Medication Adherence Measurement in Chronic Diseases: A State-of-the-Art Review of the Literature
Abstract
1. Introduction
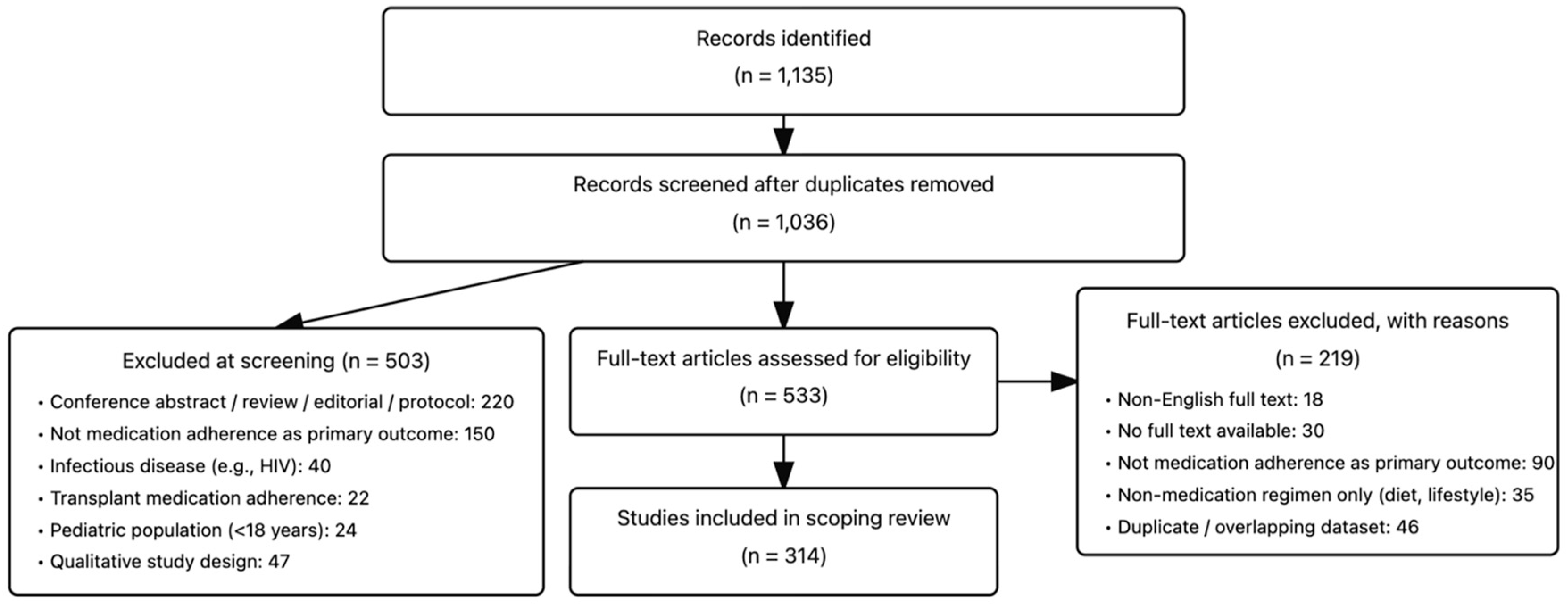
2. Methods
- Participants: Adults (≥18 years) with non-infectious chronic diseases (e.g., cardiovascular disease, diabetes, cancer, chronic respiratory disease, chronic kidney disease). Studies with mixed populations were eligible if data for eligible participants could be extracted separately.
- Concept: Medication adherence measurement was the primary outcome, including subjective (self-report questionnaires/visual analog scales) and objective methods (pharmacy refill metrics, electronic monitoring, biologic assays).
- Context: We included studies conducted in any care setting (e.g., primary care, specialty clinics, hospital outpatients, community programs) and published in the English language from 1 August 2019 to 30 July 2024.
3. Results
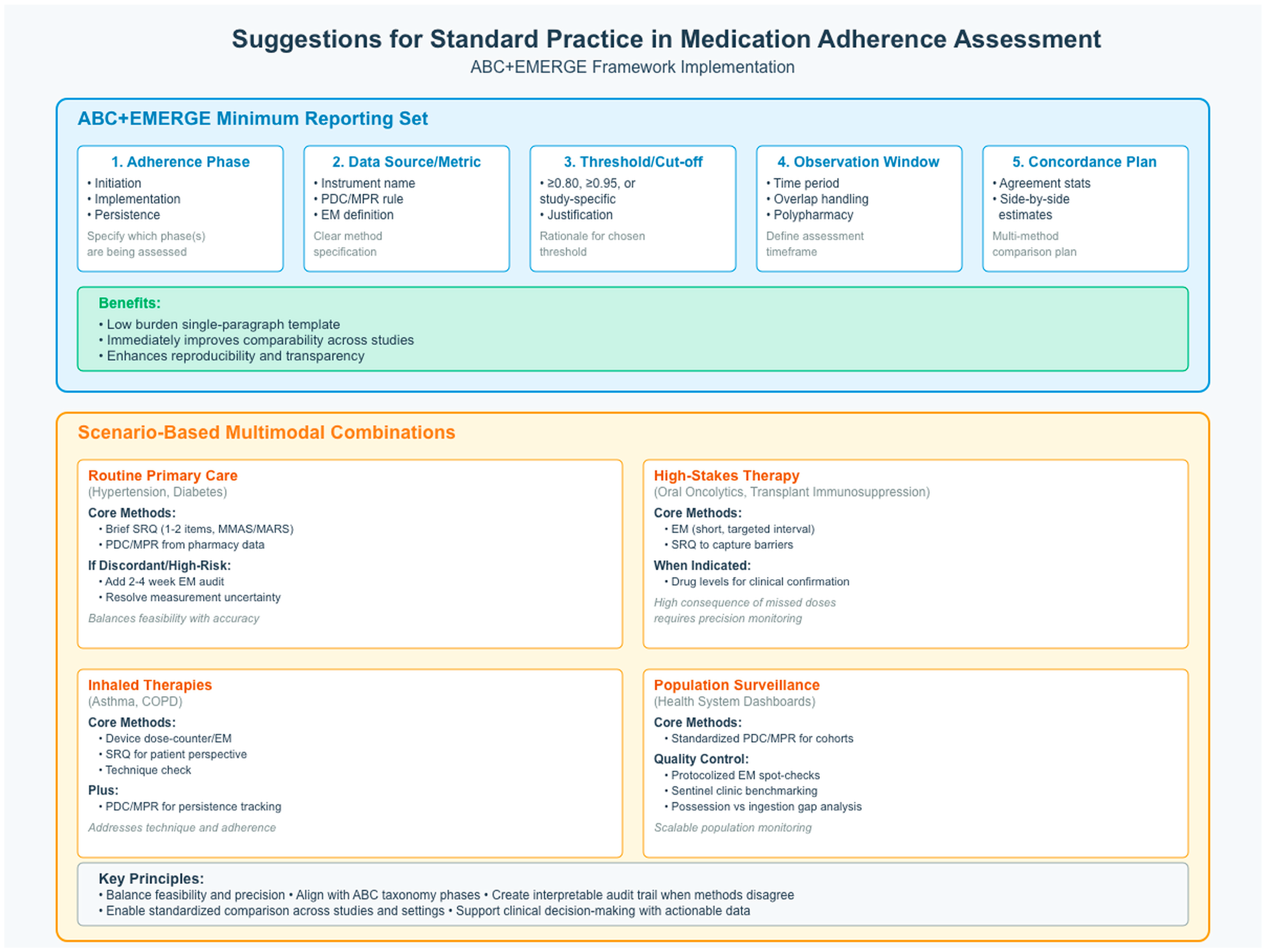
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Public Involvement Statement
Guidelines and Standards Statement
Use of Artificial Intelligence
Conflicts of Interest
References
- Brown, M.T.; Bussell, J.K. Medication adherence: Who cares? Mayo Clin. Proc. 2011, 86, 304–314. [Google Scholar] [CrossRef] [PubMed]
- De Geest, S.; Zullig, L.L.; Dunbar-Jacob, J.; Helmy, R.; Hughes, D.A.; Wilson, I.B.; Vrijens, B. ESPACOMP medication adherence reporting guideline (EMERGE). Ann. Intern. Med. 2018, 169, 30–35. [Google Scholar] [CrossRef]
- Sabaté, E. (Ed.) Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Haynes, R.B.; Taylor, D.W.; Sackett, D.L. Compliance in Health Care; Johns Hopkins University Press: Baltimore, MD, USA, 1979. [Google Scholar]
- Christensen, P.; Johansson, A.; Nielsen, V. Quantitation of protein adsorbance to glass and plastics: Investigation of a new tube with low adherence. J. Immunol. Methods 1978, 23, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S.W.; Pappius, E.M.; Harper, S. Measurement of medication compliance in a clinical setting: Comparison of three methods in patients prescribed digoxin. Arch. Intern. Med. 1979, 139, 635–638. [Google Scholar] [CrossRef]
- Rudd, P.; Tul, V.; Brown, K.; Davidson, S.M.; Bostwick, G.J. Hypertension continuation adherence: Natural history and role as an indicator condition. Arch. Intern. Med. 1979, 139, 545–549. [Google Scholar] [CrossRef]
- Dunbar-Jacob, J.; Sereika, S.M.; Houze, M.; Luyster, F.S.; Callan, J.A. Accuracy of measures of medication adherence in a cholesterol-lowering regimen. West. J. Nurs. Res. 2012, 34, 578–597. [Google Scholar] [CrossRef]
- Dunbar-Jacob, J.; Rohay, J.M. Predictors of medication adherence: Fact or artifact. J. Behav. Med. 2016, 39, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Conn, V.S.; Ruppar, T.M. Medication adherence outcomes of 771 intervention trials: Systematic review and meta-analysis. Prev. Med. 2017, 99, 269–276. [Google Scholar] [CrossRef]
- Aldeer, M.; Javanmard, M.; Martin, R.P. A review of medication adherence monitoring technologies. Appl. Syst. Innov. 2018, 1, 14. [Google Scholar] [CrossRef]
- Forbes, C.A.; Deshpande, S.; Sorio-Vilela, F.; Kutikova, L.; Duffy, S.; Gouni-Berthold, I.; Hagström, E. A systematic literature review comparing methods for the measurement of patient persistence and adherence. Curr. Med. Res. Opin. 2018, 34, 1613–1625. [Google Scholar] [CrossRef]
- Krueger, K.; Griese-Mammen, N.; Schubert, I.; Kieble, M.; Botermann, L.; Laufs, U.; Kloft, C.; Schulz, M. In search of a standard when analyzing medication adherence in patients with heart failure using claims data: A systematic review. Heart Fail. Rev. 2018, 23, 63–71. [Google Scholar] [CrossRef]
- Canfield, S.L.; Zuckerman, A.; Anguiano, R.H.; Jolly, J.A.; DeClercq, J.; Wascher, M.; Choi, L.; Knox, S.; Mitchell, D.G. Navigating the wild west of medication adherence reporting in specialty pharmacy. J. Manag. Care Spec. Pharm. 2019, 25, 1073–1077. [Google Scholar] [CrossRef]
- Stirratt, M.J.; Dunbar-Jacob, J.; Crane, H.M.; Simoni, J.M.; Czajkowski, S.; Hilliard, M.E.; Aikens, J.E.; Hunter, C.M.; Velligan, D.I.; Huntley, K.; et al. Self-report measures of medication adherence behavior: Recommendations on optimal use. Transl. Behav. Med. 2015, 5, 470–482. [Google Scholar] [CrossRef]
- Gordis, L. Conceptual and methodologic problems in measuring patient compliance. In Compliance in Health Care; Johns Hopkins University Press: Baltimore, MD, USA, 1979; pp. 23–45. [Google Scholar]
- Osterberg, L.; Blaschke, T. Adherence to medication. N. Engl. J. Med. 2005, 353, 487–497. [Google Scholar] [CrossRef]
- Vrijens, B.; De Geest, S.; Hughes, D.A.; Przemyslaw, K.; Demonceau, J.; Ruppar, T.; Dobbels, F.; Fargher, E.; Morrison, V.; Lewek, P.; et al. A new taxonomy for describing and defining adherence to medications. Br. J. Clin. Pharmacol. 2012, 73, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Barry, E.S.; Merkebu, J.; Varpio, L. State-of-the-art literature review methodology: A six-step approach for knowledge synthesis. Perspect. Med. Educ. 2022, 11, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.J.; Lee, W.-Y.; Hwang, J.S.; Hong, Y.P.; Morisky., D.E. Accuracy of a screening tool for medication adherence: A systematic review and meta-analysis of the Morisky Medication Adherence Scale-8. PLoS ONE 2017, 12, e0187139. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.J.; Booth, A. A Typology of reviews: An analysis of 14 review types and associated methodologies. Health Inf. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef]
- Tan, X.; Patel, I.; Chang, J. Review of the four item Morisky medication adherence scale (MMAS-4) and eight item Morisky medication adherence scale (MMAS-8). Innov. Pharm. 2014, 5, 5. [Google Scholar] [CrossRef]
- Kripalani, S.; Risser, J.; Gatti, M.E.; Jacobson, T.A. Development and evaluation of the Adherence to Refills and Medications Scale (ARMS) among low-literacy patients with chronic disease. Value Health 2009, 12, 118–123. [Google Scholar] [CrossRef]
- Chan, A.H.Y.; Horne, R.; Hankins, M.; Chisari, C. The medication adherence report scale: A measurement tool for eliciting patients’ reports of nonadherence. Br. J. Clin. Pharmacol. 2020, 86, 1281–1288. [Google Scholar] [CrossRef]
- Finitsis, D.J.; Pellowski, J.A.; Huedo-Medina, T.B.; Fox, M.C.; Kalichman, S.C. Visual analogue scale (VAS) measurement of antiretroviral adherence in people living with HIV (PLWH): A meta-analysis. J. Behav. Med. 2016, 39, 1043–1055. [Google Scholar] [CrossRef]
- Boons, C.C.L.M.; Timmers, L.; Janssen, J.J.W.M.; Westerweel, P.E.; Blijlevens, N.M.A.; Smit, W.M.; Bartelink, I.H.; Wilschut, J.A.; Swart, E.L.; Hendrikse, N.H.; et al. Response and Adherence to Nilotinib in Daily practice (RAND study): An in-depth observational study of chronic myeloid leukemia patients treated with nilotinib. Eur. J. Clin. Pharmacol. 2020, 76, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Hesso, I.; Nabhani Gebara, S.; Greene, G.; Co Stello, R.W.; Kayyali, R. A quantitative evaluation of adherence and inhalation technique among respiratory patients: An observational study using an electronic inhaler assessment device. Int. J. Clin. Pract. 2020, 74, e13437. [Google Scholar] [CrossRef] [PubMed]
- Arutyunov, G.P.; Arutyunov, A.G.; Ageev, F.T.; Fofanova, T.V. Use of Digital Technology Tools to Characterize Adherence to Prescription-Grade Omega-3 Polyunsaturated Fatty Acid Therapy in Postmyocardial or Hypertriglyceridemic Patients in the DIAPAsOn Study: Prospective Observational Study. JMIR Cardio 2022, 6, e37490. [Google Scholar] [CrossRef]
- Pericot-Valverde, I.; Heo, M.; Niu, J.; Rennert, L.; Norton, B.L.; Akiyama, M.J.; Arnsten, J.; Litwin, A.H. Relationship between depressive symptoms and adherence to direct-acting antivirals: Implications for Hepatitis C treatment among people who inject drugs on medications for opioid use disorder. Drug Alcohol Depend. 2022, 234, 109403. [Google Scholar] [CrossRef]
- Tangirala, N.C.; O’Conor, R.; Wolf, M.S.; Wisnivesky, J.P.; Federman, A.D. Validity of the medication adherence rating scale for adherence to inhaled corticosteroids among older adults with asthma or chronic obstructive pulmonary disease. COPD J. Chronic Obstr. Pulm. Dis. 2020, 17, 74–80. [Google Scholar] [CrossRef]
- Daud, R.; Zulkarnain, B.S.; Amu, I.V. Providing counseling through home pharmacy care (HPC) for hemodialysis patients with hypertension in lowering blood pressure. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 459–465. [Google Scholar] [CrossRef]
- Mehas, N.; Hudmon, K.S.; Jaynes, H.; Klink, S.; Downey, L.; Zillich, A.J. Impact of electronic medication reminder caps on patient adherence and blood pressure. J. Pharm. Technol. 2021, 37, 234–243. [Google Scholar] [CrossRef]
- Mielke, N.; Huscher, D.; Douros, A.; Ebert, N.; Gaedeke, J.; van der Giet, M.; Kuhlmann, M.K.; Martus, P.; Schaeffner, E. Self-reported medication in community-dwelling older adults in Germany: Results from the Berlin Initiative Study. BMC Geriatr. 2020, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Al-Lamee, R.; Foley, M.; Dehbi, H.M.; Thom, S.; Davies, J.E.; Francis, D.P.; Patel, P.; Gupta, P.; ORBITA Investigators. Achieving optimal adherence to medical therapy by telehealth: Findings from the ORBITA medication adherence sub-study. Pharmacol. Res. Perspect. 2021, 9, e00710. [Google Scholar] [CrossRef]
- Calvo-Arbeloa, M.; Insaustl-Serrano, A.M.; Arrondo-Velasco, A.; Sarobe-Carricas, M.T. Adherencia al tratamiento con adalimumab, golimumab y ustekinumab en pacientes con enfermedad inflamatoria intestinal. Farm. Hosp. 2020, 44, 62–67. [Google Scholar]
- El Alili, M.; Vrijens, B.; Demonceau, J.; Evers, S.M.; Hiligsmann, M. A scoping review of studies comparing the medication event monitoring system (MEMS) with alternative methods for measuring medication adherence. Br. J. Clin. Pharmacol. 2016, 82, 268–279. [Google Scholar] [CrossRef]
- Tourangeau, R. Remembering what happened: Memory errors and survey reports. In The Science of Self-Report; Psychology Press: Hove, UK, 1999; pp. 41–60. [Google Scholar]
- Cappelli, C.; Castello, R.; Marini, F.; Paoletta, A.; Marchetti, M.; Saullo, M.; Cristiano, A.; Pirola, I.; Gandossi, E.; Ferlin, A.; et al. Adherence to levothyroxine treatment among patients with hypothyroidism: A northeastern Italian survey. Front. Endocrinol. 2018, 9, 699. [Google Scholar] [CrossRef]
- Lam, W.Y.; Fresco, P. Medication Adherence Measures: An Overview. BioMed Res. Int. 2015, 2015, 217047. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.C.; Mandalia, S.; Gazzard, B.G. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. Aids 2002, 16, 269–277. [Google Scholar] [CrossRef] [PubMed]
- For the ABC project team; Demonceau, J.; Ruppar, T.; Kristanto, P.; Hughes, D.A.; Fargher, E.; Kardas, P.; De Geest, S.; Dobbels, F.; Lewek, P.; et al. Identification and assessment of adherence-enhancing interventions in studies assessing medication adherence through electronically compiled drug dosing histories: A systematic literature review and meta-analysis. Drugs 2013, 73, 45–62. [Google Scholar] [CrossRef] [PubMed]
| Instrument/Metric | Studies (n) | Operational Adherence Definition Used in Included Studies | Reported Adherence Range (% Adherent Unless Notes) | Pooled Mean Adherence (% Adherent) | Pooled Median Adherence (% Adherent) | |
|---|---|---|---|---|---|---|
| Subjective measures | Morisky Medication Adherence Scale (MMAS) (4/8-item) | 35 | Proportion of participants with a score above 6 in MMAS-8 or a score of 0 in MMAS-4 | 38.33–93.3% | 62.5% | 61% |
| Adherence to Refills and Medication Scale (ARMS) | 6 | Proportion of participants meeting study-defined ARMS threshold (lower score = better adherence; typical cutoffs ≤ 12–16) | 42.7–85.3% | 57.6% | 53% | |
| Visual Analog Scale (VAS) | 4 | Proportion reporting adherence ≥ study cutoff (commonly 95%) | 42% to 73% | 46% | 49% | |
| Medication Adherence Report Scale (MARS) | 6 | Proportion of participants with a score above 20–25 (cutoff value of adherence ranges from studies) in MARS | 7% to 84% | 30% | 16% | |
| Pill count | 7 | Proportion of participants who did not miss certain doses in a recall period | 53–92% | 75% | 72% | |
| Objective measures | Pharmacy refill (MPR, PDC) | 69 | Proportion of participants with PDC or MPR ≥ study cutoff (commonly 0.80–0.95) | 11% to 98% | 60% | 60% |
| Electronic monitoring | 19 | Proportion of participants above a specific ratio, which is usually calculated based on the number of observed cap openings divided by the number of prescribed doses per day in a specific period | 20% to 97% | 70% | 74% | |
| Biological test | 4 | Proportion with drug/metabolite detected within assay-defined threshold | 0.3% to 90% | 16% | 25% |
| Author | Type of Study | Population | Medication | Adherence Measurement | Definition | Adherence Outcomes |
|---|---|---|---|---|---|---|
| (Boons et al., 2020) [26] | Observational study | Chronic myeloid leukemia patients | Nilotinib | MEMS(%PDC) | Proportion of participants with PDC > 95% | 78.7% |
| Pill count(%AR) * | Proportion of participants with AR > 95% | 80% | ||||
| Medication Adherence Report Scale (MARS-5) | Proportion of participants with MARS scores above 25 at 12 months | 68% | ||||
| (Arutyunov et al., 2022a) [28] | Observational study | Post-MI patients or patients with hypertriglyceridemia | Omega-3-acid ethyl esters (OM3EE) | Electronic monitoring | Proportion of participants with ratio ≥ 0.8 (The ratio of days when the full prescribed dose of OM3EE was taken to the total number of days in the treatment period) | 34.4% |
| Inquiry at clinic visits | Proportion of participants with scores above 8 in Questionnaire of Treatment Compliance | 89.0% | ||||
| (Hesso et al., 2020) [27] | Observational study | Patients with COPD or asthma | Inhaler technique | Electronic monitoring | Proportion of participants with dose counter ≥ 80% | 42.7% |
| Medication refill adherence (MRA) | Proportion of participants with MRA ≥ 80% | 100% | ||||
| Proportion of days covered (PDC) | Proportion of participants with PDC ≥ 80% | 97.8% | ||||
| Self-reporting—inhaler adherence scale (IAS) | Proportion of participants with scores ≥ 4 in IAS | 48% | ||||
| (Pericot-Valverde et al., 2021) [29] | Secondary analysis of an RCT | Patients with HCV | Direct-acting antivirals (DAAs) | Electronic blister packs | Average blister pack adherence of the total sample | 78.2% |
| Self-reported—VAS | Average self-reported VAS (0–100%) adherence of the total sample | 94.9% | ||||
| (Tangirala et al., 2020) [30] | Cross-sectional study | Patients with COPD or asthma | Inhaled corticosteroids (ICS), long-acting beta-agonists (LABA), and long-acting anti-muscarinic agents (LAMA) | Electronic monitoring devices and dose counts from analog counters | Proportion of participants with ≥80% of total doses prescribed, per convention | 70% for asthma and 71% for COPD |
| Self-reported—10-item Medication Adherence Report Scale (MARS) (MARS assessment was defined as a score ≥ 4.5) | Proportion of participants with MARS-10 scores above 4.5 | 38% for asthma and 39.2% for COPD | ||||
| (Daud et al., 2021) [31] | Quasi-experimental study | Patients with hypertension | Medication for hypertension | Medication Adherence Questionnaire (MAQ) | Proportion of participants with MAQ scores below or equal to 2 | 89.6% on counseling group and 86.2% at non counseling group at baseline |
| Pill count | Proportion of participants with ≥80% pill count adherence scores | 55.2% on counseling group and 65.5% at non counseling group at baseline | ||||
| (Mehas et al., 2021) [32] | Randomized pilot study | Patients with hypertension | Medication for hypertension | Medication possession ratio | Average proportion of days that a patient had blood pressure medication available to take during the 6 months prior to study enrollment | 92.7% (control group) 89.1% (intervention group) |
| Electronic cap adherence | This percentage was averaged across all 6 months for each medication | 55.1% (control group) 60.7% (intervention group) | ||||
| MMAS-8 | (Range 0–8) (Mean ± SD) | 5.7 ± 1.3 (control group) 6.2 ± 1.1 (intervention group) | ||||
| (Mielke et al., 2022) [33] | Prospective cohort study | Patients with chronic kidney disease | Antihypertension medications | Self-reported medication | Average self-reported dose rate | Ranged from 1.1% (methotrexate) to 55% (β-blockers) with mean 16.6% |
| Biological test | Detectable urinary drug metabolites measured by mass spectroscopy | ranged from 0.3% (3-hydroxyvalproate) to 58% (4-acetaminophen sulfate) with mean 8.74% | ||||
| (Thompson et al., 2021) [34] | A cohort study | Patients with stable angina | Cardiovascular medication | Self-reported | Average self-reported dose rate | >96% for all drugs |
| Biological test | High-performance liquid chromatography with tandem mass spectrometry (HPLC MS/MS) | >90% for all drugs | ||||
| (Arutyunov et al., 2022b) [28] | A prospective cohort study | Patients with a history of recent myocardial infarction or endogenous hypertriglyceridemia | Omega-3 polyunsaturated fatty acids | National Questionnaire of Treatment Compliance (NQTC) | Proportion of participants with NQTC scores equal to or higher than 8 | 87.9% in post-MI group and 90.2% in hypertriglyceridemia group |
| Digital adherence monitoring system (DIAPASon) | The total number of days that a patient took the full prescribed dose of OM3EE during the specified period divided by the total number of days in that period | 37% | ||||
| (Calvo-Arbeloa et al., 2020) [35] | A cross-sectional observational analysis | Patients with inflammatory bowel disease | Adalimumab, golimumab and ustekinumab | Medication possession ratio | Proportion of patients with MPR ≥ 85% | 74.7% |
| Morisky–Green Medication Adherence Questionnaire (MMAS-8) | Proportion of patients with MMAS ≥ 6 | Only assessed for MPR non-adherent patients: 53.1% |
| Pill Count | PDC/MPR | Electronic Monitoring | Self-Reported | Biological Test | |
|---|---|---|---|---|---|
| (Boons et al., 2020) [26] | 80% | 78.7% | 68% | ||
| (Hesso et al., 2020) [27] | 97.8% | 42.7% | 100% for MRA and 48% for IAS | ||
| (Pericot-Valverde et al., 2021) [29] | 78.2% | 94.9% | |||
| (Tangirala et al., 2020) [30] | 70% for asthma and 71% for COPD | 38% for asthma and 39.2% for COPD | |||
| (Daud et al., 2021) [31] | 55.2% in counseling group and 65.5% in non-counseling group at baseline | 89.6% in counseling group and 86.2% in non-counseling group at baseline | |||
| (Mehas et al., 2021) [32] | 92.7% (control group) 89.1% (intervention group) | 55.1% (control group) 60.7% (intervention group) | 5.7 ± 1.3 (control group) 6.2 ± 1.1 (intervention group) | ||
| (Mielke et al., 2022) [33] | Ranged from 1.1% (methotrexate) to 55% (β-blockers) with mean 16.6% | Ranged from 0.3% (3-hydroxyvalproate) to 58% (4-acetaminophen sulfate) with mean 8.74% | |||
| (Thompson et al., 2021) [34] | >96% for all drugs | >90% for all drugs | |||
| (Arutyunov et al., 2022b) [28] | 37% | 87.9% in post-MI group and 90.2% in hypertriglyceridemia group | |||
| (Calvo-Arbeloa et al., 2020) [35] | 74.7% | 53.1% (for non-adherent pts based on MPR criteria) |
| Tool | Alignment with WHO Definition | ABC Phase Best Captured * | Additional Considerations |
|---|---|---|---|
| MMAS (4-/8-item) | Evaluates the act of taking medication and reasons for missed doses | Implementation | Quick, widely used; cutoffs vary, and ceiling effects occur; reflects beliefs as well as behavior → may not equal tablets consumed. Recent reviews emphasize heterogeneity and self-report bias. |
| ARMS | Assesses both medication taking and timely prescription refills. | Implementation → Persistence | Valid in chronic disease and low-literacy groups; captures refill barriers. Thresholds vary across studies. |
| MARS-5 | Focuses on specific non-adherence behaviors (e.g., forgetting, intentional omission). | Implementation | Distinguishes intentional vs. unintentional non-adherence; cutoffs differ (e.g., 20–25). New psychometric work continues to appear. |
| Electronic medication monitoring (e.g., MEMS; smart blisters/boxes, app logs) | Directly records date/time of container openings (proxy for dose taking). | Implementation → Persistence | High temporal granularity (patterns, weekends, timing); does not confirm ingestion; device cost/data burden; possible Hawthorne effect. Recent systematic reviews (incl. DOACs; digital EAM) underscore value but variable implementation/acceptability |
| Pharmacy refill records (PDC/MPR) | Compares expected vs. actual remaining doses. | Persistence → Implementation | Scalable/low cost; cannot confirm ingestion; thresholds vary (often ≥ 0.80, sometimes ≥ 0.95). Reporting per EMERGE improves comparability |
| Pill count | Detects drug/metabolite levels to confirm ingestion. | Implementation | Low cost and simple; vulnerable to pill dumping/loss; no timing info; best when paired with another method. |
| Biological tests | Drug/metabolite levels (evidence of recent ingestion). | Initiation/Implementation | Confirms exposure but depends on PK half-life, assay, sampling timing; costly and less feasible for routine use |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dunbar-Jacob, J.; Zhao, J. Medication Adherence Measurement in Chronic Diseases: A State-of-the-Art Review of the Literature. Nurs. Rep. 2025, 15, 370. https://doi.org/10.3390/nursrep15100370
Dunbar-Jacob J, Zhao J. Medication Adherence Measurement in Chronic Diseases: A State-of-the-Art Review of the Literature. Nursing Reports. 2025; 15(10):370. https://doi.org/10.3390/nursrep15100370
Chicago/Turabian StyleDunbar-Jacob, Jacqueline, and Jian Zhao. 2025. "Medication Adherence Measurement in Chronic Diseases: A State-of-the-Art Review of the Literature" Nursing Reports 15, no. 10: 370. https://doi.org/10.3390/nursrep15100370
APA StyleDunbar-Jacob, J., & Zhao, J. (2025). Medication Adherence Measurement in Chronic Diseases: A State-of-the-Art Review of the Literature. Nursing Reports, 15(10), 370. https://doi.org/10.3390/nursrep15100370






