Psychometric Properties of the Serbian Teen Version of the Problem Areas in Diabetes Scale—A Validation Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting and Participants
2.3. Sample Size Calculation
- For the Wilcoxon rank-sum test, with an effect size (d) of 0.05, study power (1 − β) of 0.80, α error of 0.05, and an allocation ratio (n2/n1) of 9, the minimum sample size required was n = 368 (with 37 participants in group 1 and 331 in group 2).
- For linear multiple regression, assuming a small effect size (f2 = 0.03), a study power (1 − β) of 0.80, an α error of 0.05, and three predictors, the minimum sample size required was also n = 368.
- Our participant sample size, n = 374, was deemed sufficient for analysing the psychometric properties of the PAID-T. Recommendations for the ratio of items to respondents in validation studies vary from 1:10 to 1:5 [45]. Additionally, the minimum sample size for conducting CFA is recommended to be larger than 200 [46,47]. In our study, the total sample was divided into two independent subsamples, both of which were sufficiently large for factor analysis. The first subsample (n = 140) was used to perform EFA, allowing us to examine the underlying factor structure of the Serbian version of the PAID-T. The second subsample (n = 234) was then used to conduct CFA to validate the factor solution identified in the EFA. This procedure ensured that the EFA and CFA were performed on separate datasets, thereby minimising the risk of overfitting and increasing the robustness of our psychometric evaluation.
2.4. Survey and Data Collection
- The development of Serbian versions of the PAID-T and DES-28 was undertaken collaboratively across three institutions (details will be elaborated further in the manuscript).
- Adolescents with T1D aged 13 to 18 (n = 374) who participated in this study attended routine check-ups or were hospitalised for various reasons, including transitioning from human insulin therapy to insulin analogues, receiving education on insulin pump usage, experiencing a deterioration in metabolic control, such as an episode of DKA, and undergoing re-education about diabetes management. All participants were in good physical and mental health at the time of the study. Adolescents with T1D were hospitalised in the Department of Endocrinology at two tertiary institutions: The Mother and Child Health Care Institute of Serbia “Dr. Vukan Čupić” and the University Children’s Hospital in Belgrade, as well as in the Department for treatment, education, and rehabilitation of children and youth at the Specialized “Bukovička Banja” Hospital in Arandjelovac, a secondary facility. Paediatric endocrinologists, co-authors of this report, and paediatric nurses referred adolescents with T1D and their parents/guardians to the principal investigator’s office.
- Of the 374 adolescents with T1D enrolled in the study, n = 289 participants agreed to complete questionnaires in two stages for test–retest analysis: initially at the beginning of the study and again 10 to 15 days later, when the Serbian versions of the PAID-T and DES-28 were re-administered. Additionally, n = 85 adolescents consented to complete questionnaires during a single stage, either during routine check-ups or hospitalisation.
2.5. Measures
2.5.1. Sociodemographic Questionnaire
2.5.2. Clinical Data
2.5.3. Assessment of Adherence to Self-Care Activities
- SMBG group: Use of SMBG, achievement of recommended target glucose values (4.0–10.0 mmol/L [70–180 mg/dL], with a narrower fasting target of 4.0–8.0 mmol/L [70–144 mg/dL]), and reporting the response option indicating glucose monitoring at least three times daily before meals, along with postprandial checks (1.5–3 h after meals) at least three times per week [1,50].
2.5.4. Perceived Diabetes-Distress and Self-Efficacy
2.5.5. Perceived Diabetes-Distress
2.5.6. Perceived Self-Efficacy
2.6. Data Analysis
3. Results
3.1. Sociodemographic and Clinical Characteristics of Participants
3.2. Description of Adherence to Self-Care Activities
3.3. Descriptive Statistics for Psychosocial Characteristics of Adolescents with T1D
3.4. Construct Validity of PAID-T: Exploratory and Confirmatory Factor Analysis
3.4.1. Determining the Number of Significant Factors
3.4.2. Exploratory Factor Analysis
3.4.3. Confirmatory Factor Analysis
3.5. Reliability: PAID-T and DES-28
3.5.1. Reliability: PAID-T
3.5.2. Reliability: DES-28
3.6. Concurrent Validity: PAID-T
3.7. Multiple Regression Analysis
4. Discussion
4.1. Perspectives for Clinical Practice
- (1)
- Augmenting the formal education of primary healthcare providers—particularly paediatricians and paediatric nurses—with additional training focused on the recognition of diabetes-related emotional distress and current guidelines for optimal disease control and self-management.
- (2)
- Implementing screening procedures to identify diabetes-specific emotional distress, thereby safeguarding and promoting adolescents’ mental health.
- (3)
- Introducing assessments to evaluate adolescents’ subjective beliefs regarding their self-management capabilities within various life contexts, including family and school environments, as well as their readiness for transitioning from paediatric to adult healthcare services.
4.2. Strengths and Weaknesses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Public Involvement Statement
Guidelines and Standards Statement
Use of Artificial Intelligence
Acknowledgments
Conflicts of Interest
Abbreviations
| β | The standardised beta coefficients |
| ADaRC | Assessing dissatisfaction and readiness to change |
| CFA | Confirmatory factor analysis |
| CFI | Comparative fit index |
| CGM | Continuous glucose monitoring |
| CI | Confidence interval |
| DES | Diabetes empowerment scale/score |
| df | Degrees of freedom |
| DSNs | Diabetes specialist nurses |
| G | One general factor |
| GAD-65 | Glutamic acid decarboxylase-65 |
| EFA | Exploratory factor analysis |
| EMA | European Medicine Agency |
| HbA1c | Glycosylated haemoglobin |
| IAA | Insulin autoantibodies |
| IA-2A | Insulinoma-associated-2 autoantibodies |
| ICC | Intraclass correlation coefficients |
| ISPAD | International Society for Pediatric and Adolescent Diabetes |
| Mad | Median absolute deviation |
| MDRC | Michigan Diabetes Research Center |
| MCDTR | Michigan Center for Diabetes Translational Research |
| MPSAD | Managing the psychosocial aspects of diabetes |
| OLS | Ordinary least squares |
| PAID | Problem areas in diabetes |
| RMSEA | Root mean square error of approximation |
| SaADG | Setting and achieving diabetes goals |
| SD | Standard deviation |
| SMBG | Self-monitoring of blood glucose |
| SRMR | Standardised root mean square residual |
| STROBE | The Strengthening the Reporting of Observational Studies in Epidemiology |
| ρ | Spearman’s rank correlation coefficient |
| T1D | Type 1 diabetes |
| TIR | Time in range |
| TLI | Tucker–Lewis index |
| W | Wilcoxon rank-sum test |
| Χ2 | Chi-square test |
| ZnT8 | Zinc transporter 8 (ZnT8) |
References
- Smudja, M.; Milenković, T.; Minaković, I.; Zdravković, V.; Javorac, J.; Milutinović, D. Self-care activities in pediatric patients with type 1 diabetes mellitus. PLoS ONE 2024, 19, e0300055. [Google Scholar] [CrossRef]
- Institute for Public Health of Serbia. “Dr. Milan Jovanovic Batut”. Serbian Diabetes Registry 2021. Report No.16. Incidence and Mortality of Diabetes in Serbia (Page 47). Available online: https://www.batut.org.rs/download/publikacije/Incidencija%20i%20mortalitet%20od%20dijabetesa%20u%20Srbiji%202021.pdf (accessed on 28 June 2024).
- Carr, A.L.J.; Evans-Molina, C.; Oram, R.A. Precision medicine in type 1 diabetes. Diabetologia 2022, 65, 1854–1866. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, S.; Chopra, A.; Nagendra, L.; Kalra, S.; Bhattacharya, S. Teplizumab in Type 1 Diabetes Mellitus: An Updated Review. TouchREV. Endocrinol. 2023, 19, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, L.; Kohls, M.; Arnolds, S.; Achenbach, P.; Bergholdt, R.; Bonifacio, E.; Bosi, E.; Gündert, M.; Hoefelschweiger, B.K.; Hummel, S.; et al. EDENT1FI consortium. EDENT1FI Master Protocol for screening of presymptomatic early-stage type 1 diabetes in children and adolescents. BMJ Open 2025, 15, e088522. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, R.M.; Eshtehardi, S.S.; Anderson, B.J.; Weissberg-Benchell, J.A.; Hilliard, M.E. Profiles of depressive symptoms and diabetes distress in preadolescents with type 1 diabetes. Can. J. Diabetes 2021, 45, 436–443. [Google Scholar] [CrossRef]
- Pantanetti, P.; Cangelosi, G.; Morales Palomares, S.; Ferrara, G.; Biondini, F.; Mancin, S.; Caggianelli, G.; Parozzi, M.; Sguanci, M.; Petrelli, F. Real-World Life Analysis of a Continuous Glucose Monitoring and Smart Insulin Pen System in Type 1 Diabetes: A Cohort Study. Diabetology 2025, 6, 7. [Google Scholar] [CrossRef]
- Hagger, V.; Hendrieckx, C.; Cameron, F.; Pouwer, F.; Skinner, T.C.; Speight, J. Diabetes distress is more strongly associated with HbA1c than depressive symptoms in adolescents with type 1 diabetes: Results from Diabetes MILES Youth-Australia. Pediatr. Diabetes 2018, 19, 840–847. [Google Scholar] [CrossRef]
- Stahl-Pehe, A.; Glaubitz, L.; Bächle, C.; Lange, K.; Castillo, K.; Tönnies, T.; Yossa, R.; Holl, R.W.; Rosenbauer, J. Diabetes distress in young adults with early-onset Type 1 diabetes and its prospective relationship with HbA1c and health status. Diabet. Med. 2019, 36, 836–846. [Google Scholar] [CrossRef]
- Aljawarneh, Y.M.; Wood, G.L.; Wardell, D.W.; Al-Jarrah, M.D. The associations between physical activity, health-related quality of life, regimen adherence, and glycemic control in adolescents with type 1 diabetes: A cross-sectional study. Prim. Care Diabetes 2023, 17, 392–400. [Google Scholar] [CrossRef]
- Vesco, A.T.; Howard, K.R.; Anderson, L.M.; Papadakis, J.L.; Hood, K.K.; Weissberg-Benchell, J. Examining indirect effects of anxiety on glycated hemoglobin via automatic negative thinking and diabetes-specific distress in adolescents with type 1 diabetes. Can. J. Diabetes 2021, 45, 473–480. [Google Scholar] [CrossRef]
- Weissberg-Benchell, J.; Rausch, J.; Iturralde, E.; Jedraszko, A.; Hood, K. A randomised clinical trial aimed at preventing poor psychosocial and glycemic outcomes in teens with type 1 diabetes (T1D). Contemp. Clin. Trials 2016, 49, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Weissberg-Benchell, J.; Antisdel-Lomaglio, J. Diabetes-specific emotional distress among adolescents: Feasibility, reliability, and validity of the problem areas in diabetes-teen version. Pediatr. Diabetes 2011, 12, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Lica, M.M.; Papai, A.; Salcudean, A.; Crainic, M.; Covaciu, C.G.; Mihai, A. Assessment of psychopathology in adolescents with insulin-dependent diabetes (IDD) and the impact on treatment management. Children 2021, 8, 414. [Google Scholar] [CrossRef]
- Geneti, Y.; Wondwossen, K.; Adimasu, M.; Deressa, D.; Aga, F.; Lami, M.; Abdisa, L.; Abebe, S.; Dinku, H. Adherence to Diabetes Self-Management and Its Associated Factors Among Adolescents Living with Type 1 Diabetes at Public Hospitals in Addis Ababa, Ethiopia: A Cross-Sectional Study. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 659–670. [Google Scholar] [CrossRef]
- Daniel, L.; Haile, D.; Egata, G. Disordered eating behaviours and body shape dissatisfaction among adolescents with type 1 diabetes: A cross sectional study. J. Eat. Disord. 2023, 11, 169. [Google Scholar] [CrossRef]
- Khadilkar, A.; Oza, C. Glycaemic control in youth and young adults: Challenges and solutions. Diabetes Metab. Syndr. Obes. 2022, 15, 121–129. [Google Scholar] [CrossRef]
- Smudja, M.; Milenkovic, T.; Minakovic, I.; Zdravkovic, V.; Mitic, S.; Milutinovic, D. Determinants of health-related quality of life in children and adolescents living with type 1 diabetes mellitus during the COVID-19 pandemic. Nurs. Open 2023, 10, 7394–7410. [Google Scholar] [CrossRef]
- Zahran, N.A.; Jadidi, S. Pediatric Hyperglycemic Hyperosmolar Syndrome: A Comprehensive Approach to Diagnosis, Management, and Complications Utilising Novel Summarising Acronyms. Children 2023, 10, 1773. [Google Scholar] [CrossRef]
- Pathak, V.; Pathak, N.M.; O’Neill, C.L.; Guduric-Fuchs, J.; Medina, R.J. Therapies for type 1 diabetes: Current scenario and future perspectives. Clin. Med. Insights Endocrinol. Diabetes 2019, 12, 1179551419844521. [Google Scholar] [CrossRef]
- Alassaf, A.; Odeh, R.; Gharaibeh, L.; Ibrahim, S.; Ajlouni, K. Personal and clinical predictors of poor metabolic control in children with type 1 diabetes in Jordan. J. Diabetes Res. 2019, 1, 4039792. [Google Scholar] [CrossRef]
- Ispriantari, A.; Agustina, R.; Konlan, K.D.; Lee, H. Family-centered interventions for children and adolescents with type 1 diabetes mellitus: An integrative review. Child. Health Nurs. Res. 2023, 29, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Montali, L.; Zulato, E.; Cornara, M.; Ausili, D.; Luciani, M. Barriers and facilitators of type 1 diabetes self-care in adolescents and young adults. J. Pediatr. Nurs. 2022, 62, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Survonen, A.; Salanterä, S.; Näntö-Salonen, K.; Sigurdardottir, A.K.; Suhonen, R. The psychosocial self-efficacy in adolescents with type 1 diabetes. Nurs. Open 2019, 6, 514–525. [Google Scholar] [CrossRef]
- Devarajooh, C.; Chinna, K. Depression, distress and self-efficacy: The impact on diabetes self-care practices. PLoS ONE 2017, 12, e0175096. [Google Scholar] [CrossRef]
- Van Allen, J.; Noser, A.E.; Littlefield, A.K.; Seegan, P.L.; Clements, M.; Patton, S.R. Measuring self-efficacy in the context of pediatric diabetes management: Psychometric properties of the Self-Efficacy for Diabetes Scale. J. Pediatr. Psychol. 2018, 43, 143–151. [Google Scholar] [CrossRef]
- Nefs, G.; Feinn, R.; Chang, A.M.; Wagner, J. Longitudinal relations of sleep quality with depressive symptoms, diabetes distress and self-efficacy in young people with type 1 diabetes. J. Psychosom. Res. 2023, 173, 111457. [Google Scholar] [CrossRef]
- Alwadiy, F.; Mok, E.; Dasgupta, K.; Rahme, E.; Frei, J.; Nakhla, M. Association of self-efficacy, transition readiness and diabetes distress with glycemic control in adolescents with type 1 diabetes Preparing to transition to adult care. Can. J. Diabetes 2021, 45, 490–495. [Google Scholar] [CrossRef]
- Sharma, V.; Feldman, M.; Sharma, R. Telehealth Technologies in Diabetes Self-management and Education. J. Diabetes Sci. Technol. 2024, 18, 148–158. [Google Scholar] [CrossRef]
- Anderson, R.M.; Funnell, M.M.; Fitzgerald, J.T.; Marrero, D.G. The Diabetes Empowerment Scale: A measure of psychosocial self-efficacy. Diabetes Care 2000, 23, 739–743. [Google Scholar] [CrossRef]
- Mahjouri, M.Y.; Arzaghi, S.M.; Heshmat, R.; Khashayar, P.; Esfahani, E.N.; Larijani, B. Psychometric properties of the Iranian version of Diabetes Empowerment Scale (IR-DES-28). J. Diabetes Metab. Disord. 2012, 11, 4. [Google Scholar] [CrossRef][Green Version]
- Younes, Z.M.H.; Abuali, A.M.; Tabba, S.; Farooqi, M.H.; Hassoun, A.A.K. Prevalence of diabetes distress and depression and their association with glycemic control in adolescents with type 1 diabetes in Dubai, United Arab Emirates. Pediatr. Diabetes 2021, 22, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Goncerz, D.; Mazurek, E.; Piasny, M.; Surówka, A.; Starzyk, B.J.; Wójcik, M.; Makara-Studzińska, M. Depressive and anxiety symptoms in adolescents with type 1 diabetes—A single-centre observational study. Pediatr. Endocrinol. Diabetes Metab. 2023, 29, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.B.; Vesco, A.T.; Weil, L.E.G.; Evans, M.A.; Hood, K.K.; Weissberg-Benchell, J. Psychometric properties of the Problem Areas in Diabetes: Teen and parent of teen versions. J. Pediatr. Psychol. 2018, 43, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, Z.; Baig, A.M.; Khawaja, K.I.; Shabbir, S.; Afzal, Z. Diabetes distress among type 1 diabetic adolescents in a tertiary care hospital in Pakistan. Cureus 2022, 14, e32392. [Google Scholar] [CrossRef]
- Delamater, A.M.; de Wit, M.; McDarby, V.; Malik, J.A.; Hilliard, M.E.; Northam, E.; Acerini, C.L. ISPAD Clinical Practice Consensus Guidelines 2018: Psychological care of children and adolescents with type 1 diabetes. Pediatr. Diabetes 2018, 19 (Suppl. S27), 237–249. [Google Scholar] [CrossRef]
- Saßmann, H.; Kim-Dorner, S.J.; Framme, J.; Heidtmann, B.; Kapellen, T.; Kordonouri, O.; Krosta, K.M.E.; Pisarek, N.; Lange, K. Psychometric properties of the German teen and parent versions of the Problem Areas in Diabetes Scale (PAID). Psychol. Assess. 2023, 35, e31–e42. [Google Scholar] [CrossRef]
- Mianowska, B.; Fedorczak, A.; Michalak, A.; Pokora, W.; Barańska-Nowicka, I.; Wilczyńska, M.; Szadkowska, A. Diabetes Related Distress in Children with Type 1 Diabetes before and during the COVID-19 Lockdown in Spring 2020. Int. J. Environ. Res. Public Health 2021, 18, 8527. [Google Scholar] [CrossRef]
- Lee, S.L.; Wu, L.M.; Chou, Y.Y.; Lai, F.C.; Lin, S.Y. Developing the Chinese version problem areas in diabetes-teen for measuring diabetes distress in adolescents with type 1 diabetes. J. Pediatr. Nurs. 2022, 64, 143–150. [Google Scholar] [CrossRef]
- Weissberg-Benchell, J.; Shapiro, J.B.; Bryant, F.B.; Hood, K.K. Supporting Teen Problem-Solving (STEPS) 3 year outcomes: Preventing diabetes-specific emotional distress and depressive symptoms in adolescents with type 1 diabetes. J. Consult Clin. Psychol. 2020, 88, 1019–1031. [Google Scholar] [CrossRef]
- Luo, J.; Wang, H.; Li, X.; Zhou, Z.; Valimaki, M.; Whittemore, R.; Grey, M.; Guo, J. Factors associated with diabetes distress among adolescents with type 1 diabetes. J. Clin. Nurs. 2021, 30, 1893–1903. [Google Scholar] [CrossRef]
- Wild, D.; Grove, A.; Martin, M.; Eremenco, S.; McElroy, S.; Verjee-Lorenz, A.; Erikson, P. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: Report of the ISPOR task force for translation and cultural adaptation. Value Health 2005, 8, 94–104. [Google Scholar] [CrossRef]
- Strengthening the Reporting of Observational Studies in Epidemiology, Strobe. Available online: https://www.strobe-statement.org/ (accessed on 29 August 2024).
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Watson, R.; Thompson, D.R. Use of factor analysis in Journal of Advanced Nursing: Literature review. J. Adv. Nurs. 2006, 55, 330–341. [Google Scholar] [CrossRef]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. (Eds.) Structural equation modeling overview. In Multivariate Data Analysis, 7th ed.; Pearson Education Limited: London, UK, 2014; pp. 541–598. [Google Scholar]
- Kyriazos, T.A. Applied Psychometrics: Sample size and sample power considerations in factor analysis (EFA, CFA) and SEM in general. Psychology 2018, 9, 2207–2230. [Google Scholar] [CrossRef]
- Law on Patient Rights in the Republic of Serbia. “Official Gazette of the RS”, no. 45/2013 and 25/2019—National Law, Article 2, Paragraphs 4 and 5. Available online: https://www.paragraf.rs/propisi/zakon_o_pravima_pacijenata.html (accessed on 10 December 2023).
- Wang, R.H.; Hsu, H.C.; Kao, C.C.; Yang, Y.M.; Lee, Y.J.; Shin, S.J. Associations of changes in psychosocial factors and their interactions with diabetes distress in patients with type 2 diabetes: A longitudinal study. J. Adv. Nurs. 2017, 73, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- de Bock, M.; Codner, E.; Craig, M.E.; Huynh, T.; Maahs, D.M.; Mahmud, F.H.; Marcovecchio, L.; DiMeglio, L.A. ISPAD Clinical practice consensus guidelines 2022: Glycemic targets and glucose monitoring for children, adolescents, and young people with diabetes. Pediatr. Diabetes 2022, 23, 1270–1276. [Google Scholar] [CrossRef]
- Gonzalez-Estrada, E.; Villasenor-Alva, J.A. MvShapirotest, version 1.0; Generalised Shapiro-Wilk Test for Multivariate Normality; CRAN, 2013. Available online: https://CRAN.R-project.org/package=mvShapiroTest (accessed on 10 June 2024).
- Herve, M. RVAideMemoire: Testing and Plotting Procedures for Biostatistics. 2023. Available online: https://CRAN.R-project.org/package=RVAideMemoire (accessed on 10 June 2024).
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis, 7th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2010; pp. 90–231. [Google Scholar]
- Horn, J.L. A rationale and test for the number of factors in factor analysis. Psychometrika 1965, 30, 79–85. [Google Scholar] [CrossRef]
- Revelle, W.; Rocklin, T. Very simple structure: An alternative procedure for estimating the optimal number of interpretable factors. Multivar. Behav. Res. 1979, 14, 403–414. [Google Scholar] [CrossRef]
- Velicer, W.F. Determining the number of components from the matrix of partial correlations. Psychometrika 1976, 41, 321–327. [Google Scholar] [CrossRef]
- Revelle, W. Psych, version 2.5.6; Procedures for Psychological, Psychometric, and Personality Research, Northwestern University, Evanston, Illinois; CRAN, 2018. Available online: https://CRAN.R-project.org/package=psych (accessed on 10 June 2024).
- Rosseel, Y. lavaan: An R package for structural equation modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef]
- Epskamp, S. Semplot: Path Diagrams and Visual Analysis of Various SEM Packages’ Output. 2022. Available online: https://CRAN.R-project.org/package=semPlot (accessed on 10 June 2024).
- Hu, L.; Bentler, P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. A Multidiscip. J. 2009, 6, 1–55. [Google Scholar] [CrossRef]
- Hu, L.; Bentler, P.M. Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychol. Methods 1998, 3, 424–453. [Google Scholar] [CrossRef]
- Tavakol, M.; Dennick, R. Making sense of Cronbach’s alpha. Int. J. Med. Educ. 2011, 2, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Jorgensen, T.D.; Pornprasertmanit, S.; Schoemann, A.M.; Rosseel, Y.; Miller, P.; Quick, C.; Garnier-Villarreal, M.; Selig, J.; Boulton, A.; Preacher, K.; et al. Semtools, R package version 0.4-14.; Useful Tools for Structural Equation Modeling. 2016. Available online: https://CRAN.R-project.org/package=semTools (accessed on 10 June 2024).
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Medica 2012, 22, 276–282. [Google Scholar] [CrossRef] [PubMed Central]
- Macbeth, G.; Razumiejczyk, E.; Ledesma, R.D. Cliff’s Delta Calculator: A non-parametric effect size program for two groups of observations. Univ. Psychol. 2011, 10, 545–555. [Google Scholar]
- Meissel, K.; Yao, E.S. Using Cliff’s Delta as a non-parametric effect size measure: An accessible web app and R tutorial. Pract. Assess. Res. Eval. 2024, 29, 2. [Google Scholar] [CrossRef]
- Kassambara, A. Rstatix. Pipe-Friendly Framework for Basic Statistical Tests. 2023. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 10 June 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.R-project.org/ (accessed on 10 June 2024).
- Berry, E.; Lockhart, S.; Davies, M.; Lindsay, J.R.; Dempster, M. Diabetes distress: Understanding the hidden struggles of living with diabetes and exploring intervention strategies. Postgrad. Med. J. 2015, 91, 278–283. [Google Scholar] [CrossRef]
- Iturralde, E.; Rausch, J.R.; Weissberg-Benchell, J.; Hood, K.K. Diabetes-related emotional distress over time. Pediatrics 2019, 143, e20183011. [Google Scholar] [CrossRef]
- Li, L.; Xu, G.; Zhou, D.; Song, P.; Wang, Y.; Bian, G. Prevalences of parental and peer support and their independent associations with mental distress and unhealthy behaviours in 53 countries. Int. J. Public Health 2022, 67, 1604648. [Google Scholar] [CrossRef]
- Hood, K.K.; Iturralde, E.; Rausch, J.; Weissberg-Benchell, J. Preventing diabetes distress in adolescents with type 1 diabetes: Results 1 year after participation in the STePS program. Diabetes Care 2018, 41, 1623–1630. [Google Scholar] [CrossRef]
- Luo, D.; Cai, X.; Wang, H.; Wang, Y.; Xu, J. The role of peer social relationships in psychological distress and quality of life among adolescents with type 1 diabetes mellitus: A longitudinal study. BMC Psychiatry 2024, 24, 270. [Google Scholar] [CrossRef] [PubMed]
- Palladino, D.K.; Helgeson, V.S. Friends or foes? A review of peer influence on self-care and glycemic control in adolescents with type 1 diabetes. J. Pediatr. Psychol. 2012, 5, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Nannsen, A.Ø.; Kristensen, K.; Johansen, L.B.; Iken, M.K.; Madsen, M.; Pilgaard, K.A.; Grabowski, D.; Hangaard, S.; Schou, A.J.; Andersen, A. Management of Diabetes during School Hours: A Cross-Sectional Questionnaire Study in Denmark. Healthcare 2023, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Ješić, M.D.; Milenković, T.; Mitrović, K.; Todorović, S.; Zdravković, V.; Ješić, M.M.; Bošnjović-Tucaković, T.; Marković, S.; Vorgučin, I.; Stanković, S.; et al. Problems in diabetes management in school setting in children and adolescents with type 1 diabetes in Serbia. Vojnosanit. Pregl. 2016, 73, 273–276. [Google Scholar] [CrossRef]
- Lawrence, S.E.; Albanese-O’Neill, A.; Besançon, S.; Black, T.; Bratina, N.; Chaney, D.; Cogen, F.R.; Cummings, E.A.; Moreau, E.; Pierce, J.S.; et al. ISPAD Clinical Practice Consensus Guidelines 2022: Management and support of children and adolescents with diabetes in school. Pediatr. Diabetes 2022, 23, 1478–1495. [Google Scholar] [CrossRef]
- Sato, A.F.; Berlin, K.S.; Hains, A.A.; Davies, W.H.; Smothers, M.K.; Clifford, L.M.; Alemzadeh, R. Teacher support of adherence for adolescents with type 1 diabetes: Preferred teacher support behaviors and youths’ perceptions of support. Diabetes Educ. 2008, 34, 866–873. [Google Scholar] [CrossRef]
- Sabbah, M.M.; Hjazeen, A.A.; Arabiat, D. Adherence to diabetes management among school-aged children and adolescents living with type 1 diabetes in Jordan. J. Pediatr. Nurs. 2024, 74, 110–115. [Google Scholar] [CrossRef]
- Tilden, D.R.; Noser, A.E.; Jaser, S.S. Sedentary behavior and physical activity associated with psychosocial outcomes in adolescents with type 1 diabetes. Pediatr. Diabetes 2023, 2023, 1395466. [Google Scholar] [CrossRef]
- Araia, E.; King, R.M.; Pouwer, F.; Speight, J.; Hendrieckx, C. Psychological correlates of disordered eating in youth with type 1 diabetes: Results from diabetes MILES Youth-Australia. Pediatr. Diabetes 2020, 21, 664–672. [Google Scholar] [CrossRef]
- Markowitz, J.T.; Garvey, K.C.; Laffel, L.M. Developmental changes in the roles of patients and families in type 1 diabetes management. Curr. Diabetes Rev. 2015, 11, 231–238. [Google Scholar] [CrossRef]
- de Wit, M.; Gajewska, K.A.; Goethals, E.R.; McDarby, V.; Zhao, X.; Hapunda, G.; Delamater, A.M.; DiMeglio, L.A. ISPAD Clinical Practice Consensus Guidelines 2022: Psychological care of children, adolescents and young adults with diabetes. Pediatr. Diabetes 2022, 23, 1373–1389. [Google Scholar] [CrossRef]
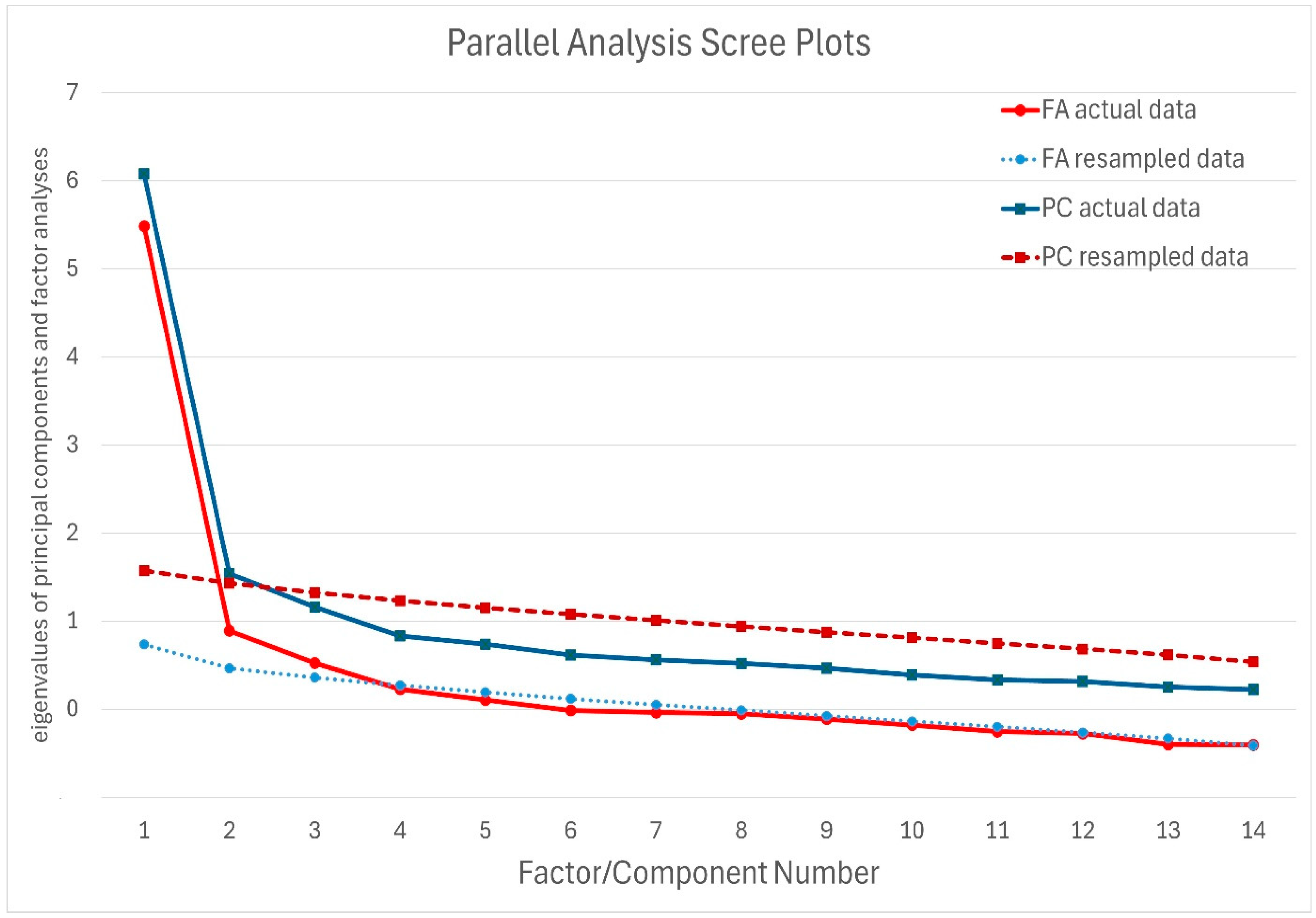
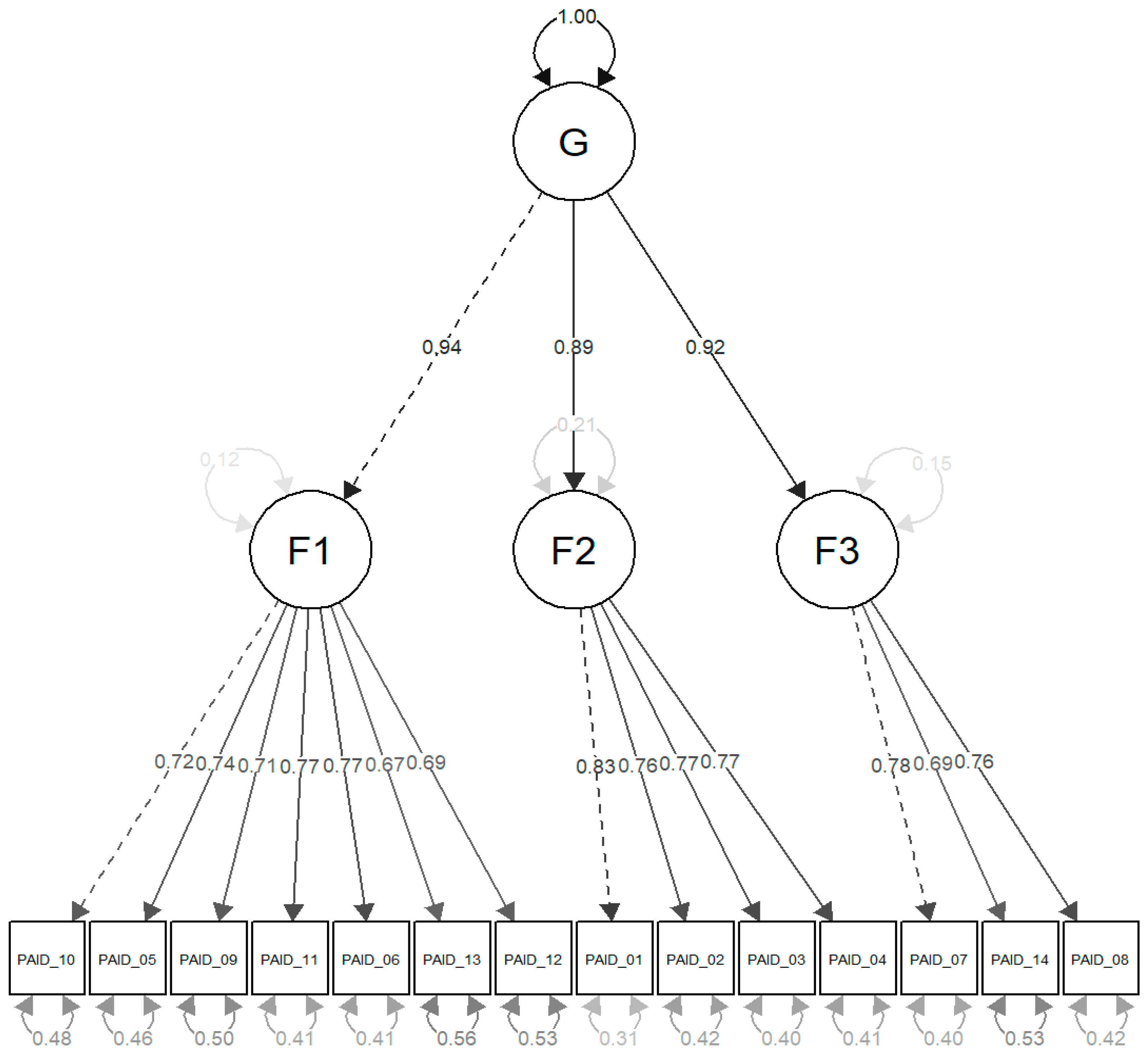
| Item | F1 | F2 | F3 |
|---|---|---|---|
| PAID_10 | 0.84 | ||
| PAID_05 | 0.81 | ||
| PAID_09 | 0.80 | ||
| PAID_11 | 0.64 | ||
| PAID_06 | 0.61 | 0.32 | |
| PAID_13 | 0.46 | 0.30 | |
| PAID_12 | 0.41 | ||
| PAID_01 | 0.87 | ||
| PAID_02 | 0.76 | ||
| PAID_03 | 0.75 | ||
| PAID_04 | 0.45 | ||
| PAID_07 | 0.89 | ||
| PAID_14 | 0.59 | ||
| PAID_08 | 0.46 |
| Factor | F1 | F2 | F3 |
|---|---|---|---|
| F1 | 1.00 | 0.62 | 0.62 |
| F2 | 0.62 | 1.00 | 0.43 |
| F3 | 0.62 | 0.43 | 1.00 |
| Model | χ2 | Df | P | CFI | TLI | RMSEA | Lower | Upper | RMSEA p | SRMR |
|---|---|---|---|---|---|---|---|---|---|---|
| Single-factor model | 205.17 | 77.00 | 0.00 | 0.90 | 0.88 | 0.10 | 0.08 | 0.12 | 0.00 | 0.06 |
| Three-factor model | 141.09 | 74.00 | 0.00 | 0.95 | 0.93 | 0.08 | 0.06 | 0.09 | 0.02 | 0.05 |
| Hierarchical three-factor model | 141.09 | 74.00 | 0.00 | 0.95 | 0.93 | 0.08 | 0.06 | 0.09 | 0.02 | 0.05 |
| Bi-factor model | 104.75 | 63.00 | 0.00 | 0.97 | 0.95 | 0.06 | 0.04 | 0.08 | 0.16 | 0.04 |
| Item | F1 | F2 | F3 | Se | P |
|---|---|---|---|---|---|
| PAID_10 | 0.72 | 0.05 | 0.00 | ||
| PAID_05 | 0.74 | 0.04 | 0.00 | ||
| PAID_09 | 0.71 | 0.04 | 0.00 | ||
| PAID_11 | 0.77 | 0.03 | 0.00 | ||
| PAID_06 | 0.77 | 0.03 | 0.00 | ||
| PAID_13 | 0.67 | 0.04 | 0.00 | ||
| PAID_12 | 0.69 | 0.04 | 0.00 | ||
| PAID_01 | 0.83 | 0.03 | 0.00 | ||
| PAID_02 | 0.76 | 0.04 | 0.00 | ||
| PAID_03 | 0.77 | 0.03 | 0.00 | ||
| PAID_04 | 0.77 | 0.04 | 0.00 | ||
| PAID_07 | 0.78 | 0.04 | 0.00 | ||
| PAID_14 | 0.69 | 0.05 | 0.00 | ||
| PAID_08 | 0.76 | 0.04 | 0.00 |
| Factor | G | Se | P |
|---|---|---|---|
| F1 | 0.94 | 0.03 | 0.00 |
| F2 | 0.89 | 0.03 | 0.00 |
| F3 | 0.92 | 0.04 | 0.00 |
| ρ | TIR | Social Support (Teachers) | Social Support (Peers) | Social Support (Family) | Social Support (Total Score) | HbA1c % | PAID-T (Test) | PAID-T (Retest) | DES-28 (Total Score) | DES MPSAD | DES ADaRC | DES SaADG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TIR | 0.11 | 0.05 | −0.01 | 0.06 | −0.62 ** | −0.06 | −0.02 | −0.32 ** | −0.30 ** | −0.29 * | −0.25 | |
| Social support (teachers) | 0.22 ** | 0.10 | 0.58 ** | −0.03 | −0.13 | −0.14 | −0.09 | −0.09 | −0.05 | −0.10 | ||
| Social support (peers) | 0.12 | 0.55 ** | −0.12 | −0.04 | −0.06 | −0.15 | −0.12 | −0.07 | −0.19 * | |||
| Social support (family) | 0.78 ** | −0.16 | −0.23 ** | −0.22 * | −0.29 ** | −0.24 ** | −0.22 ** | −0.31 ** | ||||
| Social support (total score) | −0.14 | −0.24 ** | −0.25 ** | −0.29 ** | −0.24 ** | −0.19 * | −0.32 ** | |||||
| HbA1c % | 0.09 | 0.06 | 0.43 ** | 0.45 ** | 0.35 ** | 0.34 ** | ||||||
| PAID-T (test) | 0.99 ** | 0.32 ** | 0.18 * | 0.23 ** | 0.44 ** | |||||||
| PAID-T (retest) | 0.32 ** | 0.16 | 0.23 * | 0.44 ** | ||||||||
| DES-28 (total score) | 0.91 ** | 0.88 ** | 0.84 ** | |||||||||
| DES MPSAD | 0.75 ** | 0.64 ** | ||||||||||
| DES ADaRC | 0.59 ** | |||||||||||
| DES SaADG |
| Β | seB | T | p | |
|---|---|---|---|---|
| (Intercept) | 0.024 | 0.069 | 0.348 | 0.728 |
| Adherence to glycaemic control (Adherent) | −0.034 | 0.117 | −0.293 | 0.770 |
| Self-control of T1D during school stay (No difficulties in school) | −0.287 | 0.120 | −2.391 | 0.017 |
| Social support (Overall score) | −0.239 | 0.055 | −4.326 | 0.000 |
| β | 95% CI | |
|---|---|---|
| Adherence to glycaemic control (Adherent) | −0.03 | −0.26 −0.19 |
| Self-control of T1D during school (No difficulties in school) | −0.28 | −0.51 −0.05 |
| Social support (Overall score) | −0.22 | −0.32 −0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smudja, M.; Milenković, T.; Minaković, I.; Zdravković, V.; Mitić, S.; Miljković, A.; Milutinović, D. Psychometric Properties of the Serbian Teen Version of the Problem Areas in Diabetes Scale—A Validation Study. Nurs. Rep. 2025, 15, 326. https://doi.org/10.3390/nursrep15090326
Smudja M, Milenković T, Minaković I, Zdravković V, Mitić S, Miljković A, Milutinović D. Psychometric Properties of the Serbian Teen Version of the Problem Areas in Diabetes Scale—A Validation Study. Nursing Reports. 2025; 15(9):326. https://doi.org/10.3390/nursrep15090326
Chicago/Turabian StyleSmudja, Mirjana, Tatjana Milenković, Ivana Minaković, Vera Zdravković, Sandra Mitić, Ana Miljković, and Dragana Milutinović. 2025. "Psychometric Properties of the Serbian Teen Version of the Problem Areas in Diabetes Scale—A Validation Study" Nursing Reports 15, no. 9: 326. https://doi.org/10.3390/nursrep15090326
APA StyleSmudja, M., Milenković, T., Minaković, I., Zdravković, V., Mitić, S., Miljković, A., & Milutinović, D. (2025). Psychometric Properties of the Serbian Teen Version of the Problem Areas in Diabetes Scale—A Validation Study. Nursing Reports, 15(9), 326. https://doi.org/10.3390/nursrep15090326








