Enzymatic Activity in Turkey, Duck, Quail and Chicken Liver Microsomes Against Four Human Cytochrome P450 Prototype Substrates and Aflatoxin B1
Abstract
:Introduction
Materials and Methods
Reagents
Liver samples
Microsome extraction
Microsomal incubations
Enzymatic activity of selected CYP prototype substrates
Chromatographic conditions
Determination of enzymatic kinetic parameters
Statistical analysis
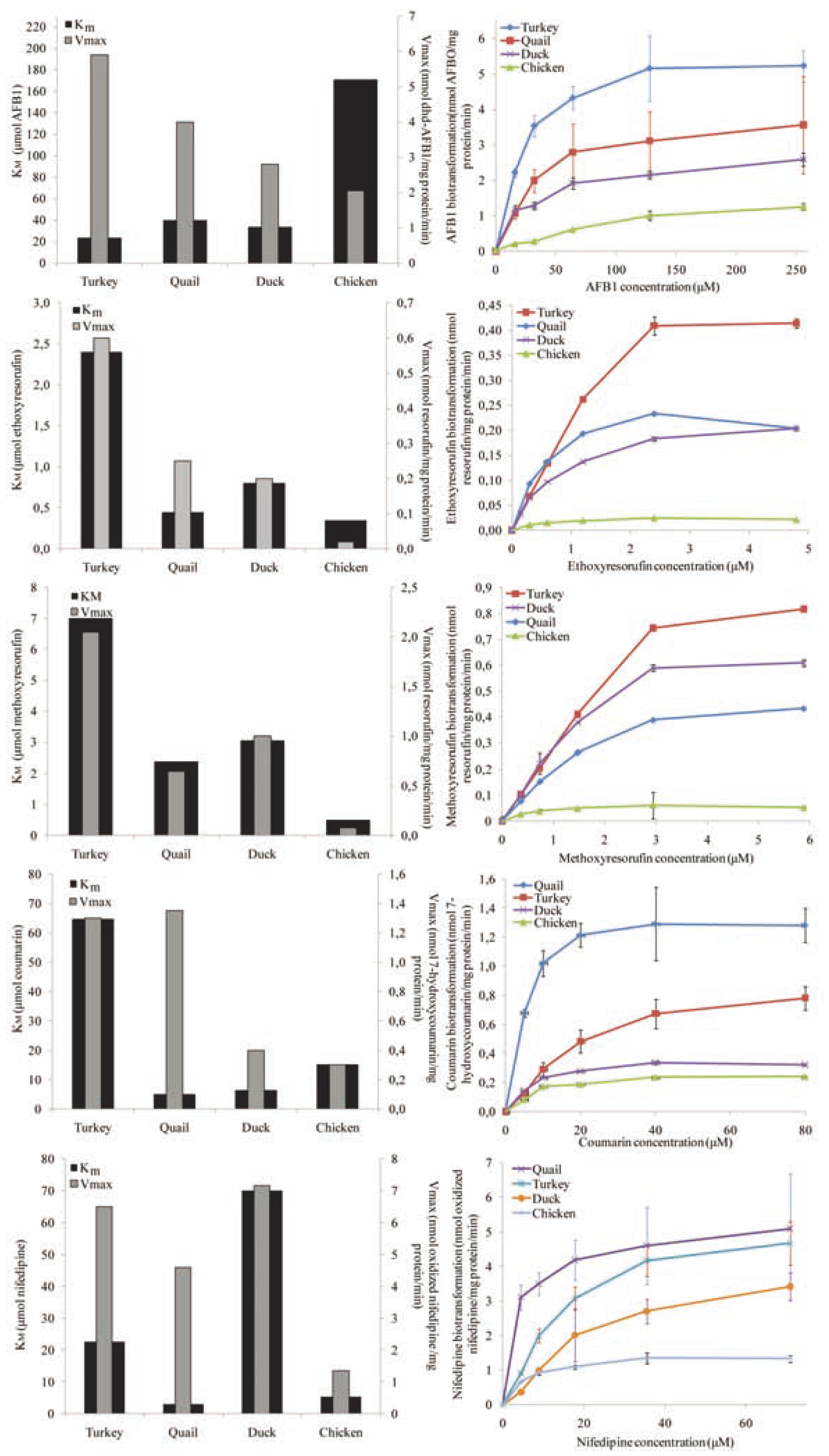
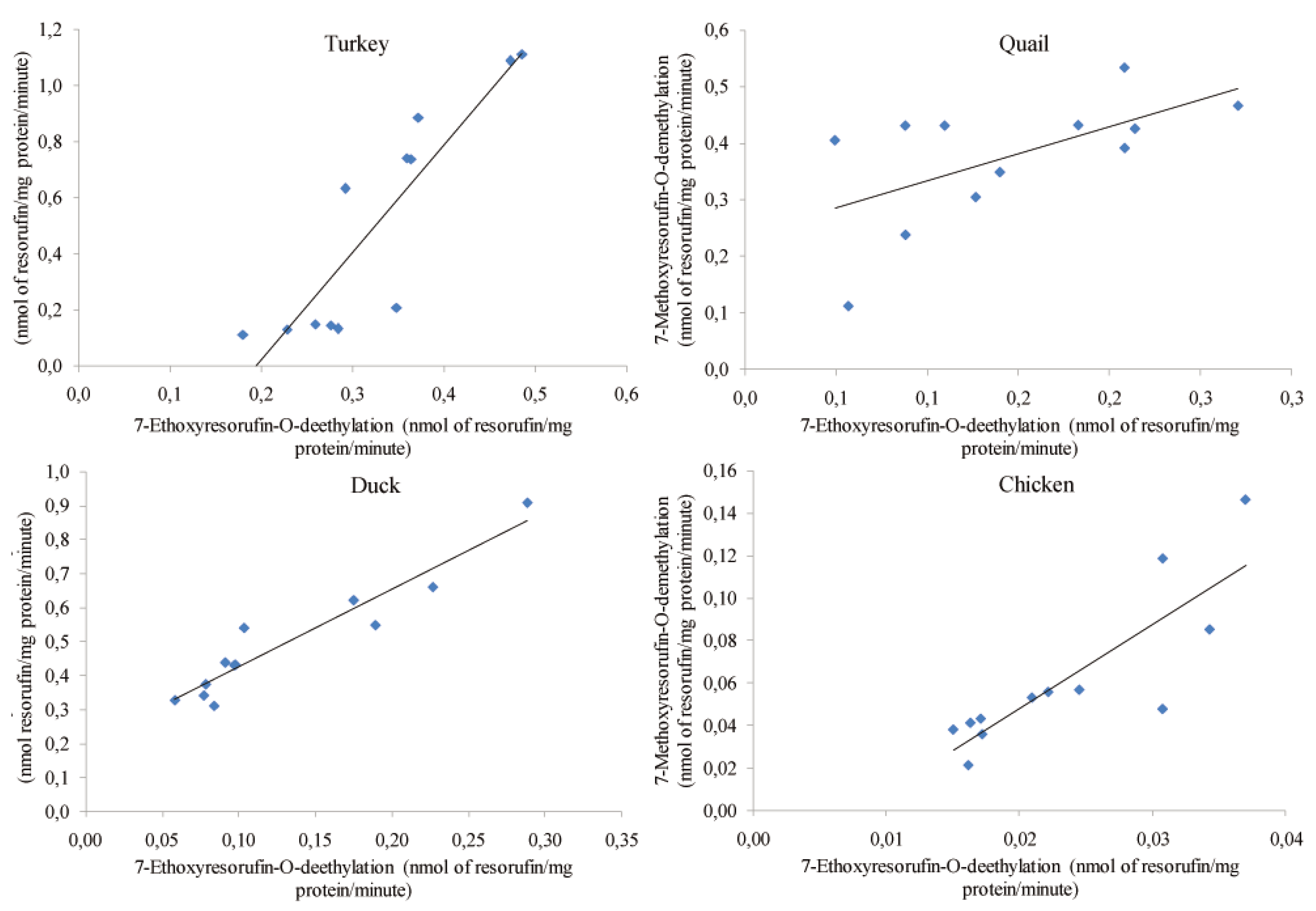
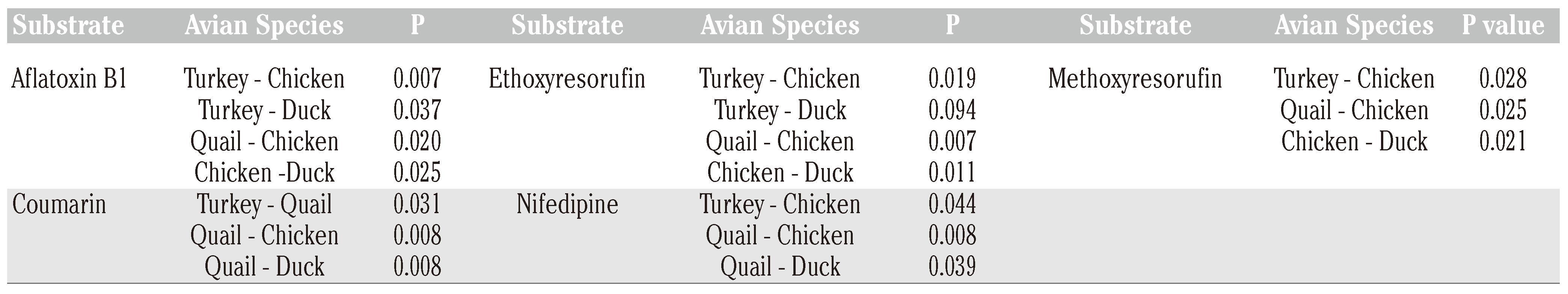
Results
Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Josephy, P.D. Molecular toxicology; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Chauret, N.; Gauthier, A.; Martin, J.; Nicoll-Griffith, D.A. In vitro comparison of cytochrome P450-mediated metabolic activities in human, dog, cat, and horse. Drug Metab. Dispos. 1997, 25, 1130–1136. [Google Scholar] [PubMed]
- Renauld, A.E.; Melancon, M.J.; Sordillo, L.M. Identification of in vitro cytochrome P450 modulators to detect induction by proto- type inducers in the mallard duckling (Anas platyrhynchos). Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1999, 122, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Halpert, J.R.; Guengerich, F.P.; Bend, J.R.; Correia, M.A. Selective inhibitors of cytochromes P450. Toxicol. Appl. Pharmacol. 1994, 125, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Pelkonen, O.; Turpeinen, M.; Hakkola, J.; et al. Inhibition and induction of human cytochrome P450 enzymes: Current status. Arch. Toxicol. 2008, 82, 667–715. [Google Scholar] [CrossRef]
- Henderson, M.C.; Miranda, C.L.; Stevens, J.U.F.; Deinzer, M.L.; Buhler, D.R. In vitro inhibition of human P450 enzymes by prenylated flavonoids from hops, Humulus lupulus. Xenobiotica 2000, 30, 235–251. [Google Scholar] [CrossRef]
- Donato, M.T.; Jiménez, N.; Castell, J.V.; Gómez-Lechón, M.J. Fluorescence-based assays for screening nine cytochrome P450 (P450) activities in intact cells expressing indi- vidual human P450 enzymes. Drug Metab. Dispos. 2004, 32, 699–706. [Google Scholar] [CrossRef]
- Peng, J.Z.; Remmel, R.P.; Sawchuk, R.J. Inhibition of murine cytochrome P4501A by tacrine: In vitro studies. Drug Metab. Dispos. 2004, 32, 805–812. [Google Scholar] [CrossRef]
- Appiah-Opong, R.; Commandeur, J.N.; van Vugt-Lussenburg, B.; Vermeulen, N.P. Inhibition of human recombinant cytochrome P450s by curcumin and cur- cumin decomposition products. Toxicology 2007, 235, 83–91. [Google Scholar] [CrossRef]
- McManus, M.E.; Boobis, A.R.; Minchin, R.F.; et al. Relationship between oxidative metab- olism of 2-acetylaminofluorene, debriso- quine, bufuralol, and aldrin in human liver microsomes. Cancer Res. 1984, 44, 5692–5697. [Google Scholar]
- Kenworthy, K.E.; Bloomer, J.C.; Clarke, S.E.; Houston, J.B. CYP3A4 drug interactions: Correlations of 10 in vitro probe substrates. Br. J. Clin. Pharmacol. 1999, 48, 716–727. [Google Scholar] [CrossRef]
- Sy, S.K.; Tang, B.; Pastrakuljic, A.; et al. Detail characterization of experimental derived human hepatic CYP1A1 activity and expression using differential inhibition of ethoxyresorufin O-deethylation by fluvox- amine. Eur. J. Pharmacol. 2001, 57, 377–386. [Google Scholar]
- Quintieri, L.; Geroni, C.; Fantin, M.; et al. Formation and antitumor activity of PNU- 159682, a major metabolite of nemoru- bicin in human liver microsomes. Clin. Cancer Res. 2005, 11, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Díaz, G.J.; Squires, E.J. Metabolism of 3- methylindole by porcine liver microsomes: Responsible cytochrome P450 enzymes. Toxicol. Sci. 2000, 55, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Šavlík, M.; Poláčkcová, L.; Szotáková, B.; et al. Activities of biotransformation enzymes in pheasant (Phasianus colchicus) and their modulation by in vivo administration of mebendazole and ubendazole. Res. Vet. Sci. 2007, 83, 20–26. [Google Scholar] [CrossRef]
- International Chicken Genome Sequencing Consortium. Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature 2004, 432, 695–716. [Google Scholar] [CrossRef]
- Dalloul, R.A.; Long, J.A.; Zimin, A.V.; et al. Multi-platform next-generation sequencing of the domestic turkey (Meleagris gallopavo): Genome assembly and analysis. PLoS Biol. 2010, 8, e1000475. [Google Scholar] [CrossRef]
- Leeson, S.; Díaz, G.J.; Summers, J.D. Poultry metabolic disorders and mycotoxins; University Books: Guelph, ON, USA, 1995. [Google Scholar]
- Hussein, H.S.; Brasel, J.M. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicol. 2001, 167, 101–134. [Google Scholar] [CrossRef]
- Eaton, D.L.; Gallagher, E.P. Mechanisms of aflatoxin carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 1994, 34, 135–172. [Google Scholar] [CrossRef]
- Verma, R.J. Aflatoxin causes DNA damage. Int. J. Hum. Genet. 2004, 4, 231–236. [Google Scholar] [CrossRef]
- Do, J.H.; Choi, D. Aflatoxins: Detection, toxicity, and biosynthesis. Biotechnol. Bioprocess. Eng. 2007, 12, 585–593. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Philpott, M. Nutrition and mutagenesis. Annu. Rev. Nutr. 2008, 28, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Redinbaugh, M.G.; Turley, R.B. Adaptation of the bicinchoninic acid protein assay for the use with microtiter plates and sucrose gradient fractions. Anal. Biochem. 1986, 153, 267–71. [Google Scholar] [CrossRef] [PubMed]
- Busby, W.F.; Ackermann, J.M.; Crespi, C.L. Effect of methanol, ethanol, dimethyl sulphoxide and acetronitrile on in vitro activities of cDNA-expressed human cytochromes P-450. Drug Metab. Dispos. 1999, 27, 246–249. [Google Scholar] [PubMed]
- Blanchard, J. Evaluation of the relate efficacy of various techniques for deproteiniz- ing plasma samples prior to high-perform- ance liquid chromatography analysis. J. Chromatogr. 1981, 226, 455–460. [Google Scholar] [CrossRef]
- Bisswanger, H. Enzyme kinetics. In Principles and methods, 2nd ed.; Revised and Updated Edition; Wiley-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Marangoni, A.G. Enzyme kinetics. A modern approach; Wiley Interscience. John Wiley & Sons, Inc: Hoboken, NJ, USA, 2008. [Google Scholar]
- Kakkar, T.; Boxenbaum, H.; Mayersohn, M. Estimation of Ki in a competitive enzyme- inhibition model: Comparisons among three methods of data analysis. Drug Metab. Dispos. 1999, 27, 756–762. [Google Scholar]
- SAS Institute Inc. SAS/STAT® 9.2 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2008. [Google Scholar]
- Hanioka, N.; Tatarazako, N.; Jinno, H.; et al. Determination of cytochrome P450 1A activities in mammalian liver microsomes by high-performance liquid chromatogra- phy with fluorescence detection. J. Chromatogr. B Biomed. Sci. Appl. 2000, 744, 399–406. [Google Scholar] [CrossRef]
- Yamasaki, H.; Tanaka, M.; Shimada, T. Highly sensitive high-performance liquid chromatographic assay for coumarin 7-hydrox-ylation and 7-ethoxycoumarin O-deethyla- tion by human liver cytochrome P450 enzymes. J. Chromatogr. B Biomed. Sci. Appl. 1999, 721, 13–19. [Google Scholar] [CrossRef]
- Patki, K.C.; von Moltke, L.L.; Greenblatt, D.J. In vitro metabolism of midazolam, triazolam, nifedipine, and testosterone by human liver microsomes and recombinant cytochromes P450: Role of CYP3A4 and CYP3A5. Drug Metab. Dispos. 2003, 31, 938–944. [Google Scholar] [CrossRef]
- Klaassen, C.D. (Ed). Casarett and Doull´s Toxicology-The Basic Science of Poisons, 7th ed.; McGraw Hill Medical: New York, NY, USA, 2008; pp. 180–190. [Google Scholar]
- Lozano, M.C.; Díaz, G.J. Microsomal and cytosolic bioactivation of aflatoxin B1 in four poultry species. Br. Poult. Sci. 2006, 47, 734–741. [Google Scholar] [CrossRef]
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© Copyright H.W. Murcia et al., 2011 This work is licensed under a Creative Commons Attribution NonCommercial 3.0 License. (CC BYNC 3.0).
Share and Cite
Murcia, H.W.; Díaz, G.J.; Cepeda, S.M. Enzymatic Activity in Turkey, Duck, Quail and Chicken Liver Microsomes Against Four Human Cytochrome P450 Prototype Substrates and Aflatoxin B1. J. Xenobiot. 2011, 1, e4. https://doi.org/10.4081/xeno.2011.e4
Murcia HW, Díaz GJ, Cepeda SM. Enzymatic Activity in Turkey, Duck, Quail and Chicken Liver Microsomes Against Four Human Cytochrome P450 Prototype Substrates and Aflatoxin B1. Journal of Xenobiotics. 2011; 1(1):e4. https://doi.org/10.4081/xeno.2011.e4
Chicago/Turabian StyleMurcia, Hansen W., Gonzalo J. Díaz, and Sandra Milena Cepeda. 2011. "Enzymatic Activity in Turkey, Duck, Quail and Chicken Liver Microsomes Against Four Human Cytochrome P450 Prototype Substrates and Aflatoxin B1" Journal of Xenobiotics 1, no. 1: e4. https://doi.org/10.4081/xeno.2011.e4
APA StyleMurcia, H. W., Díaz, G. J., & Cepeda, S. M. (2011). Enzymatic Activity in Turkey, Duck, Quail and Chicken Liver Microsomes Against Four Human Cytochrome P450 Prototype Substrates and Aflatoxin B1. Journal of Xenobiotics, 1(1), e4. https://doi.org/10.4081/xeno.2011.e4




