Brief Report: Hispanic Patients’ Trajectory of Cancer Symptom Burden, Depression, Anxiety, and Quality of Life
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.3. Data Analysis
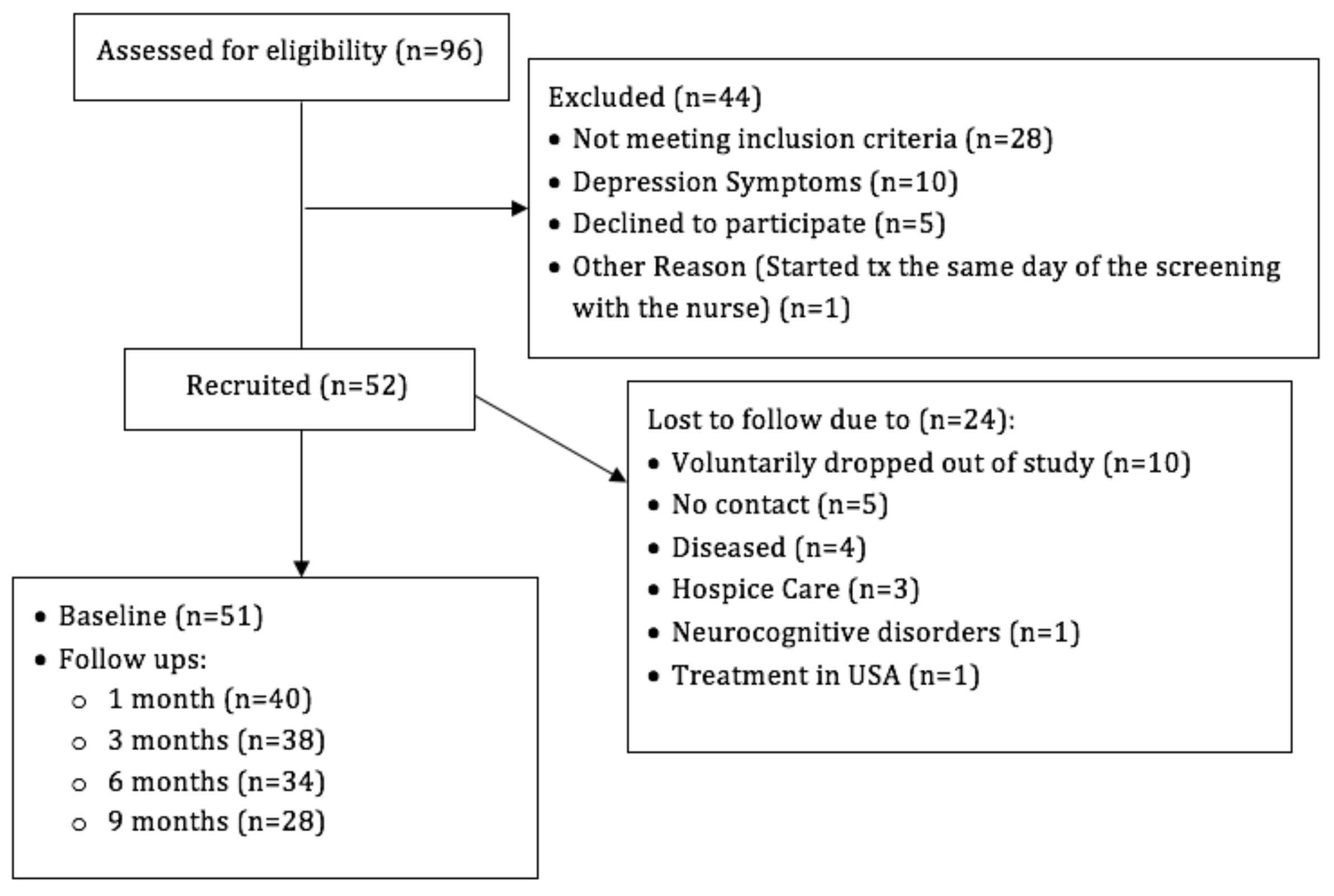
3. Results
3.1. Participants’ Socio-Demographic and Clinical Characteristics
3.2. Correlations among Anxiety, Depression, Cancer Symptom Burden, and Quality of Life
3.3. Hypothesis Testing Multilevel Mixed-Effects Linear Regression Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitchell, A.J.; Chan, M.; Bhatti, H.; Halton, M.; Grassi, L.; Johansen, C.; Meader, N. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: A meta-analysis of 94 interview-based studies. Lancet Oncol. 2011, 12, 160–174. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Ferguson, D.W.; Gill, J.; Paul, J.; Symonds, P. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 721–732. [Google Scholar] [CrossRef]
- Jacob, L.; Kalder, M.; Kostev, K. Incidence of depression and anxiety among women newly diagnosed with breast or genital organ cancer in Germany. Psycho-Oncol. 2017, 26, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Snyderman, D.; Wynn, D. Depression in cancer patients. Prim. Care 2009, 36, 703–719. [Google Scholar] [CrossRef]
- Luckett, T.; Goldstein, D.; Butow, P.N.; Gebski, V.; Aldridge, L.J.; McGrane, J.; Ng, W.; King, M.T. Psychological morbidity and quality of life of ethnic minority patients with cancer: A systematic review and meta-analysis. Lancet Oncol. 2011, 12, 1240–1248. [Google Scholar] [CrossRef]
- Costas, R.; Gany, F. Depressive symptoms in a sample of Afro-Caribbean and Latino immigrant cancer patients: A comparative analysis. Supportive Care Cancer 2013, 21, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Yanez, B.; Thompson, E.H.; Stanton, A.L. Quality of life among Latina breast cancer patients: A systematic review of the literature. J. Cancer Surviv. 2011, 5, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.; Dunn, L.B.; Phoenix, B.; Paul, S.M.; Hamolsky, D.; Levine, J.D.; Miaskowski, C. Co-occurrence of anxiety and depressive symptoms following breast cancer surgery and its impact on quality of life. Eur. J. Oncol. Nurs. 2016, 20, 97–105. [Google Scholar] [CrossRef]
- Hulbert-Williams, N.; Neal, R.; Morrison, V.; Hood, K.; Wilkinson, C. Anxiety, depression and quality of life after cancer diagnosis: What psychosocial variables best predict how patients adjust? Psycho Oncol. 2012, 21, 857–867. [Google Scholar] [CrossRef]
- Gonzalez-Mercado, V.J.; Saligan, L.N.; Ji, M.; Groer, M.; Pedro, E.; McMillan, S. Differences in the severity, distress, interference, and frequency on cancer-related symptoms between Island Hispanic Puerto Ricans and Mainland non-Hispanic Whites. J. Immigr. Minority Health 2018, 20, 1029–1039. [Google Scholar] [CrossRef]
- Krok-Schoen, J.L.; Fernandez, K.; Unzeitig, G.W.; Rubio, G.; Paskett, E.D.; Post, D.M. Hispanic breast cancer patients’ symptom experience and patient-physician communication during chemotherapy. Supportive Care Cancer 2019, 27, 697–704. [Google Scholar] [CrossRef]
- Check, D.K.; Chawla, N.; Kwan, M.L.; Pinheiro, L.; Roh, J.M.; Ergas, I.J.; Stewart, A.L.; Kolevska, T.; Ambrosone, C.; Kushi, L.H. Understanding racial/ethnic differences in breast cancer-related physical well-being: The role of patient–provider interactions. Breast Cancer Res. Treat. 2018, 170, 593–603. [Google Scholar] [CrossRef]
- Wilson, I.B.; Cleary, P.D. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA 1995, 273, 59–65. [Google Scholar] [CrossRef]
- Hyland, K.A.; Hoogland, A.I.; Gonzalez, B.D.; Nelson, A.M.; Lechner, S.; Tyson, D.M.; Barata, A.; Gomez, M.F.; Antoni, M.H.; Small, B.; et al. Evaluation of the Psychometric and Structural Properties of the Spanish Version of the Hospital Anxiety and Depression Scale in Latina Cancer Patients. J. Pain Symptom Manag. 2019. [Google Scholar] [CrossRef] [PubMed]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The validity of the Hospital Anxiety and Depression Scale: An updated literature review. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef]
- Cleeland, C.S.; Mendoza, T.R.; Wang, X.S.; Chou, C.; Harle, M.T.; Morrissey, M.; Engstrom, M.C. Assessing symptom distress in cancer patients: The MD Anderson Symptom Inventory. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2000, 89, 1634–1646. [Google Scholar] [CrossRef]
- Cella, D.F.; Tulsky, D.S.; Gray, G.; Sarafian, B.; Linn, E.; Bonomi, A.; Silberman, M.; Yellen, S.B.; Winicour, P.; Brannon, J. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1993, 11, 570–579. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 14; StataCorp LP: College Station, TX, USA, 2015. [Google Scholar]
- Linden, W.; Vodermaier, A.; MacKenzie, R.; Greig, D. Anxiety and depression after cancer diagnosis: Prevalence rates by cancer type, gender, and age. J. Affect. Disord. 2012, 141, 343–351. [Google Scholar] [CrossRef]
- Hess, C.B.; Chen, A.M. Measuring psychosocial functioning in the radiation oncology clinic: A systematic review. Psycho-Oncol. 2014, 23, 841–854. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Tyson, D.M.; Gonzalez, B.D.; Small, B.J.; Lechner, S.C.; Antoni, M.H.; Vinard, A.; Krause, M.; Meade, C.; Jacobsen, P.B. Anxiety and depression in S Spanish-speaking Latina cancer patients prior to starting chemotherapy. Psycho Oncol. 2018, 27, 333–338. [Google Scholar] [CrossRef]
- Stafford, L.; Judd, F.; Gibson, P.; Komiti, A.; Mann, G.B.; Quinn, M. Anxiety and depression symptoms in the 2 years following diagnosis of breast or gynaecologic cancer: Prevalence, course and determinants of outcome. Supportive Care Cancer 2015, 23, 2215–2224. [Google Scholar] [CrossRef]
- Chung, J.; Ju, G.; Yang, J.; Jeong, J.; Jeong, Y.; Choi, M.K.; Kwon, J.; Lee, K.H.; Kim, S.T.; Han, H.S. Prevalence of and factors associated with anxiety and depression in Korean patients with newly diagnosed advanced gastrointestinal cancer. Korean J. Intern. Med. 2018, 33, 585. [Google Scholar] [CrossRef]
- Mols, F.; Schoormans, D.; de Hingh, I.; Oerlemans, S.; Husson, O. Symptoms of anxiety and depression among colorectal cancer survivors from the population-based, longitudinal PROFILES Registry: Prevalence, predictors, and impact on quality of life. Cancer 2018, 124, 2621–2628. [Google Scholar] [CrossRef]
- Hipkins, J.; Whitworth, M.; Tarrier, N.; Jayson, G. Social support, anxiety and depression after chemotherapy for ovarian cancer: A prospective study. Br. J. Health Psychol. 2004, 9, 569–581. [Google Scholar] [CrossRef]
- Hellstadius, Y.; Lagergren, J.; Zylstra, J.; Gossage, J.; Davies, A.; Hultman, C.M.; Lagergren, P.; Wikman, A. A longitudinal assessment of psychological distress after oesophageal cancer surgery. Acta Oncol. 2017, 56, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Vahdaninia, M.; Omidvari, S.; Montazeri, A. What do predict anxiety and depression in breast cancer patients? A follow-up study. Soc. Psychiatry Psychiatr. Epidemiol. 2010, 45, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Smith, T.G.; Michonski, J.D.; Stein, K.D.; Kaw, C.; Cleeland, C.S. Symptom burden in cancer survivors 1 year after diagnosis: A report from the American Cancer Society’s Studies of Cancer Survivors. Cancer 2011, 117, 2779–2790. [Google Scholar] [CrossRef] [PubMed]
- Grotmol, K.S.; Lie, H.C.; Loge, J.H.; Aass, N.; Haugen, D.F.; Stone, P.C.; Kaasa, S.; Hjermstad, M.J. Patients with advanced cancer and depression report a significantly higher symptom burden than non-depressed patients. Palliat. Support. Care 2019, 17, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Yennurajalingam, S.; Tayjasanant, S.; Balachandran, D.; Padhye, N.S.; Williams, J.L.; Liu, D.D.; Frisbee-Hume, S.; Bruera, E. Association between daytime activity, fatigue, sleep, anxiety, depression, and symptom burden in advanced cancer patients: A preliminary report. J. Palliat. Med. 2016, 19, 849–856. [Google Scholar] [CrossRef] [PubMed]
- McFarland, D.C.; Shaffer, K.M.; Tiersten, A.; Holland, J. Physical symptom burden and its association with distress, anxiety, and depression in breast cancer. Psychosomatics 2018, 59, 464–471. [Google Scholar] [CrossRef]
- Leonhart, R.; Tang, L.; Pang, Y.; Li, J.; Song, L.; Fischer, I.; Koch, M.; Wuensch, A.; Fritzsche, K.; Schaefert, R. Physical and psychological correlates of high somatic symptom severity in Chinese breast cancer patients. Psycho-Oncol. 2017, 26, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, W.J.; Mo, L.L.; Luo, S.X.; Yu, J.Y.; Dong, Z.Q.; Liu, Y.; Huang, M.J.; Wang, Y.; Chen, L.; et al. Prevalence and strong association of high somatic symptom severity with depression and anxiety in a Chinese inpatient population. Asia-Pacific Psychiatry 2017, 9, e12282. [Google Scholar] [CrossRef] [PubMed]
| Socio-Demographics | |
|---|---|
| Age | |
| Mean ± SD | 63.3 ± 14.6 |
| Median (min, max) | 66 (23, 86) |
| Sex, n (%) | |
| Male | 30 (58.8) |
| Female | 21 (41.2) |
| Household composition, n (%) | |
| Alone | 8 (15.7) |
| Partner | 19 (37.3) |
| Son/Daughter | 5 (9.8) |
| Parents | 2 (3.9) |
| Partner and Son/Daughter | 10 (19.6) |
| Parents and Son/Daughter | 2 (3.9) |
| Son/Daughter and Grandchildren | 2 (3.9) |
| Partner, Son/Daughter, and Grandchildren | 1 (2.0) |
| Other Family Members | 2 (3.9) |
| Employment, n (%) | |
| Employed | 6 (11.8) |
| Unemployed | 8 (15.7) |
| Disabled | 4 (7.8) |
| Retired | 30 (58.8) |
| Student | 1 (2.0) |
| Other (did not specify) | 2 (3.9) |
| Education, n (%) | |
| <High School | 15 (29.4) |
| ≥High School | 36 (70.6) |
| Civil Status, n (%) | |
| Single | 7 (13.7) |
| Married/Living with Partner | 32 (62.8) |
| Divorced | 6 (11.8) |
| Widowed | 6 (11.8) |
| Medical Insurance †, n (%) | |
| Private ‡ | 14 (28.0) |
| Health Care Reform § | 14 (28.0) |
| Medicare | 22 (44.0) |
| Household Income, n (%) | |
| ≤$19,000 | 37 (72.6) |
| >$19,000 | 14 (27.4) |
| Clinical Features | |
| Tumour site (primary), n (%) | |
| Breast | 6 (11.8) |
| Prostate †† | 5 (9.8) |
| Multiple Myeloma | 2 (3.9) |
| Head and Neck | 3 (5.9) |
| Leukaemia | 2 (3.9) |
| Lung | 3 (5.9) |
| Pancreatic | 6 (11.8) |
| Lymphoma | 5 (9.8) |
| Colorectal | 10 (19.6) |
| Stomach | 2 (3.9) |
| Melanoma | 2 (3.9) |
| Other sites ‡‡ | 5 (9.8) |
| Disease stage, n (%) | |
| I †† | 6 (12.0) |
| II | 9 (18.0) |
| III | 13 (26.0) |
| IV | 22 (44.0) |
| Characteristics | Without Depressive Symptoms (n = 17) | With Depressive Symptoms (n = 11) | p-Value † |
|---|---|---|---|
| Sex | 0.14 ‡ | ||
| Male | 11 (64.7) | 4 (36.4) | |
| Female | 6 (35.3) | 7 (63.6) | |
| Age | 0.72 | ||
| Mean (+/− SD) | 59.2 (14.9) | 62.4 (15.5) | |
| Median (P25–P75) | 65 (55–67) | 63 (47–78) | |
| Household Income | 0.08 | ||
| ≤$19,000 | 15 (88.2) | 6 (54.6) | |
| >$19,000 | 2 (11.8) | 5 (45.5) | |
| Income-Enough | 0.14 ‡ | ||
| No | 6 (35.3) | 7 (63.6) | |
| Yes | 11 (64.7) | 4 (36.4) | |
| Education | 0.65 | ||
| <High School | 3 (17.7) | 3 (27.3) | |
| ≥High School | 14 (82.4) | 8 (72.7) | |
| Marital status | 0.08 | ||
| Single | 4 (23.5) | 0 (0.0) | |
| Married/Living with a partner | 9 (52.9) | 10 (90.1) | |
| Divorced | 3 (17.7) | 0 (0.0) | |
| Widowed | 1 (5.9) | 1 (9.1) |
| Fixed Effect | Depression Symptoms | Anxiety Symptoms |
|---|---|---|
| Intercept | 7.97 (4.43, 11.51) | 11.06 (6.76, 15.36) |
| Time point (visits) | ||
| Baseline | REFERENCE | REFERENCE |
| 1 | −0.23 (−0.94, 0.48) | −0.72 (−1.60, 0.16) |
| 2 | 0.19 (−0.54, 0.92) | −0.19 (−1.10, 0.71) |
| 3 | −0.00 (−0.75, 0.75) | −0.54 (−1.47, 0.39) |
| 4 | −0.39 (−1.23, 0.45) | −1.23 (−2.27, −0.19) ‡ |
| Sex | ||
| Male | REFERENCE | REFERENCE |
| Female | 0.85 (−0.26, 1.96) | 1.91 (0.58, 3.24) ‡ |
| Age† | −0.12 (−0.33, 0.10) | −0.24 (−0.50, 0.02) |
| Marital Status | ||
| Single | REFERENCE | REFERENCE |
| Married/Living with Partner | 1.57 (−0.24, 3.38) | 1.00 (−1.15, 3.16) |
| Divorced | 0.29 (−1.95, 2.54) | −0.37 (−3.05, 2.31) |
| Widowed | 0.95 (−1.74, 3.65) | 1.80 (−1.43, 5.03) |
| Quality of Life† | −0.33 (−0.47, −0.18) ‡ | −0.38 (−0.55, −0.20) ‡ |
| Burden of Cancer Symptoms† | 0.04 (−0.03, 0.11) | 0.11 (0.02, 0.19) ‡ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Figueroa, E.M.; Torres-Blasco, N.; Rosal, M.C.; Jiménez, J.C.; Castro-Rodríguez, W.P.; González-Lorenzo, M.; Vélez-Cortés, H.; Toro-Bahamonde, A.; Costas-Muñiz, R.; Armaiz-Peña, G.N.; et al. Brief Report: Hispanic Patients’ Trajectory of Cancer Symptom Burden, Depression, Anxiety, and Quality of Life. Nurs. Rep. 2021, 11, 475-483. https://doi.org/10.3390/nursrep11020044
Castro-Figueroa EM, Torres-Blasco N, Rosal MC, Jiménez JC, Castro-Rodríguez WP, González-Lorenzo M, Vélez-Cortés H, Toro-Bahamonde A, Costas-Muñiz R, Armaiz-Peña GN, et al. Brief Report: Hispanic Patients’ Trajectory of Cancer Symptom Burden, Depression, Anxiety, and Quality of Life. Nursing Reports. 2021; 11(2):475-483. https://doi.org/10.3390/nursrep11020044
Chicago/Turabian StyleCastro-Figueroa, Eida M., Normarie Torres-Blasco, Milagros C. Rosal, Julio C. Jiménez, Wallesca P. Castro-Rodríguez, Marilis González-Lorenzo, Héctor Vélez-Cortés, Alia Toro-Bahamonde, Rosario Costas-Muñiz, Guillermo N. Armaiz-Peña, and et al. 2021. "Brief Report: Hispanic Patients’ Trajectory of Cancer Symptom Burden, Depression, Anxiety, and Quality of Life" Nursing Reports 11, no. 2: 475-483. https://doi.org/10.3390/nursrep11020044
APA StyleCastro-Figueroa, E. M., Torres-Blasco, N., Rosal, M. C., Jiménez, J. C., Castro-Rodríguez, W. P., González-Lorenzo, M., Vélez-Cortés, H., Toro-Bahamonde, A., Costas-Muñiz, R., Armaiz-Peña, G. N., & Jim, H. (2021). Brief Report: Hispanic Patients’ Trajectory of Cancer Symptom Burden, Depression, Anxiety, and Quality of Life. Nursing Reports, 11(2), 475-483. https://doi.org/10.3390/nursrep11020044







