Comparison of Traditional and Ultrasound-Guided Techniques for Vascular Access in Patients with Difficult Venous Access in Emergency Departments: Randomized Clinical Trial Protocol
Abstract
1. Introduction
Hypothesis and Objetive
2. Materials and Methods
2.1. Scope of the Study
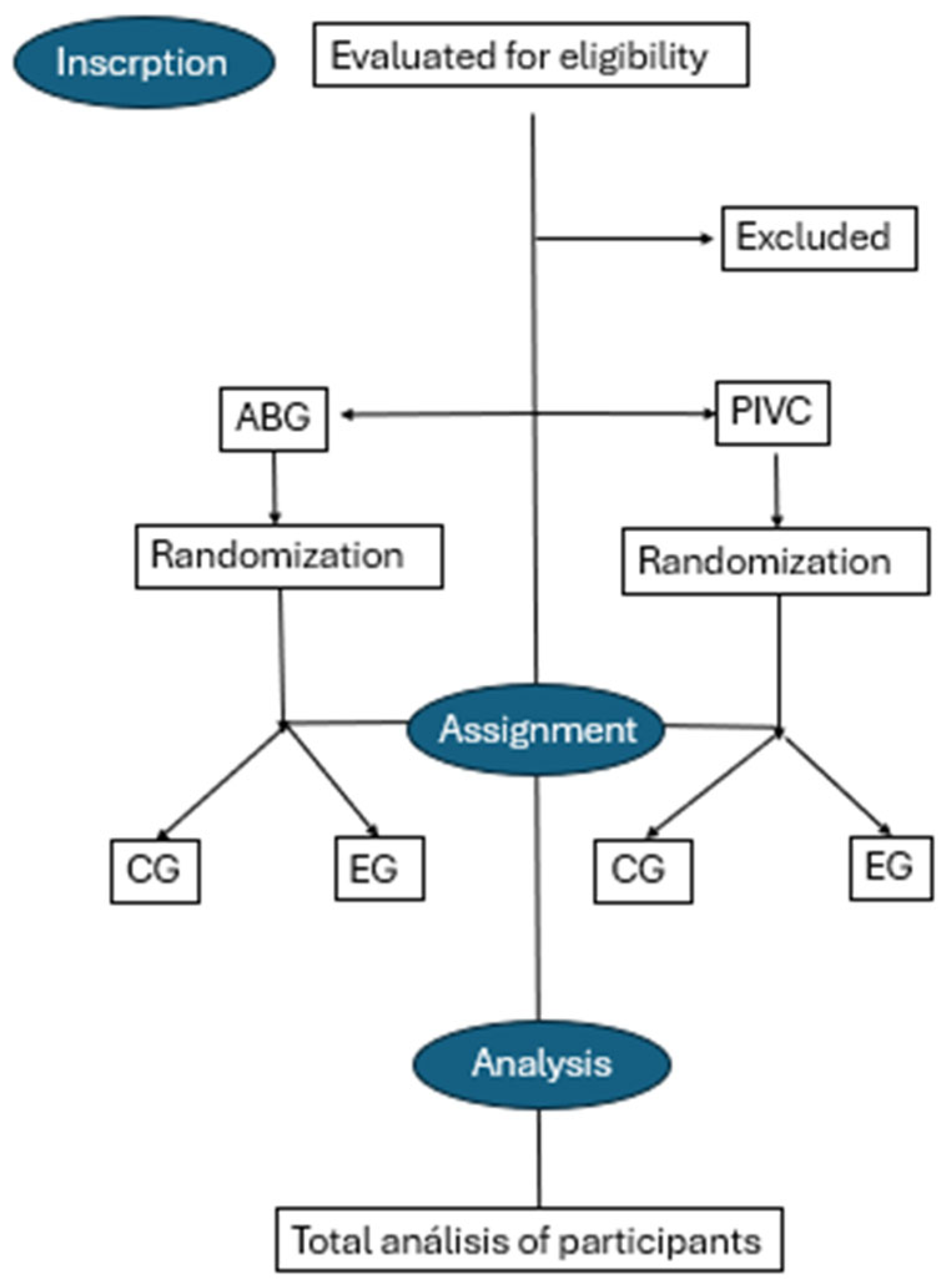
2.2. Design
2.3. Participants
- Prescription for CIP or GA cannulation.
- Informed consent signed by the patient or patient’s representative.
- A score ≥ 3 on the A-DICAVE scale or ≥4 on the DIVA scale for PIVC.
- Scores below 3 on the A-DICAVE scale or below 4 on the DIVA scale for PIVC.
- Inability to obtain informed consent from the patient or patient’s representative.
2.4. Sample Size
2.5. Randomization of the Sample
2.6. Statistical Methods
2.7. Ethical Considerations
3. Expected Results
3.1. Measurement Instruments
- Not at all satisfied.
- Not very satisfied.
- Satisfied.
- Quite satisfied.
- Very satisfied.
- ○
- For adults and children aged > 7 years, the Visual Analogue Scale (VAS) [30] will be used.
- ○
- For children aged < 5 years, the FLACC scale (Face, Legs, Activity, Cry, Consolability) [31] will be applied.
- ○
- For adults with cognitive impairments, the PAINAD scale (Pain Assessment in Advanced Dementia) [32] will be employed.
3.2. Data Collection System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Public Involvement Statement
Guidelines and Standards Statement
Use of Artificial Intelligence
Conflicts of Interest
Abbreviations
| AGB | Arterial puncture for Artery Blood Gas sampling |
| CG | Control Group |
| DIVA | Difficult venous access |
| ED | Emergency Departments |
| EG | Experimental Group |
| FLACC | Face, Legs, Activity, Cry, Consolability |
| HNSS | Hospital Nuestra Señora de Sonsoles |
| IQR | Interquartile range |
| n | Frequencies |
| PAINAD | Pain assessment in advanced dementia |
| PIVC | Peripheral intravenous catheter |
| RaPeVa | Rapid peripheral vein assessment |
| RCT | Randomized Clinical Trial |
| SD | Dispersions |
| VAS | Visual analogy scale |
References
- Rodríguez Calero, M.A.; Miquel Rodríguez Calero, C.A. Definiendo la vía venosa periférica de difícil canalización y los factores de riesgo asociados. Revisión sistemática. Med. Balear 2019, 34, 12–20. [Google Scholar]
- Riker, M.W.; Kennedy, C.; Winfrey, B.S.; Yen, K.; Dowd, M.D. Validation and Refinement of the Difficult Intravenous Access Score: A Clinical Prediction Rule for Identifying Children with Difficult Intravenous Access. Acad. Emerg. Med. 2011, 18, 1129–1134. [Google Scholar] [CrossRef]
- Grandpierre, R.G.; Bobbia, X.; Muller, L.; Markarian, T.; Occéan, B.-V.; Pommet, S.; Roger, C.; Lefrant, J.Y.; de la Coussaye, J.E.; Claret, P.-G. Ultrasound Guidance in Difficult Radial Artery Puncture for Blood Gas Analysis: A Prospective, Randomized Controlled Trial. PLoS ONE 2019, 14, e0213683. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Calero, M.A. Factores de Riesgo de Dificultad en la Canalización Venosa Periférica en Atención Hospitalaria. Estudio Caso-Control Multicéntrico. Ph.D. Thesis, Universitat de Les Illes Baleares, Palma, Spain, 2016. [Google Scholar]
- Rodríguez, J.E.R.; García, I.S.; de la Rosa Roch, B.; Martínez, B.F.M.; García, M.T.P.; Rodríguez, M.G.; García, B.R.; Muñoz, M.S. Factores asociados al éxito en la primera punción o canalización venosa en urgencias pediátricas. Metas Enferm. 2017, 20, 2. [Google Scholar] [CrossRef]
- Salleras-Duran, L.; Fuentes-Pumarola, C. Cateterización periférica ecoguiada frente a la técnica tradicional. Enferm. Clin. 2016, 26, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Laursen, C.B.; Pedersen, R.L.; Lassen, A.T. Ultrasonographically Guided Puncture of the Radial Artery for Blood Gas Analysis: A Prospective, Randomized Controlled Trial. Ann. Emerg. Med. 2015, 65, 618–619. [Google Scholar] [CrossRef]
- Yen, K.; Riegert, A.; Gorelick, M.H. Derivation of the DIVA Score: A Clinical Prediction Rule for the Identification of Children with Difficult Intravenous Access. Pediatr. Emerg. Care 2008, 24, 143–147. [Google Scholar] [CrossRef]
- Kuensting, L.L.; DeBoer, S.; Holleran, R.; Shultz, B.L.; Steinmann, R.A.; Venella, J. Difficult Venous Access in Children: Taking Control. J. Emerg. Nurs. 2009, 35, 419–424. [Google Scholar] [CrossRef]
- van Loon, F.H.J.; Puijn, L.A.P.M.; Houterman, S.; Bouwman, A.R.A. Development of the A-DIVA Scale: A Clinical Predictive Scale to Identify Difficult Intravenous Access in Adult Patients Based on Clinical Observations. Medicine 2016, 95, e3428. [Google Scholar] [CrossRef]
- Salleras-Duran, L.; Fuentes-Pumarola, C.; Ballester-Ferrando, D.; Congost-Devesa, L.; Delclós-Rabassa, J.; Fontova-Almató, A. Development, Diagnostic Sensitivity, and Prognostic Accuracy of the Adult-Difficult Venous Catheterization Scale for Emergency Departments. J. Emerg. Nurs. 2020, 46, 827–837.e2. [Google Scholar] [CrossRef]
- Blanco, P. Ultrasound-Guided Peripheral Venous Cannulation in Critically Ill Patients: A Practical Guideline. Ultrasound J. 2019, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- McKinney, A.; Steanson, K.; Lebar, K. A Standardized Training Program in Ultrasound-Guided Intravenous Line Placement: Improving Nurses’ Confidence and Success. Adv. Neonatal Care 2023, 23, 17–22. [Google Scholar] [CrossRef]
- Gil Monte, S.; Pérez Navarro, A.M.; Serrano Moreno, A.A.; García Martínez, E.M.; Sáez Royo, R. Protocolo Canalización de Vía Venosa Periférica y Punción Arterial Ecoguiada. Available online: https://www.chospab.es/publicaciones/protocolosEnfermeria/documentos/fc46edcfd8502c01729e4110c29e3378.pdf (accessed on 8 April 2025).
- Gopalasingam, N.; Obad, D.S.; Kristensen, B.S.; Lundgaard, P.; Veien, M.; Gjedsted, J.; Sloth, E.; Juhl-Olsen, P. Ultrasound-Guidance Outperforms the Palpation Technique for Peripheral Venous Catheterisation in Anaesthetised Toddlers: A Randomised Study. Acta Anaesthesiol. Scand. 2017, 61, 601–608. [Google Scholar] [CrossRef]
- Marraco-Boncompte, M.; Lorente-Roda, B.I.; Echamendi-Hernández, M.; Yagüe-Gastón, A.; Martínez-Arangoa, I.; Lerín-Lebrero, M. Incorporación de la Técnica Ecoguiada en la Inserción Periférica de Vías Centrales: Un Nuevo Reto para Enfermería en Cuidados Intensivos. Nursing 2019, 36, 53–57. [Google Scholar] [CrossRef]
- Pittiruti, M.; Van Boxtel, T.; Scoppettuolo, G.; Carr, P.; Konstantinou, E.; Miluy, G.O.; Lamperti, M.; Goossens, G.A.; Simcock, L.; Dupont, C.; et al. European Recommendations on the Proper Indication and Use of Peripheral Venous Access Devices (the ERPIUP Consensus): A WoCoVA Project. J. Vasc. Access 2023, 24, 165–182. [Google Scholar] [CrossRef]
- Lam, C.; Dunstan, L.; Sweeny, A.; Watkins, S.; George, S.; Snelling, P.J. A Survey of Paediatric Difficult Peripheral Intravenous Access in the Emergency Department and Use of Point-of-Care Ultrasound. Australas. J. Ultrasound Med. 2023, 26, 184–190. [Google Scholar] [CrossRef]
- Rodríguez-Herrera, Á.; Solaz-García, Á.; Mollá-Olmos, E.; Ferrer-Puchol, D.; Esteve-Claramunt, F.; Trujillo-Barberá, S.; García-Bermejo, P.; Casaña-Mohedo, J. Use of the Ultrasound Technique as Compared to the Standard Technique for the Improvement of Venous Cannulation in Patients with Difficult Access. Healthcare 2022, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Acuña, J.; Sorenson, J.; Gades, A.; Wyatt, R.; Stea, N.; Drachman, M.; Adhikari, S. Handheld Ultrasound: Overcoming the Challenge of Difficult Peripheral Intravenous Access in the Emergency Department. J. Ultrasound Med. 2020, 39, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Amick, A.E.; Feinsmith, S.E.M.; Davis, E.M.; Sell, J.B.; Macdonald, V.B.; Trinquero, P.; Moore, A.G.; Gappmeier, V.; Colton, K.; Cunningham, A.; et al. Simulation-Based Mastery Learning Improves Ultrasound-Guided Peripheral Intravenous Catheter Insertion Skills of Practicing Nurses. Simul. Healthc. 2022, 17, 7–14. [Google Scholar] [CrossRef]
- Ocsa, C.; Gimeno, V.; Cortés, M.; Naccarato, G.; Reinoso, G.; Nuñez, P. Desarrollo de un programa de capacitación en la colocación de catéteres venosos periféricos guiada por ecografía: Experiencia en un hospital pediátrico de tercer nivel. Emerg. Pediatr. 2024, 3, 44–48. [Google Scholar]
- Kaganovskaya, M.; Wuerz, L. Development of an Educational Program Using Ultrasonography in Vascular Access for Nurse Practitioner Students. Br. J. Nurs. 2021, 30, S34–S42. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.-W.; Tetzlaff, J.M.; Altman, D.G.; Laupacis, A.; Gøtzsche, P.C.; Krleža-Jerić, K.; Hróbja-rtsson, A.; Mann, H.; Dickersin, K.; Berlin, J.A.; et al. SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials. Ann. Intern. Med. 2013, 158, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. BMC Med. 2010, 8, 18. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadística (INE). Productos y Servicios/Publicaciones/Publicaciones de Descarga Gratuita. Available online: https://www.ine.es/ss/Satellite?L=es_ES&c=INESeccion_C&cid=1259944485770&p=1254735110672&pagename=ProductosYServicios%2FPYSLayout¶m1=PYSDetalleFichaIndicador¶m3=1259937499084 (accessed on 8 April 2025).
- Diputación Provincial de Ávila. Portal Web. Available online: https://www.diputacionavila.es/la-provincia/nuestros-pueblos/municipios/0/ (accessed on 8 April 2025).
- Junta de Castilla y León. Urgencias Hospitalarias Atendidas|Datos Abiertos. Available online: https://datosabiertos.jcyl.es/web/jcyl/set/es/salud/urgencias-hospitalarias-atendidas/1285114487305 (accessed on 8 April 2025).
- Osakidetza. Guía de Procedimiento de Punción Arterial para Gasometría en Población Adulta. Available online: https://www.researchgate.net/publication/339483856_guia_de_procedimiento_de_puncion_arterial_para_gasometria_en_poblacion_adulta (accessed on 8 April 2025).
- Shields, B.J.; Palermo, T.M.; Powers, J.D.; Grewe, S.D.; Smith, G.A. Predictors of a Child’s Ability to Use a Visual Analogue Scale. Child Care Health Dev. 2003, 29, 281–290. [Google Scholar] [CrossRef]
- Pedraza García, C.P.; Benavides Benavides, D.S. Características Psicométricas de la Escala FLACC Traducida; Universidad del Rosario: Bogotá, Colombia, 2019. [Google Scholar]
- García-Soler, Á.; Sánchez-Iglesias, I.; Buiza, C.; Alaba, J.; Navarro, A.B.; Arriola, E.; Zulaica, A.; Vaca, R.; Hernández, C. Adaptación y Validación de la Versión Española de la Escala de Evaluación de Dolor en Personas con Demencia Avanzada: PAINAD-Sp. Rev. Esp. Geriatr. Gerontol. 2014, 49, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Castellanos, B.; Jara Concha, P. Filosofía de Patricia Benner, Aplicación en la Formación de Enfermería: Propuestas de Estrategias de Aprendizaje. Educación 2019, 28, 182–202. [Google Scholar] [CrossRef][Green Version]
- Rubiera-González, R.; González-García, J.; Rodríguez-Suárez, L.M. Canalización Venosa Periférica Ecoguiada: Características y Complicaciones Comparadas con Técnica Tradicional. Rev. Esp. Urg. Emerg. 2022, 1, 87–92. [Google Scholar] [CrossRef]
- Salleras-Duran, L.; Fuentes-Pumarola, C.; Fontova-Almató, A.; Roqueta-Vall-Llosera, M.; Cámara-Liebana, D.; Ballester-Ferrando, D. Pain and Satisfaction Perceptions of Ultrasound-Guided Versus Conventional Peripheral Intravenous Catheterization: A Randomized Controlled Trial. Pain Manag. Nurs. 2024, 25, e37–e44. [Google Scholar] [CrossRef]
- Nacci, A.D. A-DIVA Score and the Implementation of the Use of Ultrasound-Guided Peripheral Intravenous Access in the Emergency Department: A Brief Literature Review. Int. J. Stud. Nurs. 2018, 3, 122. [Google Scholar] [CrossRef]
- Tran, Q.K.; Flanagan, K.; Fairchild, M.; Yardi, I.; Pourmand, A. Nurses and Efficacy of Ultrasound-Guided Versus Traditional Venous Access: A Systemic Review and Meta-Analysis. J. Emerg. Nurs. 2022, 48, 145–158.e1. [Google Scholar] [CrossRef]
- Zhao, W.; Peng, H.; Li, H.; Yi, Y.; Ma, Y.; He, Y.; Zhang, H.; Li, T. Effects of Ultrasound-Guided Techniques for Radial Arterial Catheterization: A Meta-Analysis of Randomized Controlled Trials. Am. J. Emerg. Med. 2021, 46, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Romo-Miguel, P.; Ballesteros-Peña, S. Ultrasound-Guided Puncture vs Conventional Technique for Arterial Blood Gas Analysis Sampling in Adults: A Systematic Review. Enferm. Intensiv. (Engl. Ed.) 2024, 35, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, H.; Neway, B.; Breslin, K.; Watson, A.; Poe, K.; Boniface, K.; Cohen, J.S. Utility of the DIVA Score for Experienced Emergency Department Technicians. Br. J. Nurs. 2020, 29, S35–S40. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.M.; Feinsmith, S.; Amick, A.E.; Sell, J.; McDonald, V.; Trinquero, P.; Moore, A.; Gappmaier, V.; Colton, K.; Cunningham, A.; et al. Difficult Intravenous Access in the Emergency Department: Performance and Impact of Ultrasound-Guided IV Insertion Performed by Nurses. Am. J. Emerg. Med. 2021, 46, 539–544. [Google Scholar] [CrossRef]
- Oviedo-García, A.A.; Algaba-Montes, M.; Patricio-Bordomás, M. Las Técnicas Ecoguiadas, Una Herramienta Muy Útil También para Enfermería. Semergen 2016, 42, 503–504. [Google Scholar] [CrossRef]
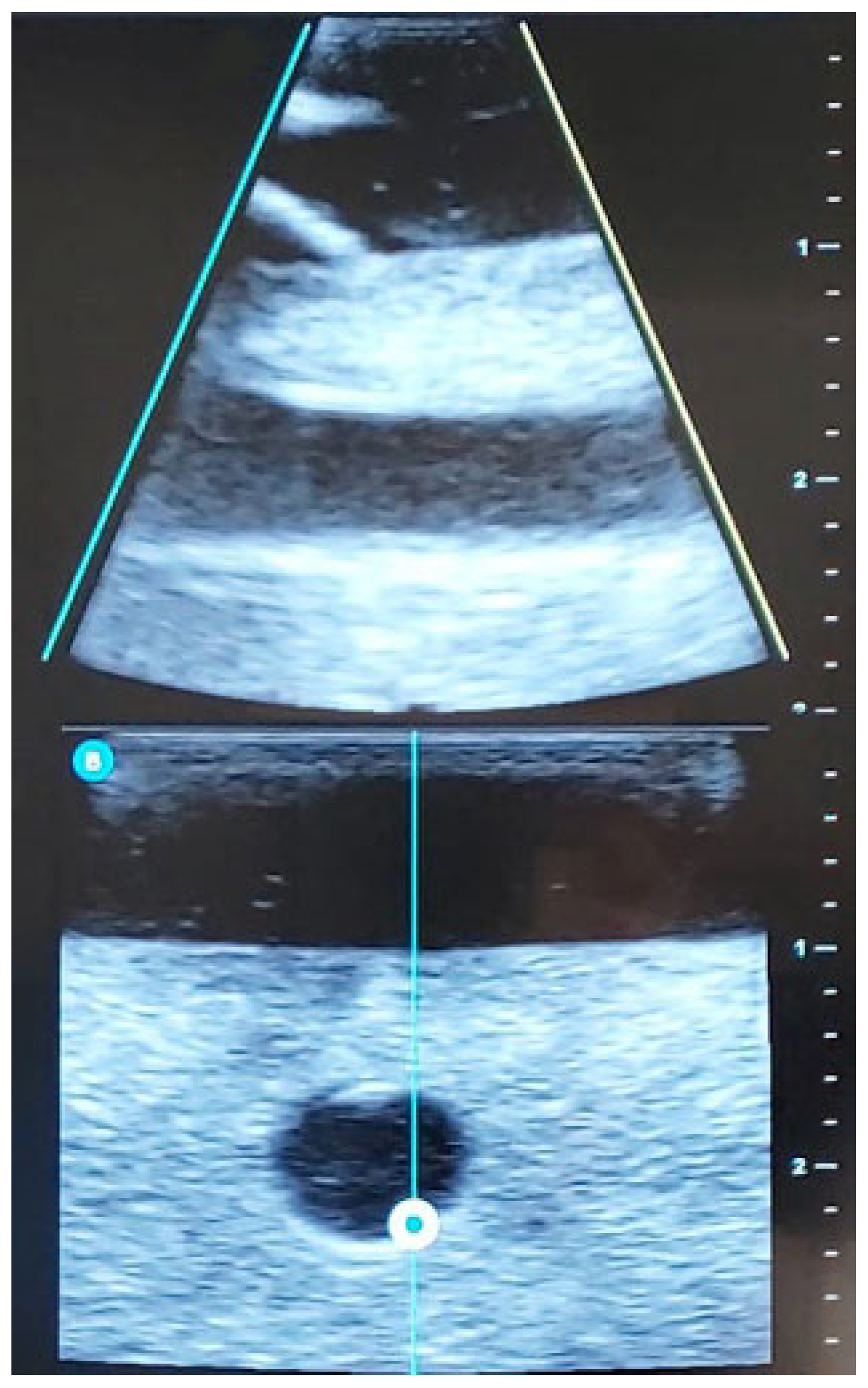
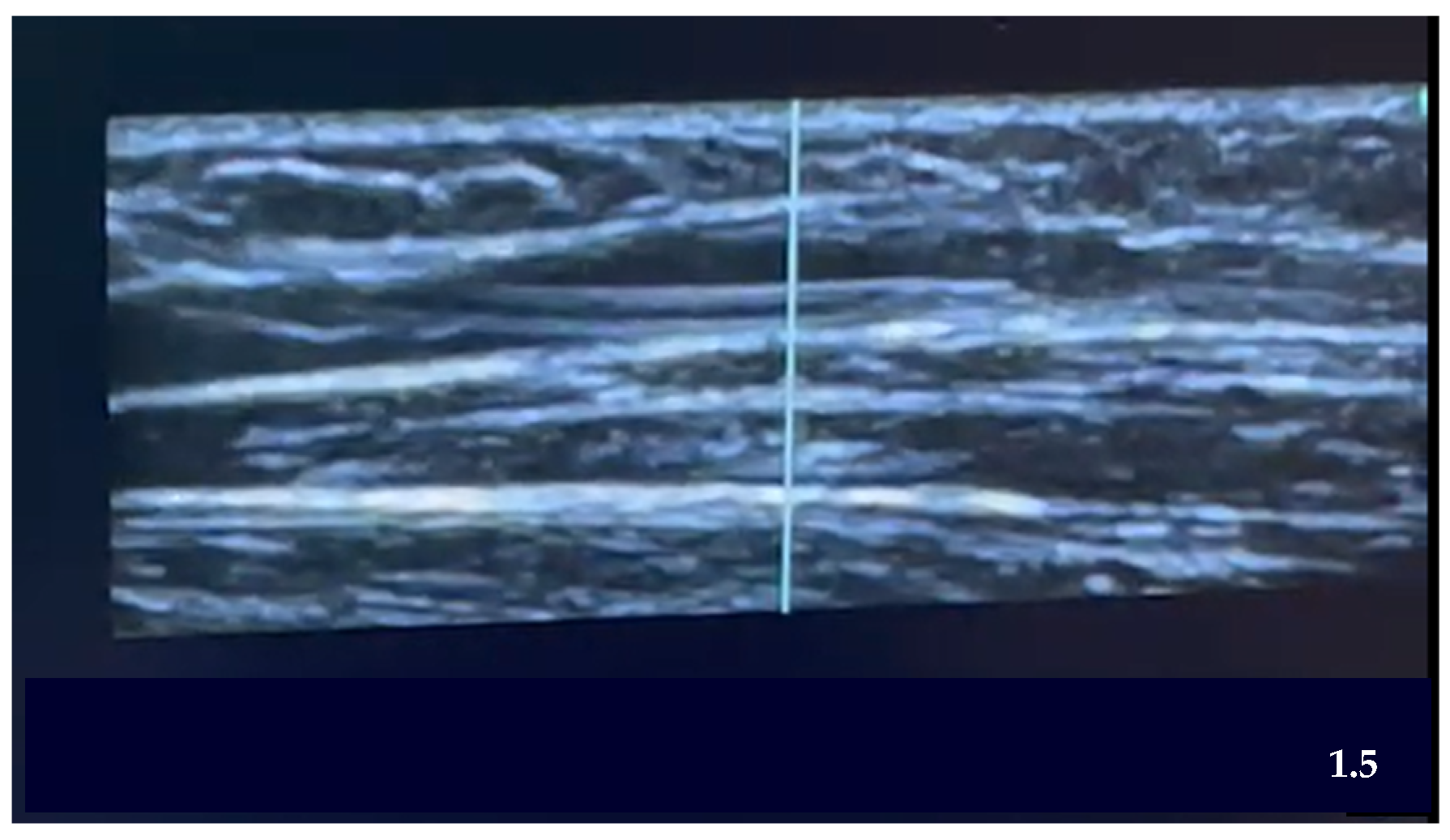
| Steps of the EG Ultrasound-Guided Technique | |
|---|---|
| STEP 1 | Patient identification and procedure explanation. |
| STEP 2 | Preparation of necessary materials. |
| STEP 3 | Ultrasound-guided examination of the limb to be punctured (RaPeVa):
|
| STEP 4 | Selection of an appropriate vessel (depth, diameter, and patency):
|
| STEP 5 | Preparation of the puncture site:
|
| STEP 6 | Ultrasound-guided puncture:
|
| STEP 7 |
|
| RTC Variables | |
|---|---|
| Dependent Variables | Description |
| Type of technique | Arterial puncture, peripheral venous cannulation with short PIVC |
| Total score on the A-DICAVE scale | From 0 to 10 score in patients below 14 |
| Score in DIVA scale | From 0 to 8 score in patients over 14 |
| Score of every item on the A-DICAVE scale | Score of every item on the scale |
| Success | Successful result (Yes/No) |
| Number of attempts | Number of punctures attempts to achieve cannulation |
| Numbers of nurses attempting | Number of nurses to achieve cannulation |
| Time taken in the technique | Time in minutes taken for the procedure |
| Cannulation zone | Pace of insertion of the catheter (hand, forearm, flexure, arm) |
| Catheter gauge | Measurement in gauges of the used catheter |
| Pain | EAV scales (oriented patient), FLACC (pediatric patient) or PAINAD (disorientated patient) |
| Permanence of the vascular access | Permanence time of the catheter |
| Secondary effects | Type of effects (extravasation, phlebitis, obstruction, others) |
| Patient satisfaction | Level of satisfaction (5-point Likert scale) |
| Family satisfaction | Level of satisfaction of the family (5-point Likert scale) |
| Professional satisfaction | Level of satisfaction of the professional who applied the technique (5-point Likert scale) |
| Independent variables | Description |
| Age | Years |
| Gender | Male, female, nonbinary |
| Reason for the consultation | Reason for the assistance according to nursing record |
| Emergency zone | Assigned emergency zone |
| Triage level | Classification SET (I, II, III, IV, V) |
| Predisposing factors of DIVA | Factors such as addiction, obesity, old age among others |
| Procedure variables | Description |
| Type of technique | Traditional, ultrasound guided |
| Transducer placement | Longitudinal, transversal, none |
| Confusion variables | Description |
| Medication given | Medication used through the access |
| Dilution volume | Volume in ml of the medication dilution |
| Time of administration | Taken time in minutes of the administration |
| Conscience level | Glasgow scale |
| Agitation | Yes/No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peralta-Gámez, M.S.; Gómez de Quero Córdoba, M.; Reverté-Villarroya, S.; Cuesta-Martínez, R. Comparison of Traditional and Ultrasound-Guided Techniques for Vascular Access in Patients with Difficult Venous Access in Emergency Departments: Randomized Clinical Trial Protocol. Nurs. Rep. 2025, 15, 177. https://doi.org/10.3390/nursrep15050177
Peralta-Gámez MS, Gómez de Quero Córdoba M, Reverté-Villarroya S, Cuesta-Martínez R. Comparison of Traditional and Ultrasound-Guided Techniques for Vascular Access in Patients with Difficult Venous Access in Emergency Departments: Randomized Clinical Trial Protocol. Nursing Reports. 2025; 15(5):177. https://doi.org/10.3390/nursrep15050177
Chicago/Turabian StylePeralta-Gámez, Mercedes S., Marina Gómez de Quero Córdoba, Silvia Reverté-Villarroya, and Roser Cuesta-Martínez. 2025. "Comparison of Traditional and Ultrasound-Guided Techniques for Vascular Access in Patients with Difficult Venous Access in Emergency Departments: Randomized Clinical Trial Protocol" Nursing Reports 15, no. 5: 177. https://doi.org/10.3390/nursrep15050177
APA StylePeralta-Gámez, M. S., Gómez de Quero Córdoba, M., Reverté-Villarroya, S., & Cuesta-Martínez, R. (2025). Comparison of Traditional and Ultrasound-Guided Techniques for Vascular Access in Patients with Difficult Venous Access in Emergency Departments: Randomized Clinical Trial Protocol. Nursing Reports, 15(5), 177. https://doi.org/10.3390/nursrep15050177








