Changes in Physical Function, Cognitive Function, Mental Health, and Sleep Quality After Cardiac Surgeries and Procedures
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
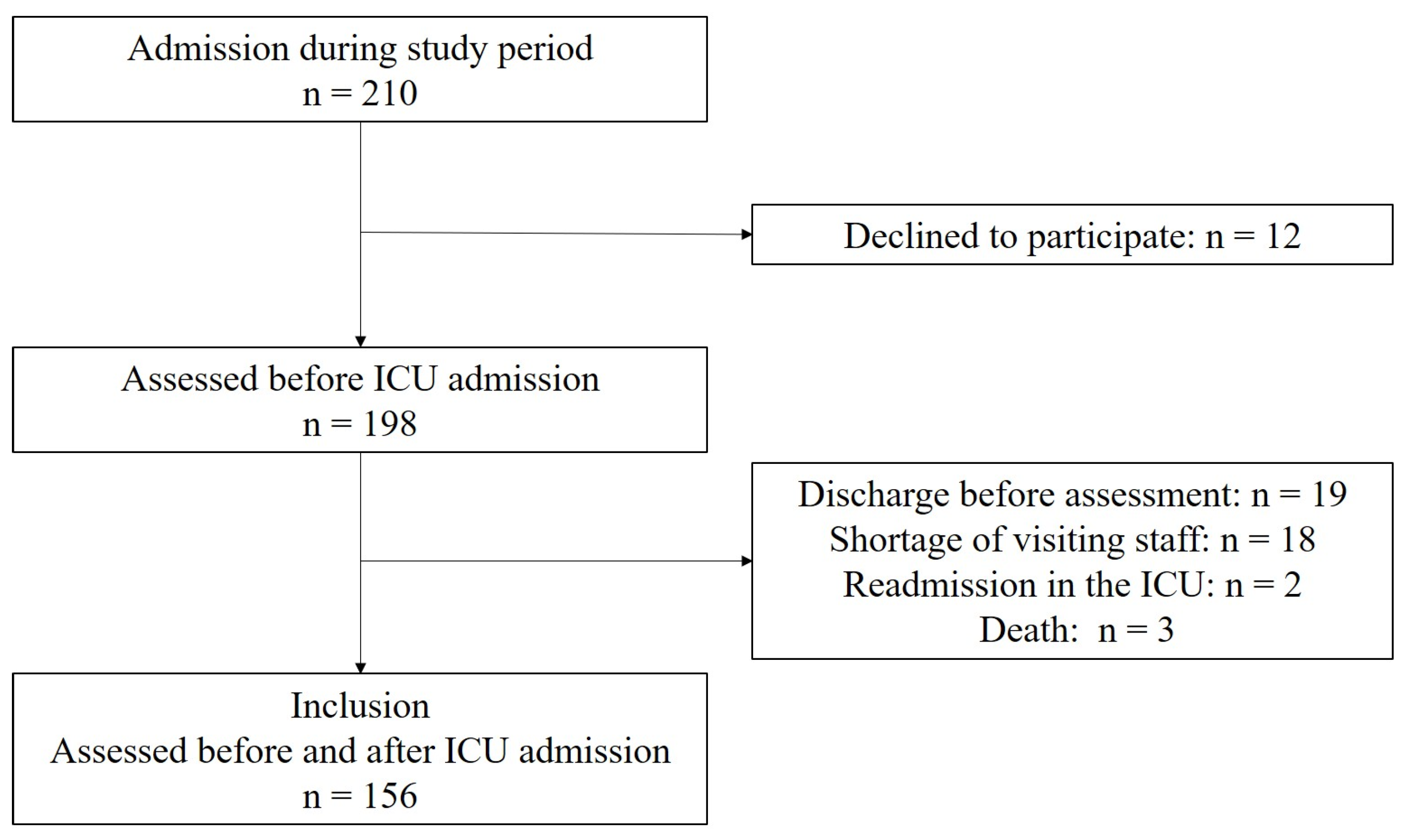
2.2. Study Population
2.3. Outcomes
2.3.1. J-CHS
2.3.2. Barthel Index
2.3.3. HADS
2.3.4. MMSE
2.3.5. 5-Point Likert Scale for Sleep
2.4. Delirium
2.5. ICU Mobility Scale (IMS)
2.6. Rehabilitation and Nutrition
2.7. Multivariate Analysis
2.8. Sample Size and Statistical Analyses
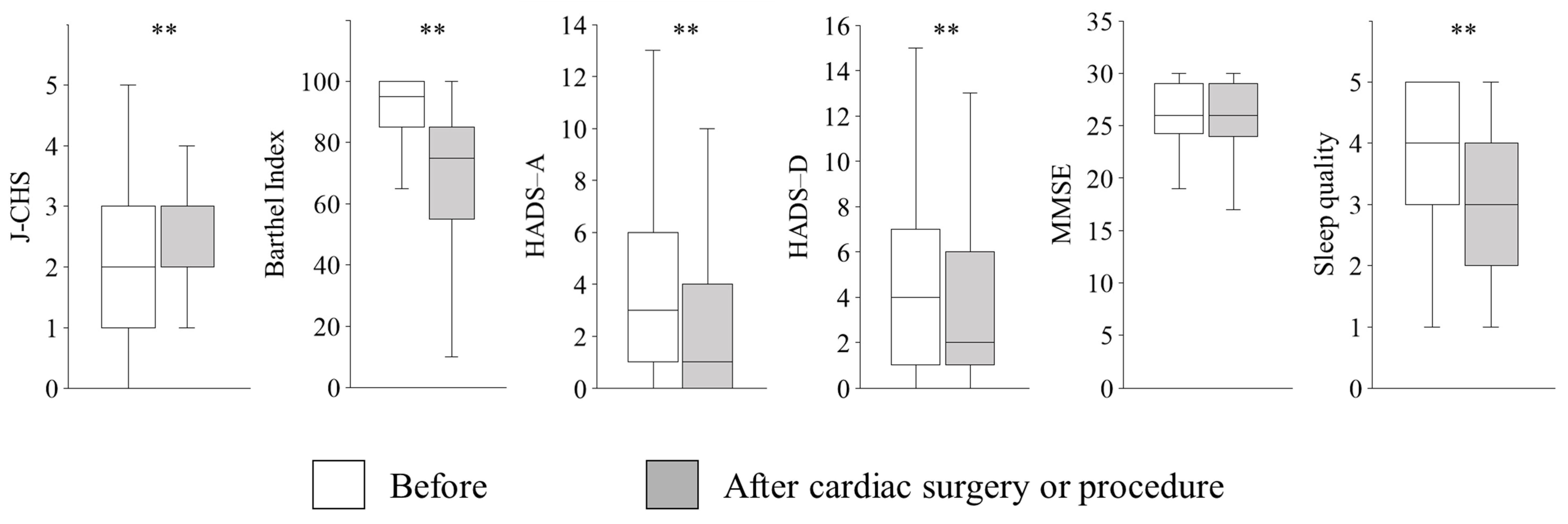
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Public Involvement Statement
Guidelines and Standards Statement
Use of Artificial Intelligence
Acknowledgments
Conflicts of Interest
Abbreviations
| ICU | intensive care unit |
| PICS | post-intensive care syndrome |
| CABG | coronary artery bypass grafting |
| TAVI | transcatheter aortic valve implantation |
| J-CHS | Japanese version of the Cardiovascular Health Study |
| ADL | activities of daily living |
| HADS | Hospital Anxiety and Depression Scale |
| MMSE | Mini-Mental State Examination |
References
- Kawakami, D.; Fujitani, S.; Morimoto, T.; Dote, H.; Takita, M.; Takaba, A.; Hino, M.; Nakamura, M.; Irie, H.; Adachi, T.; et al. Prevalence of post-intensive care syndrome among Japanese intensive care unit patients: A prospective, multicenter, observational J-PICS study. Crit. Care 2021, 25, 69. [Google Scholar] [CrossRef] [PubMed]
- Rea, A.; Holler, S.; Hottle, R.; Fonner, C.; Salenger, R. Incidence of post intensive care syndrome (PICS) in cardiac surgery. Clin. Nutr. ESPEN 2023, 57, 802–803. [Google Scholar] [CrossRef]
- Ayenew, T.; Gete, M.; Gedfew, M.; Getie, A.; Afenigus, A.D.; Edmealem, A.; Amha, H.; Alem, G.; Tiruneh, B.G.; Messelu, M.A. Prevalence of post-intensive care syndrome among intensive care unit-survivors and its association with intensive care unit length of stay: Systematic review and meta-analysis. PLoS ONE 2025, 20, e0323311. [Google Scholar] [CrossRef]
- Lui, K.Y.; Luo, G.; Li, S.; Song, X.; Qian, X.; Dou, R.; Li, L.; Guan, X.; Cai, C. Incidence and risk factors of post-intensive care syndrome (PICS) in surgical ICU survivors: A prospective Chinese cohort study. BMC Public Health 2024, 24, 3277. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, L.E.; Pisani, M.A.; Murphy, T.E.; Gahbauer, E.A.; Leo-Summers, L.S.; Gill, T.M. Functional trajectories among older persons before and after critical illness. JAMA Intern. Med. 2015, 175, 523–529. [Google Scholar] [CrossRef]
- Fujinaga, J.; Otake, T.; Umeda, T.; Fukuoka, T. Case volume and specialization in critically ill emergency patients: A nationwide cohort study in Japanese ICUs. J. Intensive Care 2024, 12, 20. [Google Scholar] [CrossRef]
- Dhillon, N.K.; Ko, A.; Smith, E.J.T.; Kharabi, M.; Castongia, J.; Nurok, M.; Gewertz, B.L.; Ley, E.J. Potentially avoidable surgical intensive care unit admissions and disposition delays. JAMA Surg. 2017, 152, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Mazer, C.D.; Byrick, R.J.; Sibbald, W.J.; Chovaz, P.M.; Goodman, S.J.; Girotti, M.J.; Hall, J.K.; Pagliarello, J. Postoperative utilization of critical care services by cardiac surgery: A multicenter study in the Canadian healthcare system. Crit. Care Med. 1993, 21, 851–859. [Google Scholar] [CrossRef]
- Phillips, E.K.; Monnin, C.; Gregora, A.; Smith, K.; Schultz, A.S.H.; O’Keefe-McCarthy, S.; Arora, R.C.; Duhamel, T.A.; Chudyk, A.M. A scoping review of incidence and assessment tools for post-intensive care syndrome following cardiac surgery. Intensive Crit. Care Nurs. 2024, 83, 103718. [Google Scholar] [CrossRef]
- Rousseau, A.-F.; Prescott, H.C.; Brett, S.J.; Weiss, B.; Azoulay, E.; Creteur, J.; Latronico, N.; Hough, C.L.; Weber-Carstens, S.; Vincent, J.-L.; et al. Long-term outcomes after critical illness: Recent insights. Crit. Care 2021, 25, 108. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Satake, S.; Arai, H. The revised Japanese version of the Cardiovascular Health Study criteria (revised J-CHS criteria). Geriatr. Gerontol. Int. 2020, 20, 992–993. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The barthel index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, S.; Lapierre, S.; Vasiliadis, H.M.; Hudon, C. Evaluation of the effects of an intervention intended to optimize the sleep environment among the elderly: An exploratory study. Clin. Interv. Aging 2020, 15, 2117–2127. [Google Scholar] [CrossRef]
- Huang, M.; Ma, H.; Spruyt, K.; Dzierzewski, J.M.; Jiang, C.; He, J.; Yang, N.; Ying, Y.; Ola, B.A.; Meng, R. Assessing psychometric properties and measurement invariance of the Sleep Quality Questionnaire among healthcare students. BMC Psychol. 2024, 12, 41. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Sugishita, M.; Koshizuka, Y.; Sudou, S.; Sugishita, K.; Hemmi, I.; Karasawa, H.; Ihara, M.; Asada, T.; Mihara, B. The validity and reliability of the Japanese version of the Mini-Mental State Examination (MMSE-J) with the original procedure of the attention and calculation task (2001). Jpn. J. Cogn. Neurosci. 2018, 20, 91–110. [Google Scholar] [CrossRef]
- Grewal, S.; Theijse, R.T.; Dunlop, G.; van Deurzen, D.F.P.; van den Bekerom, M.P.J.; Klautz, R.J.M.; Lefebvre, R.P.; Munsami, D.; Grewal, N. Exploring the impact of sleep on emotional and physical well-being in professional cricketers: A cohort study over an in-season training period. Front. Sports Act. Living 2024, 6, 1389565. [Google Scholar] [CrossRef]
- Ely, E.W.; Inouye, S.K.; Bernard, G.R.; Gordon, S.; Francis, J.; May, L.; Truman, B.; Speroff, T.; Gautam, S.; Margolin, R.; et al. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001, 286, 2703–2710. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, C.; Needham, D.; Haines, K.; Bailey, M.; Ward, A.; Harrold, M.; Young, P.; Zanni, J.; Buhr, H.; Higgins, A.; et al. Feasibility and inter-rater reliability of the ICU mobility scale. Heart Lung 2014, 43, 19–24. [Google Scholar] [CrossRef]
- Yasumura, D.; Katsukawa, H.; Matsuo, R.; Kawano, R.; Taito, S.; Liu, K.; Hodgson, C. Feasibility and inter-rater reliability of the Japanese version of the intensive care unit mobility scale. Cureus 2024, 16, e59135. [Google Scholar] [CrossRef]
- Kim, R.Y.; Murphy, T.E.; Doyle, M.; Pulaski, C.; Singh, M.; Tsang, S.; Wicker, D.; Pisani, M.A.; Connors, G.R.; Ferrante, L.E. Factors associated with discharge home among medical ICU patients in an early mobilization program. Crit. Care Explor. 2019, 1, e0060. [Google Scholar] [CrossRef] [PubMed]
- Devlin, J.W.; Skrobik, Y.; Gélinas, C.; Needham, D.M.; Slooter, A.J.C.; Pandharipande, P.P.; Watson, P.L.; Weinhouse, G.L.; Nunnally, M.E.; Rochwerg, B.; et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit. Care Med. 2018, 46, e825–e873. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Farajzadeh, M.; Nasrollahi, E.; Bahramvand, Y.; Mohammadkarimi, V.; Dalfardi, B.; Anushiravani, A. The use of APACHE II scoring system for predicting clinical outcome of patients admitted to the intensive care unit: A report from a resource-limited center. Shiraz Med. J. 2021, 22, e102858. [Google Scholar] [CrossRef]
- Liu, K.; Shibata, J.; Fukuchi, K.; Takahashi, K.; Sonoo, T.; Ogura, T.; Goto, T. Optimal timing of introducing mobilization therapy for ICU patients with sepsis. J. Intensive Care 2022, 10, 22. [Google Scholar] [CrossRef]
- Itagaki, A.; Saitoh, M.; Okamura, D.; Kawamura, T.; Otsuka, S.; Tahara, M.; Mori, Y.; Kamisaka, K.; Ochi, Y.; Yuguchi, S.; et al. Factors related to physical functioning decline after cardiac surgery in older patients: A multicenter retrospective study. J. Cardiol. 2019, 74, 279–283. [Google Scholar] [CrossRef]
- Henderson, P.; Quasim, T.; Asher, A.; Campbell, L.; Daniel, M.; Davey, L.; Devine, H.; Gall, M.; Mactavish, P.; McGroarty, K.; et al. Post-intensive care syndrome following cardiothoracic critical care: Feasibility of a complex intervention. J. Rehabil. Med. 2021, 53, jrm00206. [Google Scholar] [CrossRef]
- Pandharipande, P.P.; Girard, T.D.; Jackson, J.C.; Morandi, A.; Thompson, J.L.; Pun, B.T.; Brummel, N.E.; Hughes, C.G.; Vasilevskis, E.E.; Shintani, A.K.; et al. Long-Term Cognitive Impairment after Critical Illness. N. Engl. J. Med. 2013, 369, 1306–1316. [Google Scholar] [CrossRef]
- Iwashyna, T.J.; Ely, E.W.; Smith, D.M.; Langa, K.M. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010, 304, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Ebersoldt, M.; Sharshar, T.; Annane, D. Sepsis-associated delirium. Intensive Care Med. 2007, 33, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Gofton, T.E.; Young, G.B. Sepsis-associated encephalopathy. Nat. Rev. Neurol. 2012, 8, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Bowden, T.; Hurt, C.S.; Sanders, J.; Aitken, L.M. Predictors of cognitive dysfunction after cardiac surgery: A systematic review. Eur. J. Cardiovasc. Nurs. 2021, 21, 192–204. [Google Scholar] [CrossRef]
- Bruggemans, E.F. Cognitive dysfunction after cardiac surgery: Pathophysiological mechanisms and preventive strategies. Neth. Heart J. 2013, 21, 70–73. [Google Scholar] [CrossRef]
- Shih, C.-Y.; Wang, A.-Y.; Chang, K.-M.; Yang, C.-C.; Tsai, Y.-C.; Fan, C.-C.; Chuang, H.-J.; Thi Phuc, N.; Chiu, H.-Y. Dynamic prevalence of sleep disturbance among critically ill patients in intensive care units and after hospitalisation: A systematic review and meta-analysis. Intensive Crit. Care Nurs. 2023, 75, 103349. [Google Scholar] [CrossRef]
- Kamdar, B.B.; Needham, D.M.; Collop, N.A. Sleep deprivation in critical illness: Its role in physical and psychological recovery. J. Intensive Care Med. 2012, 27, 97–111. [Google Scholar] [CrossRef]
- Lin, T.R.; Cheng, C.H.; Wei, J.; Wang, T.J. Factors influencing sleep quality in open-heart patients in the postoperative intensive care unit. Healthcare 2022, 10, 2311. [Google Scholar] [CrossRef]
- Unoki, T.; Hayashida, K.; Kawai, Y.; Taito, S.; Ando, M.; Iida, Y.; Kasai, F.; Kawasaki, T.; Kozu, R.; Kondo, Y.; et al. Japanese Clinical Practice Guidelines for Rehabilitation in Critically Ill Patients 2023 (J-ReCIP 2023). J. Intensive Care 2023, 11, 47. [Google Scholar] [CrossRef]
- Nakamura, K.; Yamamoto, R.; Higashibeppu, N.; Yoshida, M.; Tatsumi, H.; Shimizu, Y.; Izumino, H.; Oshima, T.; Hatakeyama, J.; Ouchi, A.; et al. The Japanese critical care nutrition guideline 2024. J. Intensive Care 2025, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Herbst, N.P.; Danesh, V.; Lewis, A.; Sevin, C.M. Multidisciplinary team approaches to assessing and addressing post intensive care syndrome. Crit. Care Clin. 2025, 41, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, N.; Abraham, B.K.; Barokar, R.; Chanchalani, G.; Jagathkar, G.; Shetty, R.M.; Tripathy, S.; Vijayaraghavan, B.K.T. Post-ICU care: Why, What, When and How? ISCCM position statement. Indian J. Crit. Care Med. 2024, 28, S279–S287. [Google Scholar] [CrossRef] [PubMed]
- Matthews, E.E. Sleep disturbances and fatigue in critically ill patients. AACN Adv. Crit. Care 2011, 22, 204–224. [Google Scholar] [CrossRef]
- Nakanishi, N.; Liu, K.; Hatakeyama, J.; Kawauchi, A.; Yoshida, M.; Sumita, H.; Miyamoto, K.; Nakamura, K. Post-intensive care syndrome follow-up system after hospital discharge: A narrative review. J. Intensive Care 2024, 12, 2. [Google Scholar] [CrossRef]
| All Patients | |
|---|---|
| Variables | n = 156 |
| Age, mean ± SD, y | 74 ± 16 |
| Male/female | 64/92 |
| Body mass index, mean ± SD, kg/m2 | 23.1 ± 3.9 |
| APACHE II score | 14 (12–17) |
| SOFA | 5 (3–7) |
| Treatment type, n (%) | |
| Valve replacement or valvuloplasty | 67 (43) |
| CABG | 14 (9) |
| Combination of valve and CABG | 6 (4) |
| TAVI | 60 (39) |
| Others | 9 (6) |
| Thoracotomy, n (%) | 89 (57) |
| Mechanical ventilation, n (%) | 99 (64) |
| Length of ICU stay | 4 (2–5) |
| Comorbidities, n (%) | |
| High blood pressure | 103 (66) |
| Diabetes mellitus | 44 (28) |
| Stroke | 28 (18) |
| Liver disease | 11 (7) |
| Kidney disease | 49 (31) |
| Lung disease | 16 (1) |
| J-CHS Deterioration | Barthel Index Deterioration | Sleep Quality Deterioration | ||||
|---|---|---|---|---|---|---|
| Variables | Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate |
| Age ≥ 65 years | 1.85 (0.75–4.57) | 1.91 (0.68–5.40) | 0.78 (0.27–2.25) | 0.95 (0.28–3.21) | 0.89 (0.39–2.05) | 1.67 (0.60–4.65) |
| Male/female | 0.93 (0.42–2.06) | 1.09 (0.46–2.58) | 1.61 (0.72–3.58) | 1.62 (0.70–3.76) | 1.49 (0.78–2.85) | 1.20 (0.58–2.51) |
| Body mass index ≥ 22 kg/m2 | 1.15 (0.51–2.60) | 1.05 (0.44–2.55) | 1.32 (0.62–2.85) | 1.13 (0.51–2.52) | 1.90 (0.98–3.67) | 1.57 (0.77–3.22) |
| APACHE II score ≥ 20 | 0.73 (0.26–2.08) | 0.60 (0.20–1.82) | 6.09 (0.79–47.23) | 5.99 (0.75–48.15) | 0.97 (0.38–2.48) | 0.59 (0.19–1.79) |
| Thoracotomy | 0.80 (0.24–2.72) | 0.84 (0.24–2.98) | 1.95 (0.91–4.21) | 1.68 (0.63–4.50) | 3.77 (1.89–7.52) | 3.43 (1.37–8.62) |
| Maximum IMS ≥ 3 | 0.76 (0.12–4.74) | 0.77 (0.11–5.40) | 1.90 (0.74–4.88) | 1.47 (0.52–4.20) | 2.95 (1.10–7.92) | 1.75 (0.58–5.23) |
| Delirium | 1.49 (0.62–3.58) | 1.28 (0.48–3.47) | 1.26 (0.59–2.70) | 0.86 (0.35–2.12) | 2.13 (1.11–4.09) | 1.28 (0.56–2.95) |
| Physical restraints | 1.17 (0.25–5.52) | 1.28 (0.24–6.94) | 0.68 (0.13–3.69) | 0.58 (0.09–3.68) | 0.88 (0.19–4.09) | 0.48 (0.09–2.70) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawahara, Y.; Nakanishi, N.; Nomura, K.; Doi, S.; Oto, J. Changes in Physical Function, Cognitive Function, Mental Health, and Sleep Quality After Cardiac Surgeries and Procedures. Nurs. Rep. 2025, 15, 209. https://doi.org/10.3390/nursrep15060209
Kawahara Y, Nakanishi N, Nomura K, Doi S, Oto J. Changes in Physical Function, Cognitive Function, Mental Health, and Sleep Quality After Cardiac Surgeries and Procedures. Nursing Reports. 2025; 15(6):209. https://doi.org/10.3390/nursrep15060209
Chicago/Turabian StyleKawahara, Yoshimi, Nobuto Nakanishi, Keiko Nomura, Satoshi Doi, and Jun Oto. 2025. "Changes in Physical Function, Cognitive Function, Mental Health, and Sleep Quality After Cardiac Surgeries and Procedures" Nursing Reports 15, no. 6: 209. https://doi.org/10.3390/nursrep15060209
APA StyleKawahara, Y., Nakanishi, N., Nomura, K., Doi, S., & Oto, J. (2025). Changes in Physical Function, Cognitive Function, Mental Health, and Sleep Quality After Cardiac Surgeries and Procedures. Nursing Reports, 15(6), 209. https://doi.org/10.3390/nursrep15060209







