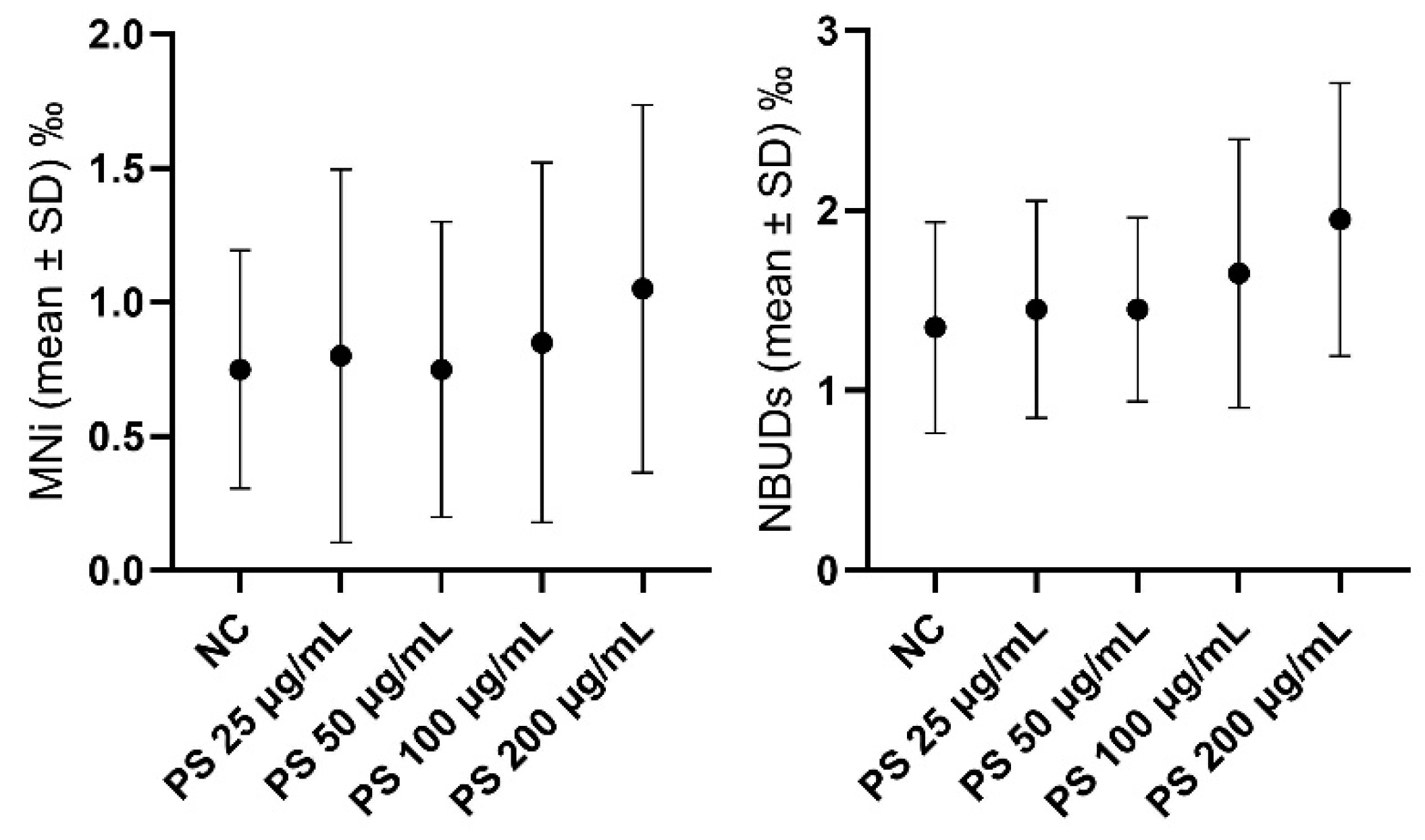
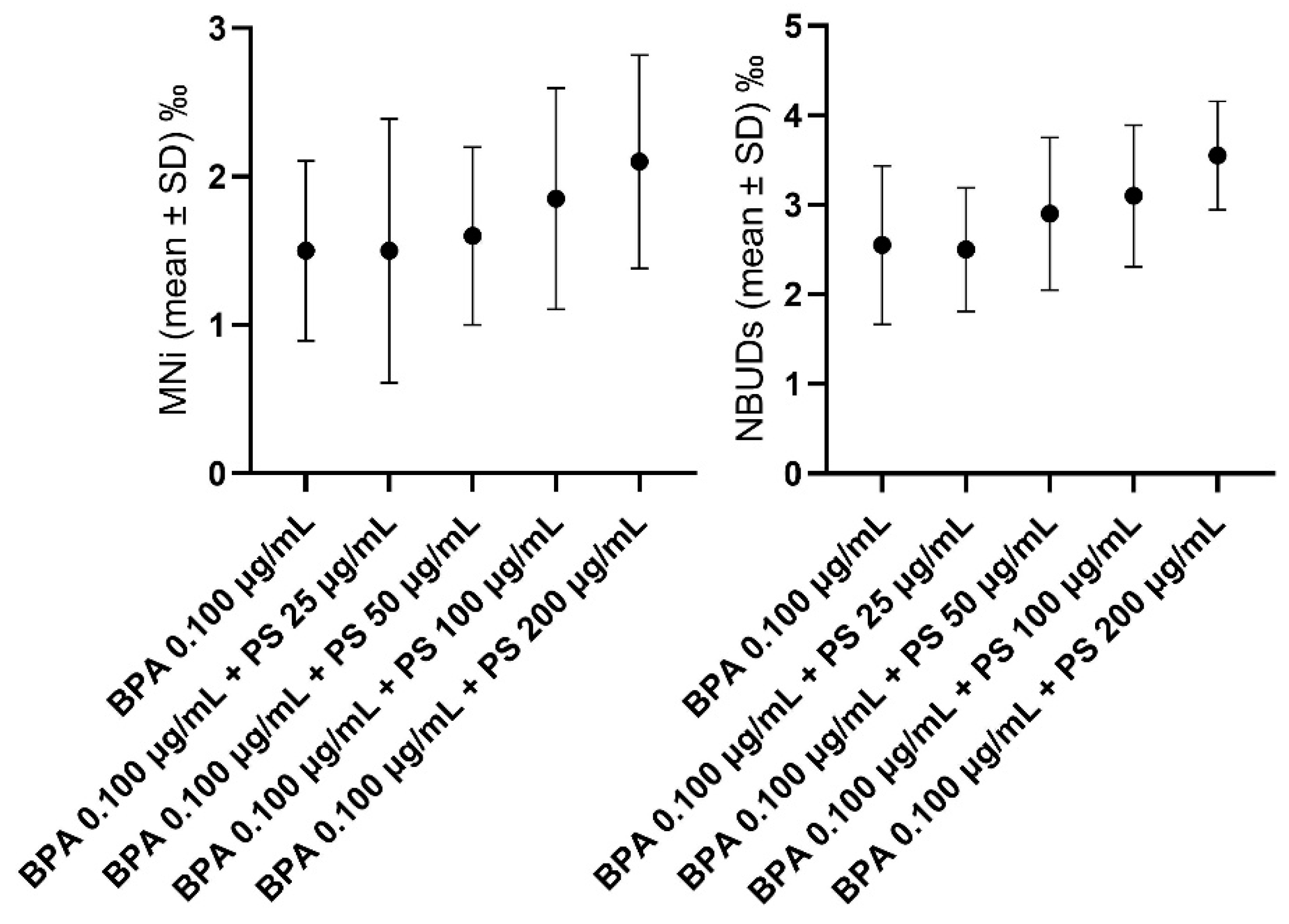
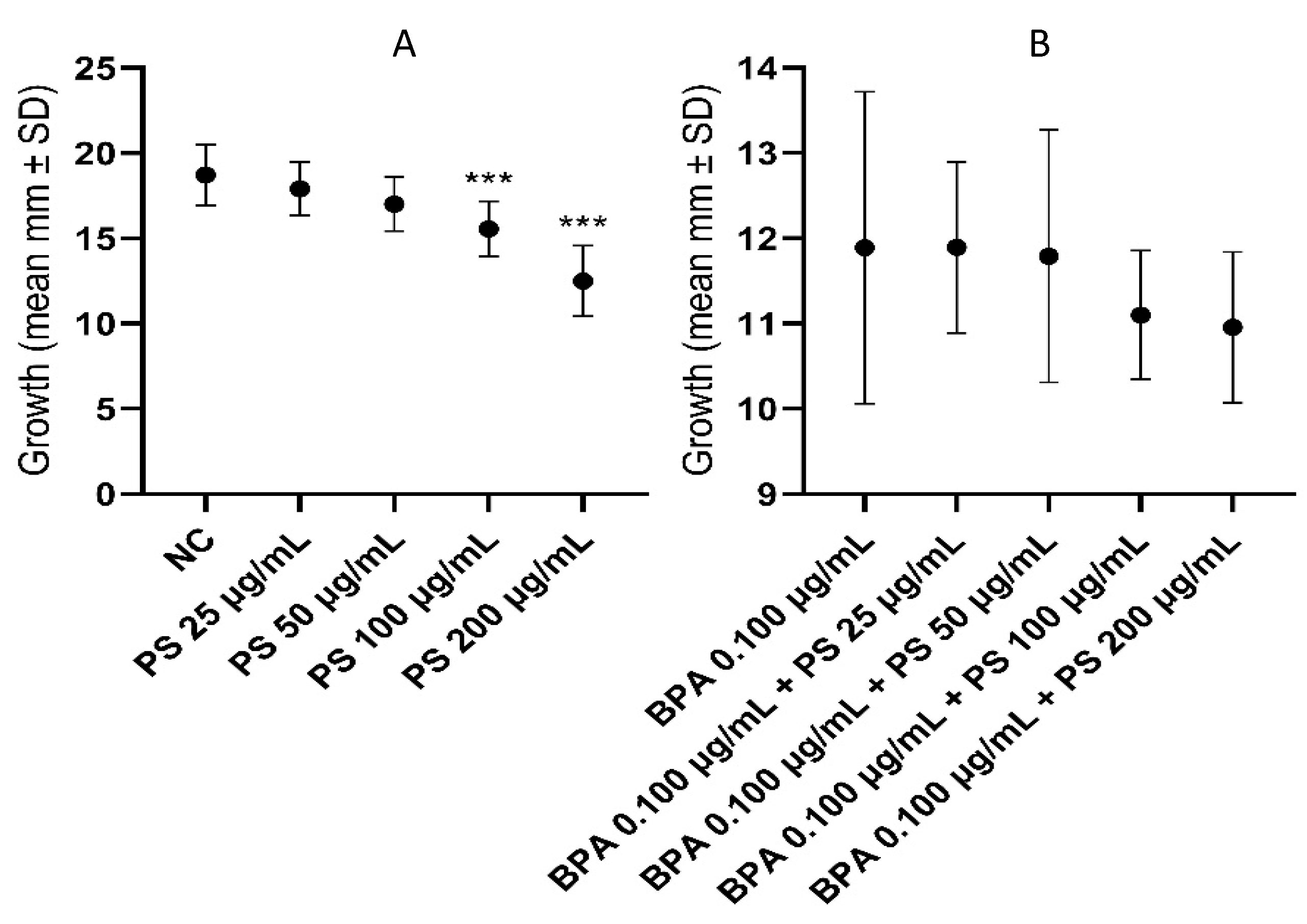
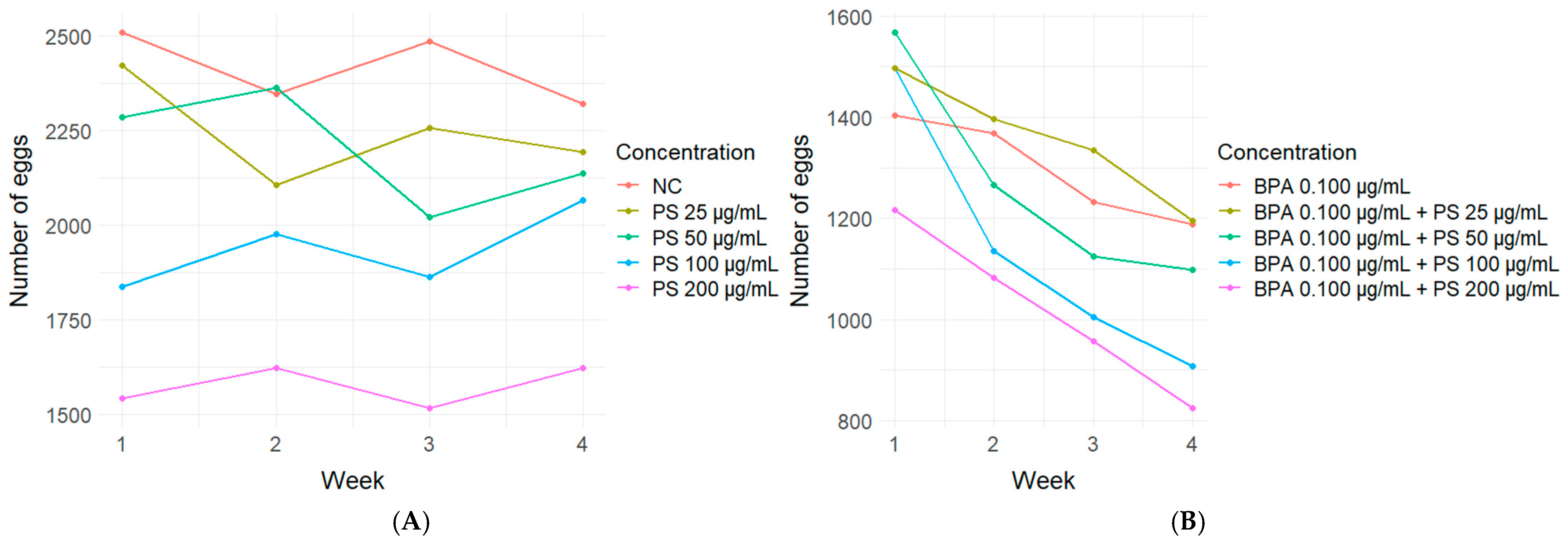
3.2. Hemocytes
4. Discussion
4.1. In Vitro Experiment
4.2. In Vivo Experiment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarma, D.K.; Dubey, R.; Samarth, R.M.; Shubham, S.; Chowdhury, P.; Kumawat, M.; Verma, V.; Tiwari, R.R.; Kumar, M. The biological effects of polystyrene nanoplastics on human peripheral blood lymphocytes. Nanomaterials 2022, 12, 1632. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, R.; Borghesi, G.; Ronzano, A.; Vignali, G. Plastic or glass: A new environmental assessment with a marine litter indicator for the comparison of pasteurized milk bottles. Int. J. Life Cycle Assess. 2021, 26, 767–784. [Google Scholar] [CrossRef]
- Nugnes, R.; Lavorgna, M.; Orlo, E.; Russo, C.; Isidori, M. Toxic impact of polystyrene microplastic particles in freshwater organisms. Chemosphere 2022, 299, 134373. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.; Kim, D.; Kim, S.W.; An, Y.-J. Trophic transfer and individual impact of nano-sized polystyrene in a four-species freshwater food chain. Sci. Rep. 2018, 8, 284. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.; Piazza, V.; Lavorano, S.; Faimali, M.; Garaventa, F.; Gambardella, C. Trophic transfer of microplastics from copepods to jellyfish in the marine environment. Front. Environ. Sci. 2020, 8, 571732. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of various microplastics in human stool. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Pironti, C.; Notarstefano, V.; Ricciardi, M.; Motta, O.; Giorgini, E.; Montano, L. First evidence of microplastics in human urine, a preliminary study of intake in the human body. Toxics 2023, 11, 40. [Google Scholar] [CrossRef]
- Huang, S.; Huang, X.; Bi, R.; Guo, Q.; Yu, X.; Zeng, Q.; Huang, Z.; Liu, T.; Wu, H.; Chen, Y.; et al. Detection and analysis of microplastics in human sputum. Environ. Sci. Technol. 2022, 56, 2476–2486. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, L.; Weng, J.; Jin, Z.; Cao, Y.; Jiang, H.; Zhang, Z. Detection and characterization of microplastics in the human testis and semen. Sci. Total Environ. 2023, 877, 162713. [Google Scholar] [CrossRef]
- Cetin, M.; Demirkaya Miloglu, F.; Kilic Baygutalp, N.; Ceylan, O.; Yildirim, S.; Eser, G.; Gul, H.İ. Higher number of microplastics in tumoral colon tissues from patients with colorectal adenocarcinoma. Environ. Chem. Lett. 2023, 21, 639–646. [Google Scholar] [CrossRef]
- Kik, K.; Bukowska, B.; Krokosz, A.; Sicińska, P. Oxidative properties of polystyrene nanoparticles with different diameters in human peripheral blood mononuclear cells (in vitro study). Int. J. Mol. Sci. 2021, 22, 4406. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, K.; Bukowska, B.; Piwoński, I.; Foksiński, M.; Kisielewska, A.; Zarakowska, E.; Gackowski, D.; Sicińska, P. Polystyrene nanoparticles: The mechanism of their genotoxicity in human peripheral blood mononuclear cells. Nanotoxicology 2022, 16, 791–811. [Google Scholar] [CrossRef] [PubMed]
- Guševac Stojanović, I.; Drakulić, D.; Todorović, A.; Martinović, J.; Filipović, N.; Stojanović, Z. Acute toxicity assessment of orally administered microplastic particles in adult male Wistar rats. Toxics 2024, 12, 167. [Google Scholar] [CrossRef]
- Płuciennik, K.; Sicińska, P.; Misztal, W.; Bukowska, B. Important factors affecting induction of cell death, oxidative stress and DNA damage by nano- and microplastic particles in vitro. Cells 2024, 13, 768. [Google Scholar] [CrossRef]
- Rubio, L.; Barguilla, I.; Domenech, J.; Marcos, R.; Hernández, A. Biological effects, including oxidative stress and genotoxic damage, of polystyrene nanoparticles in different human hematopoietic cell lines. J. Hazard. Mater. 2020, 398, 122900. [Google Scholar] [CrossRef]
- Nakai, M.; Tsubokura, M.; Suzuki, M.; Fujishima, S.; Watanabe, Y.; Hasegawa, Y.; Oyama, K.; Ogura, S. Genotoxicity of styrene oligomers extracted from polystyrene intended for use in contact with food. Toxicol. Rep. 2014, 1, 1175–1180. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.; Hollman, P.C.H.; Peters, R.J.B. Potential health impact of environmentally released micro- and nanoplastics in the human food production chain: Experiences from nanotoxicology. Environ. Sci. Technol. 2015, 49, 8932–8947. [Google Scholar] [CrossRef]
- Khan, N.S.; Bari, J.B.A.; Mahatab Uddin, S.M.; Rahman, M.S.; Uddin, M.; Bhowmik, S.; Nisa, S.A.; Alam, M.d.A.; Hossain, M.N. A first record on microplastic ingestion by tropical estuarine copepods of Bangladesh. Bull. Environ. Contam. Toxicol. 2024, 113, 1. [Google Scholar] [CrossRef]
- Kneel, S.; Stephens, C.G.; Rolston, A.; Mendes, A.M.; Morrison, L.; Linnane, S. Microplastic contamination of intertidal sediment and cockles (Cerastoderma edule). Mar. Pollut. Bull. 2024, 205, 116568. [Google Scholar] [CrossRef]
- Leuenberger, K.; Erni-Cassola, G.; Leistenschneider, C.; Burkhardt-Holm, P. Microplastic ingestion in five demersal, bathydemersal and bathypelagic fish species from the easstern Weddell Sea, Antarctica. Sci. Total Environ. 2024, 946, 174320. [Google Scholar] [CrossRef]
- Gray, A.; Mayer, K.; Gore, B.; Gaesser, M.; Ferguson, N. Microplastic burden in native (Cambarus appalachiensis) and non-native (Faxonius cristavarius) crayfish along semi-rural and urban streams in southwest Virginia, USA. Environ. Res. 2024, 258, 119494. [Google Scholar] [CrossRef]
- Ziani, K.; Ioniță-Mîndrican, C.-B.; Mititelu, M.; Neacșu, S.M.; Negrei, C.; Moroșan, E.; Drăgănescu, D.; Preda, O.-T. Microplastics: Areal global threat for environment and food safety: A state of the art review. Nutrients 2023, 15, 617. [Google Scholar] [CrossRef]
- Marcharla, E.; Vinayagam, S.; Gnanasekaran, L.; Soto-Moscoso, M.; Chen, W.-H.; Thanigaivel, S.; Ganesan, S. Microplastics in marine ecosystems: A comprehensive review of biological and ecological implications and its mitigation approach using nanotechnology for the sustainable environment. Environ. Res. 2024, 256, 119181. [Google Scholar] [CrossRef]
- Gao, H.; Yang, B.-J.; Li, N.; Feng, L.-M.; Shi, X.-Y.; Zhao, W.-H.; Liu, S.-J. Bisphenol A and hormone-associated cancers: Current progress and perspectives. Medicine 2015, 94, e211. [Google Scholar] [CrossRef]
- Hafezi, S.A.; Abdel-Rahman, W.M. The endocrine disruptor bisphenol A (BPA) exerts a wide range of effects in carcinogenesis and response to therapy. Curr. Mol. Pharmacol. 2019, 12, 230–238. [Google Scholar] [CrossRef]
- Negi, A.; Kesari, K.K.; Voisin-Chiret, A.S. Estrogen receptor-α targeting: PROTACs, SNIPERs, peptide-PROTACs, antibody conjugated PROTACs and SNIPERs. Pharmaceutics 2022, 14, 2523. [Google Scholar] [CrossRef]
- Zhivotovsky, B.; Orrenius, S. Calcium and cell death mechanisms: A perspective from the cell death community. Cell Calcium 2011, 50, 211–221. [Google Scholar] [CrossRef]
- Wu, C.; Guo, W.-B.; Liu, Y.-Y.; Yang, L.; Miao, A.-J. Perturbation of calcium homeostasis and multixenobiotic resistance by nanoplastics in the ciliate Tetrahymena thermophila. J. Hazard. Mater. 2021, 403, 123923. [Google Scholar] [CrossRef]
- Das, A. The emerging role of microplastics in systemic toxicity: Involvement of reactive oxygen species (ROS). Sci. Total Environ. 2023, 895, 165076. [Google Scholar] [CrossRef]
- An, Q.; Sun, C.; Li, D.; Xu, K.; Guo, J.; Wang, C. Peroxidase-like activity of Fe3O4@Carbon nanoparticles enhances ascorbic acid-induced oxidative stress and selective damage to PC-3 prostate cancer cells. ACS Appl. Mater. Interfaces 2013, 5, 13248–13257. [Google Scholar] [CrossRef]
- Liu, P.; Zhan, X.; Wu, X.; Li, J.; Wang, H.; Gao, S. Effect of weathering on environmental behavior of microplastics: Properties, sorption and potential risks. Chemosphere 2020, 242, 125193. [Google Scholar] [CrossRef]
- Gao, D.; Li, X.; Liu, H. Source, Occurrence, Migration and potential environmental risk of microplastics in sewage sludge and during sludge amendment to soil. Sci. Total Environ. 2020, 742, 140355. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Y.; Liu, G.; He, G.; Liu, W. Adsorption mechanism of cadmium on microplastics and their desorption behavior in sediment and gut environments: The roles of water pH, lead ions, natural organic matter and phenanthrene. Water Res. 2020, 184, 116209. [Google Scholar] [CrossRef]
- Turner, A.; Holmes, L.; Thompson, R.C.; Fisher, A.S. Metals and marine microplastics: Adsorption from the environment versus addition during manufacture, exemplified with lead. Water Res. 2020, 173, 115577. [Google Scholar] [CrossRef]
- Liao, Y.-L.; Yang, J.-Y. Microplastic serves as a potential vector for Cr in an in-vitro human digestive model. Sci. Total Environ. 2020, 703, 134805. [Google Scholar] [CrossRef]
- Liu, X.; Shi, H.; Xie, B.; Dionysiou, D.D.; Zhao, Y. Microplastics as both a sink and a source of bisphenol A in the marine environment. Environ. Sci. Technol. 2019, 53, 10188–10196. [Google Scholar] [CrossRef]
- Liu, X.; Sun, P.; Qu, G.; Jing, J.; Zhang, T.; Shi, H.; Zhao, Y. Insight into the characteristics and sorption behaviors of aged polystyrene microplastics through three type of accelerated oxidation processes. J. Hazard. Mater. 2021, 407, 124836. [Google Scholar] [CrossRef]
- Cortés-Arriagada, D.; Ortega, D.E.; Miranda-Rojas, S. Mechanistic Insights into the Adsorption of Endocrine Disruptors onto Polystyrene Microplastics in Water. Environ. Pollut. 2023, 319, 121017. [Google Scholar] [CrossRef]
- Cortés-Arriagada, D.; Miranda-Rojas, S.; Camarada, M.B.; Ortega, D.E.; Alarcón-Palacio, V.B. The Interaction mechanism of polystyrene microplastics with pharmaceuticals and personal care products. Sci. Total Environ. 2023, 861, 160632. [Google Scholar] [CrossRef]
- Huang, W.; Song, B.; Liang, J.; Niu, Q.; Zeng, G.; Shen, M.; Deng, J.; Luo, Y.; Wen, X.; Zhang, Y. Microplastics and associated contaminants in the aquatic environment: A review on their ecotoxicological effects, trophic transfer, and potential impacts to human health. J. Hazard. Mater. 2021, 405, 124187. [Google Scholar] [CrossRef]
- Menéndez-Pedriza, A.; Jaumot, J. Interaction of environmental pollutants with microplastics: A critical review of sorption factors, bioaccumulation and ecotoxicological effects. Toxics 2020, 8, 40. [Google Scholar] [CrossRef]
- Katsumiti, A.; Losada-Carrillo, M.P.; Barros, M.; Cajaraville, M.P. Polystyrene nanoplastics and microplastics can act as Trojan Horse carriers of benzo(a)pyrene to mussel hemocytes in vitro. Sci. Rep. 2021, 11, 22396. [Google Scholar] [CrossRef]
- Santos, L.H.M.L.M.; Rodríguez-Mozaz, S.; Barceló, D. Microplastics as vectors of pharmaceuticals in aquatic organisms—An overview of their environmental implications. Case Stud. Chem. Environ. Eng. 2021, 3, 100079. [Google Scholar] [CrossRef]
- Paul-Pont, I.; Lacroix, C.; González Fernández, C.; Hégaret, H.; Lambert, C.; Le Goïc, N.; Frère, L.; Cassone, A.-L.; Sussarellu, R.; Fabioux, C.; et al. Exposure of marine mussels Mytilus spp. to polystyrene microplastics: Toxicity and influence on fluoranthene bioaccumulation. Environ. Pollut. 2016, 216, 724–737. [Google Scholar] [CrossRef]
- Pittura, L.; Avio, C.G.; Giuliani, M.E.; d’Errico, G.; Keiter, S.H.; Cormier, B.; Gorbi, S.; Regoli, F. Microplastics as vehicles of environmental PAHs to marine organisms: Combined chemical and physical hazards to the mediterranean mussels, Mytilus galloprovincialis. Front. Mar. Sci. 2018, 5, 103. [Google Scholar] [CrossRef]
- Magara, G.; Khan, F.R.; Pinti, M.; Syberg, K.; Inzirillo, A.; Elia, A.C. Effects of combined exposures of fluoranthene and polyethylene or polyhydroxybutyrate microplastics on oxidative stress biomarkers in the blue mussel (Mytilus edulis). J. Toxicol. Environ. Health Part A 2019, 82, 616–625. [Google Scholar] [CrossRef]
- González-Soto, N.; Hatfield, J.; Katsumiti, A.; Duroudier, N.; Lacave, J.M.; Bilbao, E.; Orbea, A.; Navarro, E.; Cajaraville, M.P. Impacts of dietary exposure to different sized polystyrene microplastics alone and with sorbed benzo[a]pyrene on biomarkers and whole organism responses in mussels Mytilus galloprovincialis. Sci. Total Environ. 2019, 684, 548–566. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef]
- de Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Futter, M.N. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef]
- Santovito, A.; Cannarsa, E.; Schleicherova, D.; Cervella, P. Clastogenic effects of bisphenol A on human cultured lymphocytes. Hum. Exp. Toxicol. 2018, 37, 69–77. [Google Scholar] [CrossRef]
- Rainieri, S.; Conlledo, N.; Larsen, B.K.; Granby, K.; Barranco, A. Combined effects of microplastics and chemical contaminants on the organ toxicity of zebrafish (Danio rerio). Environ. Res. 2018, 162, 135–143. [Google Scholar] [CrossRef]
- Fenech, M. The in vitro micronucleus technique. Mutat. Res. 2000, 455, 81–95. [Google Scholar] [CrossRef]
- Fenech, M.; Chang, W.P.; Kirsch-Volders, M.; Holland, N.; Bonassi, S.; Zeiger, E. HUMN Project: Detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat. Res. 2003, 534, 65–75. [Google Scholar] [CrossRef]
- Thomas, P.; Fenech, M. Cytokinesis-block micronucleus cytome assay in lymphocytes. In DNA Damage Detection In Situ, Ex Vivo, and In Vivo: Methods and Protocols; Didenko, V.V., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 217–234. ISBN 978-1-60327-409-8. [Google Scholar]
- Santovito, A.; Gendusa, C. Micronuclei frequency in peripheral blood lymphocytes of healthy subjects living in Turin (north-Italy): Contribution of body mass index, age and sex. Ann. Hum. Biol. 2020, 47, 48–54. [Google Scholar] [CrossRef]
- Fenech, M.; Crott, J.W. Micronuclei, nucleoplasmic bridges and nuclear buds induced in folic acid deficient human lymphocytes—Evidence for breakage–fusion-bridge cycles in the cytokinesis-block micronucleus assay. Mutat. Res. 2002, 504, 131–136. [Google Scholar] [CrossRef]
- Santovito, A.; Pappalardo, A.; Nota, A.; Prearo, M.; Schleicherová, D. Lymnaea stagnalis and Ophryotrocha diadema as model organisms for studying genotoxicological and physiological effects of benzophenone-3. Toxics 2023, 11, 827. [Google Scholar] [CrossRef]
- Amorim, J.; Abreu, I.; Rodrigues, P.; Peixoto, D.; Pinheiro, C.; Saraiva, A.; Carvalho, A.P.; Guimarães, L.; Oliva-Teles, L. Lymnaea stagnalis as a freshwater model invertebrate for ecotoxicological studies. Sci. Total Environ. 2019, 669, 11–28. [Google Scholar] [CrossRef]
- Fodor, I.; Hussein, A.A.; Benjamin, P.R.; Koene, J.M.; Pirger, Z. The unlimited potential of the great pond snail, Lymnaea stagnalis. eLife 2020, 9, e56962. [Google Scholar] [CrossRef]
- Santovito, A.; Lambertini, M.; Schleicherová, D.; Mirone, E.; Nota, A. Cellular and genomic instability induced by the herbicide glufosinate-ammonium: An in vitro and in vivo approach. Cells 2024, 13, 909. [Google Scholar] [CrossRef]
- Schleicherová, D.; Pastorino, P.; Pappalardo, A.; Nota, A.; Gendusa, C.; Mirone, E.; Prearo, M.; Santovito, A. Genotoxicological and physiological effects of glyphosate and its metabolite, aminomethylphosphonic acid, on the freshwater invertebrate Lymnaea stagnalis. Aquat. Toxicol. 2024, 271, 106940. [Google Scholar] [CrossRef]
- GraphPad. Available online: https://www.graphpad.com/ (accessed on 28 August 2024).
- Cortés, C.; Domenech, J.; Salazar, M.; Pastor, S.; Marcos, R.; Hernández, A. Nanoplastics as a potential environmental health factor: Effects of polystyrene nanoparticles on human intestinal epithelial Caco-2 cells. Environ. Sci. Nano 2020, 7, 272–285. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, D.; Han, S.; Jung, S.Y.; Choi, J.; Hong, J. Potential toxicity of polystyrene microplastic particles. Sci. Rep. 2020, 10, 7391. [Google Scholar] [CrossRef]
- Leso, V.; Battistini, B.; Vetrani, I.; Reppuccia, L.; Fedele, M.; Ruggieri, F.; Bocca, B.; Iavicoli, I. The endocrine disrupting effects of nanoplastic exposure: A systematic review. Toxicol. Ind. Health 2023, 39, 613–629. [Google Scholar] [CrossRef]
- Martín-Pozo, L.; Mejías, C.; Santos, J.L.; Martín, J.; Aparicio, I.; Alonso, E. Influence of microplastic contamination on the dissipation of endocrine disrupting chemicals in soil environment. Environ. Pollut. 2024, 349, 123919. [Google Scholar] [CrossRef]
- Inkielewicz-Stepniak, I.; Tajber, L.; Behan, G.; Zhang, H.; Radomski, M.W.; Medina, C.; Santos-Martinez, M.J. The role of mucin in the toxicological impact of polystyrene nanoparticles. Materials 2018, 11, 724. [Google Scholar] [CrossRef]
- Yee, M.S.-L.; Hii, L.-W.; Looi, C.K.; Lim, W.-M.; Wong, S.-F.; Kok, Y.-Y.; Tan, B.-K.; Wong, C.-Y.; Leong, C.-O. Impact of microplastics and nanoplastics on human health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef]
- Bouki, E.; Dimitriadis, V.K.; Kaloyianni, M.; Dailianis, S. Antioxidant and pro-oxidant challenge of tannic acid in mussel hemocytes exposed to cadmium. Mar. Environ. Res. 2013, 85, 13–20. [Google Scholar] [CrossRef]
- Tsiaka, P.; Tsarpali, V.; Ntaikou, I.; Kostopoulou, M.N.; Lyberatos, G.; Dailianis, S. Carbamazepine-mediated pro-oxidant effects on the unicellular marine algal species Dunaliella tertiolecta and the hemocytes of mussel Mytilus galloprovincialis. Ecotoxicology 2013, 22, 1208–1220. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; d’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef]
- Ribeiro, F.; Garcia, A.R.; Pereira, B.P.; Fonseca, M.; Mestre, N.C.; Fonseca, T.G.; Ilharco, L.M.; Bebianno, M.J. Microplastics effects in Scrobicularia plana. Mar. Pollut. Bull. 2017, 122, 379–391. [Google Scholar] [CrossRef]
- Ansoar-Rodríguez, Y.; Christofoletti, C.A.; Marcato, A.C.; Correia, J.E.; Bueno, O.C.; Malaspina, O.; Fontanetti, C.S. Genotoxic potential of the insecticide imidacloprid in a non-target organism (Oreochromis niloticus-Pisces). J. Environ. Prot. 2015, 6, 1360–1367. [Google Scholar] [CrossRef]
- Albendín, M.G.; Aranda, V.; Coello, M.D.; González-Gómez, C.; Rodríguez-Barroso, R.; Quiroga, J.M.; Arellano, J.M. Pharmaceutical products and pesticides toxicity associated with microplastics (polyvinyl chloride) in Artemia salina. Int. J. Environ. Res. Public Health 2021, 18, 10773. [Google Scholar] [CrossRef] [PubMed]
- Christudoss, A.C.; Chandrasekaran, N.; Mukherjee, A. Polystyrene nanoplastics alter the ecotoxicological effects of diclofenac on freshwater microalgae Scenedesmus obliquus. Environ. Sci. Process. Impacts 2024, 26, 56–70. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, W.; Sun, S.; Du, X.; Han, Y.; Shi, W.; Liu, G. Immunotoxicity and neurotoxicity of bisphenol A and microplastics alone or in combination to a bivalve species, Tegillarca granosa. Environ. Pollut. 2020, 265, 115115. [Google Scholar] [CrossRef]
- Zhou, W.; Tang, Y.; Du, X.; Han, Y.; Shi, W.; Sun, S.; Zhang, W.; Zheng, H.; Liu, G. Fine polystyrene microplastics render immune responses more vulnerable to two veterinary antibiotics in a bivalve species. Mar. Pollut. Bull. 2021, 164, 111995. [Google Scholar] [CrossRef]
- Brandts, I.; Teles, M.; Gonçalves, A.P.; Barreto, A.; Franco-Martinez, L.; Tvarijonaviciute, A.; Martins, M.A.; Soares, A.M.V.M.; Tort, L.; Oliveira, M. Effects of nanoplastics on Mytilus galloprovincialis after individual and combined exposure with carbamazepine. Sci. Total Environ. 2018, 643, 775–784. [Google Scholar] [CrossRef]
- Sun, W.; Meng, Z.; Li, R.; Zhang, R.; Jia, M.; Yan, S.; Tian, S.; Zhou, Z.; Zhu, W. Joint effects of microplastic and dufulin on bioaccumulation, oxidative stress and metabolic profile of the earthworm (Eisenia fetida). Chemosphere 2021, 263, 128171. [Google Scholar] [CrossRef]
- Jiang, H.; Li, Q.; Sun, J.; Mao, Y.; Liu, X.; Que, S.; Yu, W.; Kan, Y. The Adsorption and desorption behavior of Bisphenol A on five microplastics under simulated gastrointestinal conditions. Water Air Soil. Pollut. 2023, 234, 106. [Google Scholar] [CrossRef]
- Bråte, I.L.; Halsband, C.; Allan, I.; Thomas, K. Report Made for the Norwegian Environment Agency: Microplastics in Marine Environments; Occurrence, Distribution and Effects. 2014. Norwegian Institute for Water Research. Available online: https://www.miljodirektoratet.no/globalassets/publikasjoner/m319/m319.pdf (accessed on 15 September 2024).
- Nobre, C.R.; Moreno, B.B.; Alves, A.V.; de Lima Rosa, J.; da Rosa Franco, H.; Abessa, D.M.d.S.; Maranho, L.A.; Choueri, R.B.; Gusso-Choueri, P.K.; Pereira, C.D.S. Effects of microplastics associated with triclosan on the oyster Crassostrea brasiliana: An integrated biomarker approach. Arch. Environ. Contam. Toxicol. 2020, 79, 101–110. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. WAO J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Weng, Y.; Huang, Z.; Zhao, Y.; Jin, Y. Combined hepatotoxicity of imidacloprid and microplastics in adult zebrafish: Endpoints at gene transcription. CBPC 2021, 246, 109043. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wang, S.; Zhao, F.; Wang, S.; Liu, F.; Liu, G. Joint toxicity of microplastics with triclosan to marine microalgae Skeletonema costatum. Environ. Pollut. 2019, 246, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Nugnes, R.; Russo, C.; Lavorgna, M.; Orlo, E.; Kundi, M.; Isidori, M. Polystyrene microplastic particles in combination with pesticides and antiviral drugs: Toxicity and genotoxicity in Ceriodaphnia dubia. Environ. Pollut. 2022, 313, 120088. [Google Scholar] [CrossRef]
- Felten, V.; Toumi, H.; Masfaraud, J.-F.; Billoir, E.; Camara, B.I.; Férard, J.-F. Microplastics enhance Daphnia magna sensitivity to the pyrethroid insecticide deltamethrin: Effects on life history traits. Sci. Total Environ. 2020, 714, 136567. [Google Scholar] [CrossRef]


| Group | Treatment |
|---|---|
| Negative control | 0.000 μg/mL of fresh water |
| Positive control | 0.100 µg/mL of mytomicin-C |
| Culture 1 | 0.100 μg/mL of BPA |
| Culture 2 | 200 μg/mL of PS |
| Culture 3 | 100 μg/mL of PS |
| Culture 4 | 50 μg/mL of PS |
| Culture 5 | 25 μg/mL of PS |
| Culture 6 | 200 μg/mL of PS + 0.100 μg/mL of BPA |
| Culture 7 | 100 μg/mL of PS + 0.100 μg/mL of BPA |
| Culture 8 | 50 μg/mL of PS + 0.100 μg/mL of BPA |
| Culture 9 | 25 μg/mL of PS + 0.100 μg/mL of BPA |
| Group—20 Individuals per Container | Treatment |
|---|---|
| Controls | 0.000 μg/mL of fresh water |
| Group 1 | 0.100 μg/mL of BPA |
| Group 2 | 200 μg/mL of PS |
| Group 3 | 100 μg/mL of PS |
| Group 4 | 50 μg/mL of PS |
| Group 5 | 25 μg/mL of PS |
| Group 6 | 200 μg/mL of PS + 0.100 μg/mL of BPA |
| Group 7 | 100 μg/mL of PS + 0.100 μg/mL of BPA |
| Group 8 | 50 μg/mL of PS + 0.100 μg/mL of BPA |
| Group 9 | 25 μg/mL of PS + 0.100 μg/mL of BPA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santovito, A.; Lambertini, M.; Nota, A. In Vitro and In Vivo Genotoxicity of Polystyrene Microplastics: Evaluation of a Possible Synergistic Action with Bisphenol A. J. Xenobiot. 2024, 14, 1415-1431. https://doi.org/10.3390/jox14040079
Santovito A, Lambertini M, Nota A. In Vitro and In Vivo Genotoxicity of Polystyrene Microplastics: Evaluation of a Possible Synergistic Action with Bisphenol A. Journal of Xenobiotics. 2024; 14(4):1415-1431. https://doi.org/10.3390/jox14040079
Chicago/Turabian StyleSantovito, Alfredo, Mattia Lambertini, and Alessandro Nota. 2024. "In Vitro and In Vivo Genotoxicity of Polystyrene Microplastics: Evaluation of a Possible Synergistic Action with Bisphenol A" Journal of Xenobiotics 14, no. 4: 1415-1431. https://doi.org/10.3390/jox14040079
APA StyleSantovito, A., Lambertini, M., & Nota, A. (2024). In Vitro and In Vivo Genotoxicity of Polystyrene Microplastics: Evaluation of a Possible Synergistic Action with Bisphenol A. Journal of Xenobiotics, 14(4), 1415-1431. https://doi.org/10.3390/jox14040079







