Perceived Pain in People Living with Amyotrophic Lateral Sclerosis—A Scoping Review
Abstract
1. Introduction
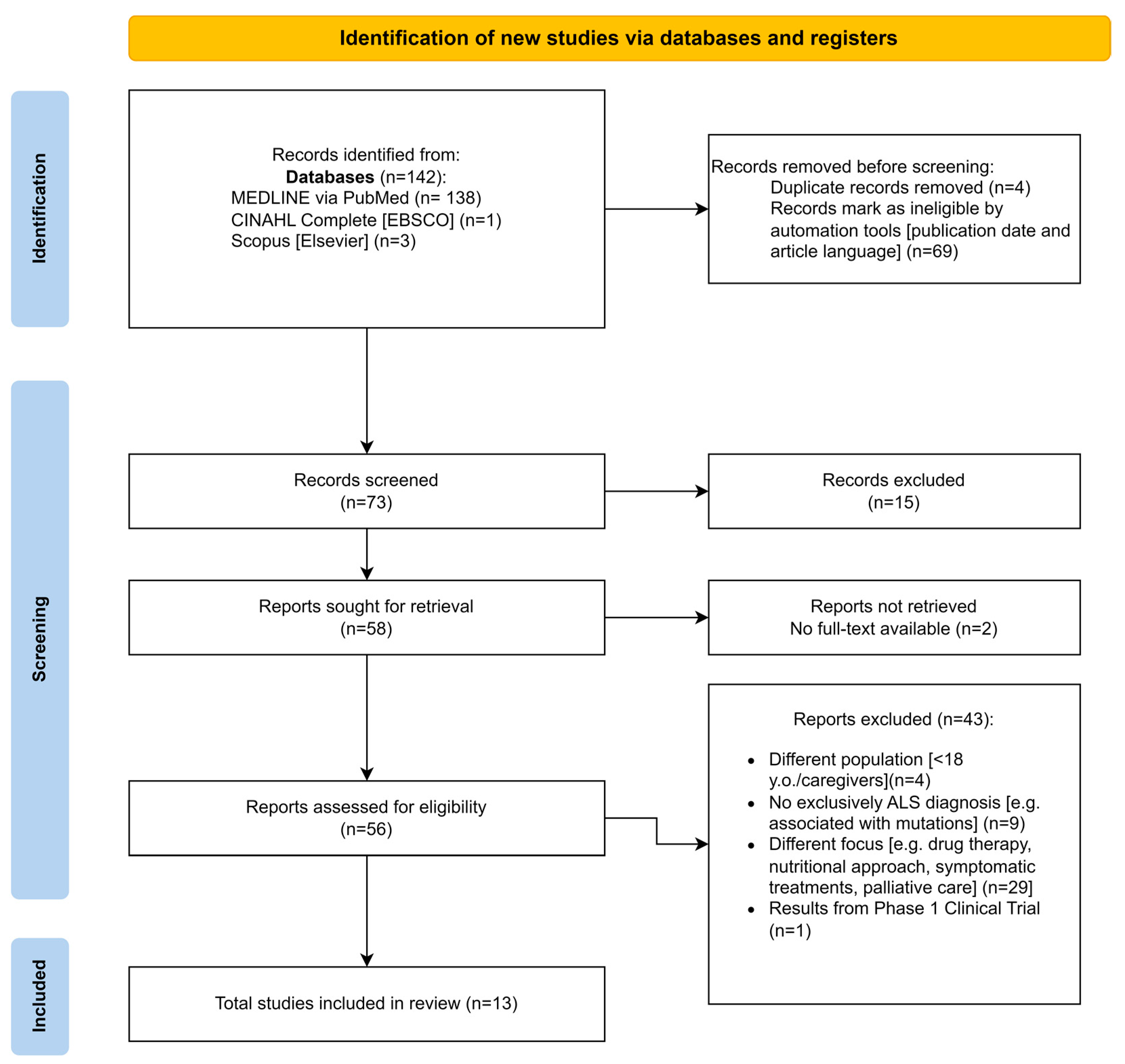
2. Materials and Methods
- How do ALS patients perceive pain?
- What are the characteristics of pain perceived by ALS patients?
2.1. Eligibility Criteria
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.1.3. Filters
2.2. Research Strategy
2.3. Article Selection
Data Extraction
3. Results
3.1. Study Characteristics
3.2. Data Collection Tools
3.3. The Way Pain Is Expressed
3.4. Pain Intensity
3.5. Pain Localisation
3.6. Pain Management
3.7. The Impact of Pain on Activities of Daily Living (ADLs)
3.8. The Impact of Pain on Social Relationships
3.9. The Relationship between Pain and Other Pathologies
3.10. Interference with Compliance and Standard of Care
3.11. Pain and Palliative Care
4. Discussion
4.1. Pain Perception
4.2. Pain Intensity
4.3. Interference of Pain in ADLs and Social Relationships
4.4. Pain Management
4.5. Localisation of Pain
4.6. Areas for Improvement and Recommendation
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Public Involvement Statement
Guidelines and Standards Statement
Use of Artificial Intelligence
Conflicts of Interest
References
- Brown, R.H.; Al-Chalabi, A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017, 377, 162–172. [Google Scholar] [CrossRef]
- Kong, Z.; Chen, P.; Jiang, J.; Wang, X.; Wang, Y.; Shi, Y.; Zhao, B.; Zhu, J. Pain Characteristics in Amyotrophic Lateral Sclerosis Patients and Its Impact on Quality of Life: A Prospective Observational Study in a Northern City of China. Ann. Palliat. Med. 2021, 10, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- Longinetti, E.; Fang, F. Epidemiology of Amyotrophic Lateral Sclerosis: An Update of Recent Lit Erature. Curr. Opin. Neurol. 2019, 32, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S. Pain in Amyotrophic Lateral Sclerosis: A Narrative Review. J. Yeungnam Med. Sci. 2022, 39, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Talbott, E.O.; Malek, A.M.; Arena, V.C.; Wu, F.; Steffes, K.; Sharma, R.K.; Buchanich, J.; Rager, J.R.; Bear, T.; Hoffman, C.A.; et al. Case-Control Study of Environmental Toxins and Risk of Amyotrophic Lat Eral Sclerosis Involving the National ALS Registry. Amyotroph. Lateral Scler. Front. Degener. 2024, 1–10. [Google Scholar] [CrossRef]
- Chiò, A.; Moglia, C.; Canosa, A.; Manera, U.; Vasta, R.; Brunetti, M.; Barberis, M.; Corrado, L.; D’Alfonso, S.; Bersano, E.; et al. Cognitive Impairment across ALS Clinical Stages in a Population-Based Cohort. Neurology 2019, 93. [Google Scholar] [CrossRef]
- Crockford, C.; Newton, J.; Lonergan, K.; Chiwera, T.; Booth, T.; Chandran, S.; Colville, S.; Heverin, M.; Mays, I.; Pal, S.; et al. ALS-Specific Cognitive and Behavior Changes Associated with Advancing Disease Stage in ALS. Neurology 2018, 91. [Google Scholar] [CrossRef]
- Dubbioso, R.; Provitera, V.; Pacella, D.; Santoro, L.; Manganelli, F.; Nolano, M. Autonomic Dysfunction Is Associated with Disease Progression and Survival in Amyotrophic Lateral Sclerosis: A Prospective Longitudinal Cohort Study. J. Neurol. 2023, 270, 4968–4977. [Google Scholar] [CrossRef]
- Nolano, M.; Provitera, V.; Caporaso, G.; Fasolino, I.; Borreca, I.; Stancanelli, A.; Iuzzolino, V.V.; Senerchia, G.; Vitale, F.; Tozza, S.; et al. Skin Innervation across Amyotrophic Lateral Sclerosis Clinical Stages: New Prognostic Biomarkers. Brain 2024, 147, 1740–1750. [Google Scholar] [CrossRef]
- Miller, R.G.; Jackson, C.E.; Kasarskis, E.J.; England, J.D.; Forshew, D.; Johnston, W.; Kalra, S.; Katz, J.S.; Mitsumoto, H.; Rosenfeld, J.; et al. Practice Parameter Update: The Care of the Patient with Amyotrophic Lateral Sclerosis: Drug, Nutritional, and Respiratory Therapies (an Evidence-Based Review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2009, 73, 1218–1226. [Google Scholar] [CrossRef]
- Chiò, A.; Canosa, A.; Gallo, S.; Moglia, C.; Ilardi, A.; Cammarosano, S.; Papurello, D.; Calvo, A. Pain in Amyotrophic Lateral Sclerosis: A Population-Based Controlleds Tudy. Eur. J. Neurol. 2012, 19, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Brettschneider, J.; Kurent, J.; Ludolph, A.; Mitchell, J.D. Drug Therapy for Pain in Amyotrophic Lateral Sclerosis or Motor Neuron Disease. In Cochrane Database of Systematic Reviews; The Cochrane Collaboration, Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2008; p. CD005226.pub2. [Google Scholar]
- Hanisch, F.; Skudlarek, A.; Berndt, J.; Kornhuber, M.E. Characteristics of Pain in Amyotrophic Lateral Sclerosis. Brain Behav. 2015, 5, e00296. [Google Scholar] [CrossRef]
- Åkerblom, Y.; Zetterberg, L.; Larsson, B.J.; Nyholm, D.; Nygren, I.; Åsenlöf, P. Pain, Disease Severity and Associations with Individual Quality of Life in Patients with Motor Neuron Diseases. BMC Palliat. Care 2021, 20, 154. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping Studies: Advancing the Methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Aromataris, E.; Munn, Z. (Eds.) JBI Manual for Evidence Synthesis; JBI: Adelaide, Australia, 2020; ISBN 978-0-648-84880-6. [Google Scholar]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Expla Nation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systemat Ic Reviews. BMJ 2021, n71. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, N.; Radakovic, R.; Boyce, E.; Peryer, G. Prevalence of Pain in Amyotrophic Lateral Sclerosis: A Systematic Revi Ew and Meta-Analysis. Amyotroph. Lateral Scler. Front. Degener. 2021, 22, 449–458. [Google Scholar] [CrossRef]
- Åkerblom, Y.; Larsson, B.J.; Zetterberg, L.; Åsenlöf, P. The Multiple Faces of Pain in Motor Neuron Disease: A Qualitative Study to Inform Pain Assessment and Pain Management. Disabil. Rehabil. 2020, 42, 2123–2132. [Google Scholar] [CrossRef]
- Ishida, N.; Hongo, S.; Kumano, A.; Hatta, H.; Zakoji, N.; Hirutani, M.; Yamamoto, Y.; Aono, H.; Tuigi, M.; Suzuki, R.; et al. Relationship between Pain and Functional Status in Patients with Amyot Rophic Lateral Sclerosis: A Multicenter Cross-Sectional Study. J. Palliat. Med. 2018, 21, 588–591. [Google Scholar] [CrossRef]
- Pagnini, F.; Lunetta, C.; Banfi, P.; Rossi, G.; Fossati, F.; Marconi, A.; Castelnuovo, G.; Corbo, M.; Molinari, E. Pain in Amyotrophic Lateral Sclerosis: A Psychological Perspective. Neurol. Sci. 2012, 33, 1193–1196. [Google Scholar] [CrossRef]
- Pizzimenti, A.; Aragona, M.; Onesti, E.; Inghilleri, M. Depression, Pain and Quality of Life in Patients with Amyotrophic Late Ral Sclerosis: A Cross-Sectional Study. Funct. Neurol. 2013, 28, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Edge, R.; Mills, R.; Tennant, A.; Diggle, P.J.; Young, C.A.; TONiC Study Group. Do Pain, Anxiety and Depression Influence Quality of Life for People w Ith Amyotrophic Lateral Sclerosis/Motor Neuron Disease? A National Stu Dy Reconciling Previous Conflicting Literature. J. Neurol. 2020, 267, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Stephens, H.E.; Lehman, E.; Raheja, D.; Yang, C.; Walsh, S.; Simmons, Z. The Role of Mental Health and Self-Efficacy in the Pain Experience of Patients with Amyotrophic Lateral Sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2016, 17, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Wallace, V.C.J.; Ellis, C.M.; Burman, R.; Knights, C.; Shaw, C.E.; Al-Chalabi, A. The Evaluation of Pain in Amyotrophic Lateral Sclerosis: A Case Contro Lled Observational Study. Amyotroph. Lateral Scler. Front. Degener. 2014, 15, 520–527. [Google Scholar] [CrossRef]
- Hulisz, D. Amyotrophic Lateral Sclerosis: Disease State Overview. Am. J. Manag. Care 2018, 24, S320–S326. [Google Scholar]
- Poquet, N.; Lin, C. The Brief Pain Inventory (BPI). J. Physiother. 2016, 62, 52. [Google Scholar] [CrossRef]
- Cedarbaum, J.M.; Stambler, N.; Malta, E.; Fuller, C.; Hilt, D.; Thurmond, B.; Nakanishi, A. The ALSFRS-R: A Revised ALS Functional Rating Scale That Incorporates Assessments of Respiratory Function. BDNF ALS Study Group (Phase III). J. Neurol. Sci. 1999, 169, 13–21. [Google Scholar] [CrossRef]
- Snaith, R.P. The Hospital Anxiety And Depression Scale. Health Qual. Life Outcomes 2003, 1, 29. [Google Scholar] [CrossRef]
- Vahedi, S. World Health Organization Quality-of-Life Scale (WHOQOL-BREF): Analyse s of Their Item Response Theory Properties Based on the Graded Respons Es Model. Iran. J. Psychiatry 2010, 5, 140–153. [Google Scholar]
- Spitzer, W.O.; Dobson, A.J.; Hall, J.; Chesterman, E.; Levi, J.; Shepherd, R.; Battista, R.N.; Catchlove, B.R. Measuring the Quality of Life of Cancer Patients. J. Chronic Dis. 1981, 34, 585–597. [Google Scholar] [CrossRef]
- Zung, W.W.K. A Self-Rating Depression Scale. Arch. Gen. Psychiatry 1965, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.; Huskisson, E.C. Graphic Representation of Pain. Pain 1976, 2, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Felgoise, S.H.; Stewart, J.L.; Bremer, B.A.; Walsh, S.M.; Bromberg, M.B.; Simmons, Z. The SEIQoL-DW for Assessing Quality of Life in ALS: Strengths and Limi Tations. Amyotroph. Lateral Scler. 2009, 10, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Garra, G.; Singer, A.J.; Domingo, A.; Thode, H.C. The Wong-Baker Pain FACES Scale Measures Pain, Not Fear. Pediatr. Emerg. Care 2013, 29, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index: A Simple Index of Independen Ce Useful in Scoring Improvement in the Rehabilitation of the Chronica Lly Ill. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Benedittis, G.D.; Massel, R.; Nobili, R.; Pieri, A. The Italian Pain Questionnaire. Pain 1988, 33, 53–62. [Google Scholar] [CrossRef]
- Cohen, M.H. Ethical Issues in Discharge Planning for Vulnerable Infants and Childr En. Ethics Behav. 1995, 5, 1–13. [Google Scholar] [CrossRef]
- Freynhagen, R.; Baron, R.; Gockel, U.; Tölle, T.R. painDETECT: A New Screening Questionnaire to Identify Neuropathic Comp Onents in Patients with Back Pain. Curr. Med. Res. Opin. 2006, 22, 1911–1920. [Google Scholar] [CrossRef]
- Anderson, K.O.; Dowds, B.N.; Pelletz, R.E.; Edwards, T.W.; Peeters-Asdourian, C. Development and Initial Validation of a Scale to Measure Self-Efficacy Beliefs in Patients with Chronic Pain. Pain 1995, 63, 77–83. [Google Scholar] [CrossRef]
- Sandstedt, P.; Littorin, S.; Johansson, S.; Gottberg, K.; Ytterberg, C.; Kierkegaard, M. Disability and Contextual Factors in Patients with Amyotrophic Lateral Sclerosis—A Three-Year Observational Study. J. Neuromuscul. Dis. 2018, 5, 439–449. [Google Scholar] [CrossRef]
- Tosas, M.R. The Downgrading of Pain Sufferers’ Credibility. Philos. Ethics Humanit. Med. 2021, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Hoxhaj, P.; Hastings, N.; Kachhadia, M.P.; Gupta, R.; Sindhu, U.; Durve, S.A.; Azam, A.; Auz Vinueza, M.J.; Bhuvan; Win, S.H.; et al. Exploring Advancements in the Treatment of Amyotrophic Lateral Scleros Is: A Comprehensive Review of Current Modalities and Future Prospects. Cureus 2023, 15, e45489. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.E.; Baria, A.T. Assessing Pain Research: A Narrative Review of Emerging Pain Methods, Their Technosocial Implications, and Opportunities for Multidisciplina Ry Approaches. Front. Pain Res. 2022, 3, 896276. [Google Scholar] [CrossRef] [PubMed]
- Pota, V.; Sansone, P.; De Sarno, S.; Aurilio, C.; Coppolino, F.; Barbarisi, M.; Barbato, F.; Fiore, M.; Cosenza, G.; Passavanti, M.B.; et al. Amyotrophic Lateral Sclerosis and Pain: A Narrative Review from Pain A Ssessment to Therapy. Behav. Neurol. 2024, 2024, 1–23. [Google Scholar] [CrossRef]
- Pagnini, F.; Castelnuovo, G. (Eds.) Psychological Issues in Amyotrophic Lateral Sclerosis; Frontiers Media SA: Lausanne, Switzerland, 2016; ISBN 978-2-88919-758-3. [Google Scholar]
- Rivera, I.; Ajroud-Driss, S.; Casey, P.; Heller, S.; Allen, J.; Siddique, T.; Sufit, R. Prevalence and Characteristics of Pain in Early and Late Stages of ALS. Amyotroph. Lateral Scler. Front. Degener. 2013, 14, 369–372. [Google Scholar] [CrossRef]
- Moretta, P.; Spisto, M.; Ausiello, F.P.; Iodice, R.; De Lucia, N.; Santangelo, G.; Trojano, L.; Salvatore, E.; Dubbioso, R. Alteration of Interoceptive Sensitivity: Expanding the Spectrum of Behavioural Disorders in Amyotrophic Lateral Sclerosis. Neurol. Sci. 2022, 43, 5403–5410. [Google Scholar] [CrossRef]
- Ikeda, S. Interoceptive Sensitivity and Perception of Others’ Emotions: An Investigation Based on a Two-Stage Model. Cogn. Process. 2024, 25, 229–239. [Google Scholar] [CrossRef]
| PCC | |
|---|---|
| Population | Patients (≥18 years old) with a diagnosis of spinal/bulbar ALS. |
| Concept | Pain |
| Context | No geographical limitations were imposed, ensuring that all contexts where people with ALS live and frequent are included, such as hospitals, communities, hospices, and homes. |
| Authors/Year | Aim | Sample | Study Design | Interventions | Results |
|---|---|---|---|---|---|
| Åkerblom et al., 2020 [20] | Exploring personal experiences of pain in people with primary lateral sclerosis | 16 participants diagnosed with MND e.g., primary lateral sclerosis, with upper and lower motor neuron signs and symptoms | Exploratory qualitative study | In-depth interviews | Of the 16 participants recruited, 1 refused due to lack of interest in the study. The important findings were the experiences of unpredictability of pain outbreaks, the efforts required to manage pain, the consequences for activity and quality of life, and the suffering induced by decreased attention to and neglect of pain on the part of both patients and staff. |
| Åkerblom et al., 2021 [14] | To study associations between pain, disease severity, and individual quality of life (IQOL) in patients with primary lateral sclerosis | 61 patients recruited from four multidisciplinary teams in Sweden, of whom 55 responded to the pain measure (The Brief Pain Inventory—Short form) and were included in the main analyses | Cross-sectional study | Disease severity was measured with the Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R), and individual quality of life was measured with study-specific SEIQoL-DW. Pain was measured with the Short Form of Brief Pain Inventory (BPI-SF). | Forty-one (74%) of the participants who responded to BPI-SF reported pain. Thirty-nine (71%) of those reported pain in the last 24 h. Pain severity was moderate on average, with eight participants (14%) reporting severe pain. Satisfaction with IQOL for the whole sample was good, and there was no difference in satisfaction with IQOL between those who reported pain/no pain. There was no correlation between severity of pain and satisfaction with IQOL, nor between severity of illness and satisfaction with IQOL. |
| Chiò et al., 2012 [11] | To assess the prevalence and characteristics of pain in an epidemiological series of patients with Amyotrophic Lateral Sclerosis (unspecified whether of spinal or bulbar origin) compared to population-based controls | 160 ALS patients residing in the province of Turin; the controls were randomly selected from the lists of general practitioners. | Controlled population study | Interview and assessment of pain using the Brief Pain Inventory | Patients with ALS reported pain more frequently than controls, healthy subjects, with the same gender and age (±3 years). In ALS patients, pain was more frequently localised to the extremities. |
| Hanisch et al., 2015 [13] | To determine the prevalence, severity, interference, location, and type of pain experienced by 46 German patients with Amyotrophic Lateral Sclerosis (ALS) and to correlate this information with disease duration and severity parameters; to this end, the Brief Pain Questionnaire (BPI) was administered to the patients. | 46 ALS patients and 23 controls with myotonic dystrophy type 2 (DM2) | Cross-sectional | Administration of the BPI and the ALS-FRS-R scale | 78% of the 46 patients (n = 36) reported pain, compared to 54% of the controls. Patients with ALS, compared to controls, reported moderate to severe pain (42% vs. 20%). Pain in ALS patients interfered significantly more with daily activities than in controls. |
| Hurwitz et al., 2021 [19] | To determine the aggregate prevalence of pain in ALS, with respect to its measurement method and pain characteristics | Patients with Amyotrophic Lateral Sclerosis (not specified whether of spinal or bulbar origin) | Systematic review and meta-analysis | A critical examination of the pain measures employed; the measures were classified into three categories: validated (e.g., scales, questionnaires, and structured instruments), customised (e.g., interviews, single-question surveys, and multiple-question surveys), and customised with validated measures. | The overall prevalence of pain in all included studies was 60%, indicating that between half and two-thirds of all ALS patients experience pain. The most frequently reported site of pain in ALS patients is the upper extremities, although it may also manifest in other regions, including the lower extremities, head, back, and neck. The type of pain was frequently associated with cramps or spasms. |
| Ishida et al., 2018 [21] | Determining the frequency and characteristics of pain and its treatment in patients with ALS | 80 patients diagnosed with ALS according to the El Escorial criteria were recruited for this study between 1 May and 31 May 2015. | Multicentre cross-sectional study | Administration of the Wong–Baker Faces Pain Rating Scale (WBS) and assessment of functional status using the Barthel Index | Pain was reported by 53.8% of ALS patients and 36.3% reported receiving painkillers. Opioids are the most commonly used drugs to treat pain. Differences in pain frequency according to functional status were not statistically significant (p = 0.38). Pain intensity in patients whose functional status was total dependence (BI 0–20, 2.5–1.2) was significantly worse than that in those with a better functional status (BI 21–60, 1.4–0.7; BI 61–99, 1.4–0.5; p < 0.01). |
| Kong et al., 2021 [2] | Studying the characteristics of pain in patients with ALS using standardised pain questionnaires | 89 patients with ALS (not specified whether of spinal or bulbar origin) and 89 control subjects with peripheral neuropathy | Prospective observational study | In-person interviews with 89 ALS patients between January 2010 and June 2018; data including gender, age, ALSFRS-R scale score, and pain severity index (PSI) were collected. Characteristics were compared between ALS and peripheral neuropathy, and between ALS patients with and without pain. | There were no significant differences in the sex and age ratio between the two groups. There were many more patients with pain symptoms in the ALS group (35/89.39%) than in the peripheral neuropathy group (20/89.22%). Quality of life was significantly affected in ALS patients with pain (using ALS patients without pain as control subjects). |
| Kwak, 2022 [4] | To provide an overview of the epidemiology, clinical characteristics, underlying mechanisms, and management approaches to pain in ALS, with the objective of optimising clinical practice and patient outcomes | Narrative review | |||
| Pagnini et al., 2012 [22] | Investigating pain in ALS patients and its influence on their quality of life (QoL) | 40 patients with sporadic ALS, recruited from the Neuromuscular Omnicentre (NEMO) in Milan | Observational Study | Administration of the Italian Pain Questionnaire (QUID) and the McGill Quality of Life Questionnaire (MQoL) at time 0 and at follow-up after 4 months | Approximately half of the patients with ALS reported pain, which was described as bothersome, tiresome, and exhausting. These symptoms occurred intermittently, but persisted over time. Pain was found to be correlated with quality of life (QoL), and its intensity was found to be able to predict a worsening of QoL. |
| Pizzimenti et al., 2013 [23] | To assess the prevalence of pain in ALS patients, to compare depression and QoL measures in patients with and without pain, and to study the influence of depression and pain scores on the QoL of ALS patients with pain | Forty patients with ALS were enrolled, and thirty-six were included in the analysis. | Cross-sectional study | Quality of life (QoL) was assessed using the Quality of Life Index (QL- Index), depression using the Zung Self-Rating Depression Scale (SDS), and pain intensity using a visual analogue scale (VAS). | Twenty-six patients (72.2%) reported pain, localised as follows: scapulohumeral area (15 patients, 57.7%), lower limb (8 patients, 30.8%), and cervical–dorsal area (3 patients, 11.5%). The average pain intensity was 7.6 (±2.7), while the average duration of pain in the last 24 h was assessed as 1.4, which corresponds to approximately 2 h per day. The frequency of pain episodes in the last 24 h was 0.6 (approximately 2 per day). A total SDS score of 50 or higher, indicating depression, was found in only one patient. Sixteen patients self-reported several depressive symptoms, but remained in the non-depressed range (score between 35 and 47). Ten of the patients with a score of 35 or higher on the SDS were taking antidepressants. |
| Edge et al., 2020 [24] | To examine the prevalence of pain, anxiety, and depression in a large sample of people with ALS/ MND and to examine their interrelationships and effect on QoL, using a measure that recognises the multifaceted nature of QoL | 636 participants with ALS/MND, diagnosed according to the criteria of the El Escorial World Federation of Neurology using a convenience sampling strategy | Cross-sectional study | Focus groups and 1:1 interviews; administration of the NRS numeric scale for pain assessment, the World Health Organisation Quality of Life Questionnaire (WHOQOL-BREF), and the Hospital Anxiety and Depression Scale for MND (mHADS) | 636 persons with ALS, 69%, reported pain, 7% of the participants exceeded the published cutoffs for probable depression, and 14% had probable anxiety. Pain, depression, and anxiety all affect quality of life; depression has a significant effect on the physical and psychological domains of QoL, while pain affects physical QoL and psychological anxiety QoL. |
| Stephens et al., 2016 [25] | Exploring the relationship between depression, anxiety, self-efficacy, and the experience of pain in ALS patients | The patients included individuals registered with the Agency for Toxic Substances and Disease Registry (ATSDR) who indicated that they wished to be informed about the research studies. | Cross-sectional study | Online, anonymous public survey; ALS Functional Rating Scale-Review (ALSFRS-R); Brief Pain Inventory-Short Form (BPI); Hospital Anxiety and Depression Scale (HADS); and Chronic Pain Self-Efficacy Scale (CPSS) | 197 participants responded to the survey. Borderline cases of depression and anxiety were common. Average pain levels were moderate. Higher pain self-efficacy scores predicted less pain severity, less pain interference and greater pain relief with treatment. As depression scores increased, pain interference with daily life was greater. In conclusion, anxiety and depression are common in patients with ALS and pain. Self-efficacy seems to attenuate pain. |
| Wallace et al., 2014 [26] | To gather information regarding pain in ALS using standardised questionnaires | 42 patients with ALS; the control subjects included 41 healthy volunteers and 42 patients with neurological problems other than ALS. | Observational case-control study | Pain data were collected using The Brief Pain Inventory and The PainDetect Questionnaire. | 85% of subjects with ALS reported pain compared to 50% of controls with neurological problems other than ALS and 35% of healthy controls (p < 0.01). Pain in ALS included cramps, aches, and fatigue, and was non-neuropathic. Pain had a significant impact on mood, general activity, relationships, and overall enjoyment of life. Of those with painful ALS, 54% used analgesic drugs regularly and 29% used opiates on a regular basis. Other non-motor symptoms included fatigue, constipation, urinary problems, itching, and drowsiness. |
| Authors | Country | Sample | M/F | Severity of Disease ALSFRS-R † or Stage |
|---|---|---|---|---|
| Åkerblom et al., 2020 [20] | Sweden | 16 | 11/5 | 32.50 |
| Åkerblom et al., 2021 [14] | Sweden | 61 | 39/22 | 37 |
| Chiò et al., 2012 [11] | Italy | 160 | 91/69 | 28.70 |
| Edge et al., 2020 [24] | United Kingdom | 636 | 390/246 | _ |
| Hanisch et al., 2015 [13] | Germany | 46 | 20/26 | 33.10 |
| Hurwitz et al., 2021 [19] | United Kingdom | _ | _ | _ |
| Ishida et al., 2018 [21] | Japan | 80 | 45/35 | _ |
| Kong et al., 2021 [2] | China | 178 | 54/35 ALS 57/32 peripheral neuropathy (control group) | _ |
| Kwak, 2022 [4] | Korea | _ | _ | _ |
| Pagnini et al., 2012 [22] | Italy | 40 | _ | 34.87 |
| Pizzimenti et al., 2013 [23] | Italy | 36 | 22/14 | 35.10 |
| Stephens et al., 2016 [25] | United States | 287 | 184/103 | 31.29 |
| Wallace et al., 2014 [26] | United Kingdom | 24 | 17/7 | 5 people were at stage 1 16 people were at stage 2 12 people were at stage 3 9 people were at stage 4 |
| Åkerblom et al., 2020 [20] | Åkerblom et al., 2021 [14] | Chiò et al., 2012 [11] | Edge et al., 2020 [24] | Hanisch et al., 2015 [13] | Hurwitz et al., 2021 [19] | Ishida et al., 2018 [21] | Kong et al., 2021 [2] | Kwak, 2022 [4] | Pagnini et al., 2012 [22] | Pizzimenti et al., 2013 [23] | Stephens et al., 2016 [25] | Wallace et al., 2014 [26] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Face-to-face interview | X | X | X | X | |||||||||
| Brief Pain Inventory (BPI) [28] | X | X | X | X | X | X | |||||||
| ALS Functional Rating Scale-Review (ALSFRS-R) [29] | X | X | X | X | X | ||||||||
| Focus group | X | ||||||||||||
| Scala di valutazione del dolore NRS | X | ||||||||||||
| World Health Organization Quality-of-Life Scale (WHOQOL-BREF) [31] | X | ||||||||||||
| Anxiety and depression scale (HADS) (HADS) [30] | X | X | |||||||||||
| Quality of Life Index (QL-Index) [32] | X | ||||||||||||
| Zung Self-Rating Depression Scale (SDS) [33] | X | ||||||||||||
| Visual analogical scale (VAS) [34] | X | ||||||||||||
| Schedule for the Evaluation of the Individual Quality of Life-Direct Weighting (SEIQoL-DW) [35] | X | ||||||||||||
| Wong–Baker Faces Pain Rating Scale (WBS) [36] | X | ||||||||||||
| Barthel Index [37] | X | ||||||||||||
| Italian Pain Questionnaire (QUID) [38] | X | ||||||||||||
| McGill Quality of Life Questionnaire (MQoL) [39] | X | ||||||||||||
| Pain Detect Questionnaire [40] | X | ||||||||||||
| Survey online | X | ||||||||||||
| Chronic Pain Self-Efficacy Scale (CPSS) [41] | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, D.; Ingrande, L.; Marcomini, I.; Poliani, A.; Villa, G.; Sodano, M.; Manara, D.F. Perceived Pain in People Living with Amyotrophic Lateral Sclerosis—A Scoping Review. Nurs. Rep. 2024, 14, 3023-3039. https://doi.org/10.3390/nursrep14040220
Rosa D, Ingrande L, Marcomini I, Poliani A, Villa G, Sodano M, Manara DF. Perceived Pain in People Living with Amyotrophic Lateral Sclerosis—A Scoping Review. Nursing Reports. 2024; 14(4):3023-3039. https://doi.org/10.3390/nursrep14040220
Chicago/Turabian StyleRosa, Debora, Laura Ingrande, Ilaria Marcomini, Andrea Poliani, Giulia Villa, Martina Sodano, and Duilio Fiorenzo Manara. 2024. "Perceived Pain in People Living with Amyotrophic Lateral Sclerosis—A Scoping Review" Nursing Reports 14, no. 4: 3023-3039. https://doi.org/10.3390/nursrep14040220
APA StyleRosa, D., Ingrande, L., Marcomini, I., Poliani, A., Villa, G., Sodano, M., & Manara, D. F. (2024). Perceived Pain in People Living with Amyotrophic Lateral Sclerosis—A Scoping Review. Nursing Reports, 14(4), 3023-3039. https://doi.org/10.3390/nursrep14040220







