Abstract
Venous thromboembolism (VTE) is a life-threatening complication, especially in case of recurrence. The appropriate duration of anticoagulant treatment following the first event is crucial. Risk factors that increase the risk of recurrence of VTE are many, and include medications, kidney disease, renal transplantation (RT), and a diagnosis of sickle cell disease (SCD). There are currently no guidelines that define the duration of anticoagulant therapy after the first event in a patient with RT. We report a case of recurring episodes of VTE after RT in a SCD patient. Our case suggests that the use of a long-term anticoagulant treatment may be recommended in patients with SCD and RT after the first event of VTE.
1. Introduction
Sickle cell disease (SCD) is one of the most common hemoglobinopathies [1,2,3]. Its pathology is characterized by various systemic sequelae, including renal manifestations [4,5], that may result in end-stage renal disease (ESRD) [6,7,8], requiring hemodialysis and transplantation. Venous thromboembolism (VTE) is also common in these patients due to an underlying hemolysis-induced hypercoagulable state [4,9,10,11,12]. VTE risk is increased in renal disease, irrespective of medications or transplantation history [13,14].
This report describes a case of a 21-year-old man with SCD suffering from recurrent venous thromboembolism (VTE) after renal transplantation (RT).
2. Clinical Case
A 21-year-old man with SCD (genotype: homozygous point mutation GAG→GTG at the sixth codon of beta-globin) and a history of hemolytic and vaso-occlusive crises (VOC) phenotype and renal transplantation was hospitalized in our department for recurrent VTE. Splenectomy was performed at 6 years of age, and he received transfusion treatment until the age of 12 years because he had a low Hb level without VOC. He was first managed with periodic erythrocytapheresis from 12 to 19 years, followed by hydroxyurea (HU) treatment because of poor symptom control, with frequent VOC. Despite HU therapy, after one year, the patient was again admitted for VOC, acute chest syndrome, and acute renal failure associated with nephrotic syndrome. He was treated with analgesic therapy, hydration, erythrocytapheresis, and transfusion support. Despite treatment, the renal damage did not improve. Negative autoimmunity, hypoalbuminemia, and nephrotic proteinuria were found on laboratory examination. The kidneys showed normal morphology on ultrasonography. Thus, HU treatment was stopped, and the patient was treated with ACE-I and periodic erythrocytapheresis.
High doses of steroid were used, without benefit, because of the nephrotic syndrome persistence, which led to frequent hospitalizations for anasarca. Therefore, a renal biopsy was performed. The histological report of the renal biopsy showed a pattern of crescentic pauci-immune glomerulonephritis, suggestive of renal damage secondary to SCD. Therefore, there were no indications to continue immunosuppressive treatment with steroid therapy or rituximab. A treatment attempt of compassionate use with crizanlizumab, on the basis of SCD damage, was performed without any benefit. The patient proceeded to peritoneal dialysis because of worsening kidney function, as well as erythropoietin therapy.
In 2021, he underwent RT, which was complicated by a post-surgical perirenal hematoma, which gradually resolved. After the transplant, there was a gradual decrease in his creatinine level. The patient received immunosuppressive therapy with steroids, mycophenolate, and tacrolimus. He was treated with erythrocytapheresis for 5 months after transplantation to prevent VOC. In September 2021, the patient was admitted for VOC and severe thrombocytosis (platelets = 1,410,000/mm3). No evidence of myeloproliferative disease was shown (negativity of JAK2V617F and BCR-ABL and of a bone marrow biopsy). Calreticulin was not checked. Therefore, on the basis of thrombocytosis, reactive to inflammation, the patient received HU treatment and acetylsalicylic prophylaxis.
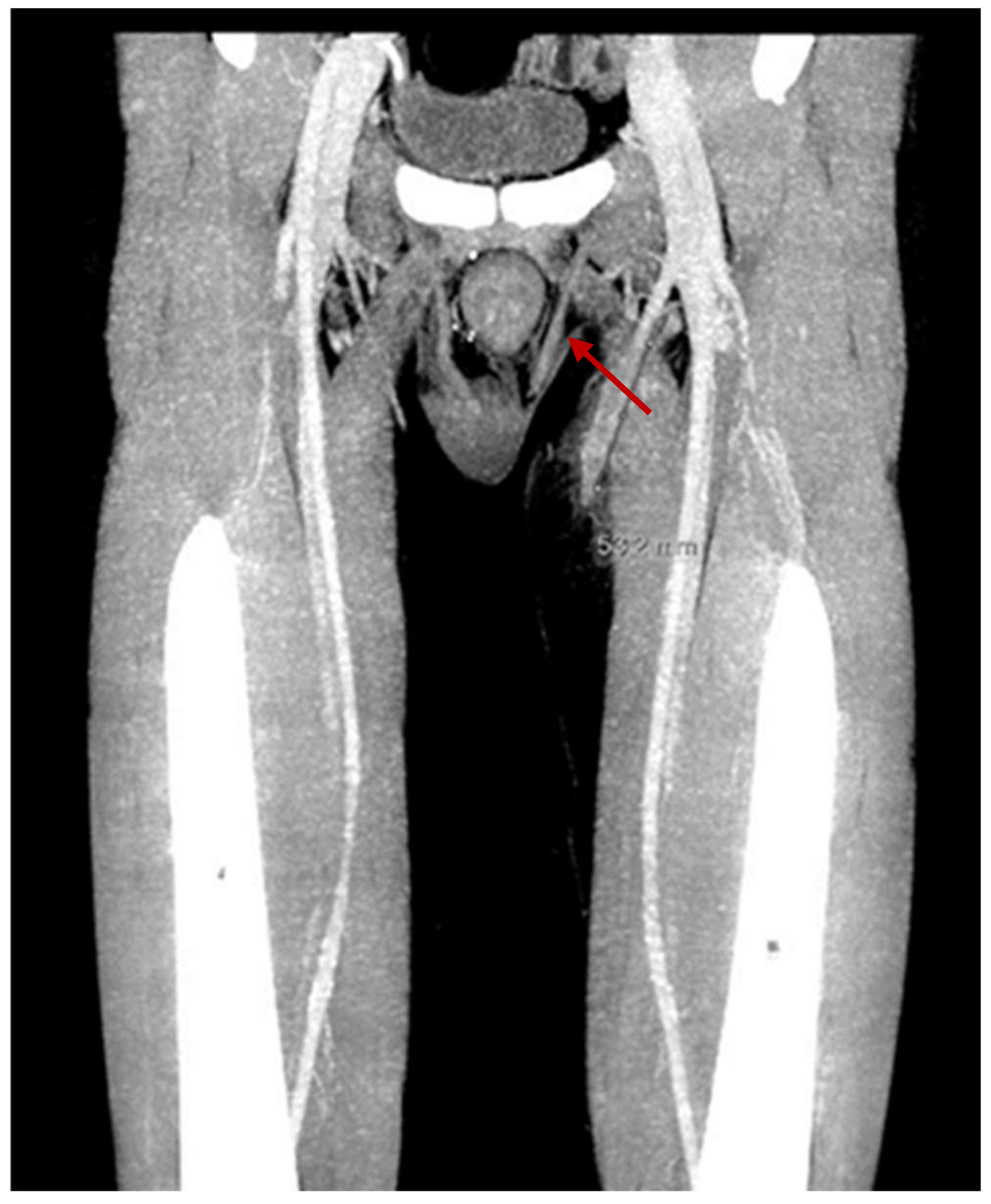
The patient had his first episode of VTE, in two superficial veins of the right forearm with 10 cm extension, six months after renal transplantation. There was no thrombocytosis at the time of VTE, and the level of antithrombin III was 90%. Furthermore, no instrumental maneuvers were performed at the site of VTE. Family history for VTE was negative. The patient received anticoagulant treatment with subcutaneous injection of enoxaparin 100 UI/kg twice a day, which was discontinued after two months. In January 2022, he had another episode of VTE, which was 10 cm long, in the small and large saphenous veins of the left inferior limb. The same anticoagulant treatment was started again. The search for mutations at the G20210A Factor II and Factor V was negative, as was the search for antiphospholipid antibodies. There were no varicose veins. A hematuria episode required discontinuation of anticoagulation within a month of the start of treatment. An ultrasound was performed on the left leg, and it showed the resolution of superficial VTE. Fifteen days after stopping anticoagulant treatment, a further episode of superficial VTE occurred, with extension for 5 cm in the great saphenous of the left lower limb, above the knee, up to 2 cm from the origin of the deep vein system (see Figure 1). Because of the recurrence of superficial VTE, it was decided to use long-term anticoagulant treatment with vitamin K inhibitors. No further thrombosis was observed after two months of follow-up.
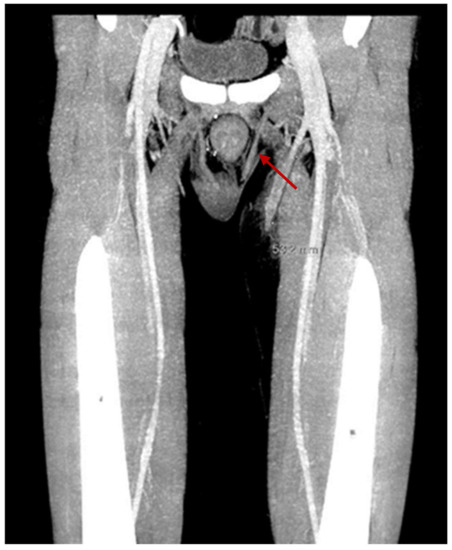
Figure 1.
Superficial venous thrombosis in the great saphenous of the left lower limb with 5 cm extension, above the knee, up to 2 cm from the origin of the deep vein system.
3. Discussion
Episodes of recurring superficial VTE were observed in a patient with SCD after RT. As the patient had never experienced thrombotic events prior to transplantation, we examined what risk factors might be responsible for the increased tendency for thrombosis (see Table 1). Superficial and deep venous thrombosis depend on hereditary and acquired risk factors [11,12]. Among the congenital factors, the patient had SCD [4,9,10]. However, the absent recurrence of thrombotic events before transplantation made us think that SCD alone could not be the only cause of VTE. The patient was also screened for thrombophilia, and the result was negative for G20210A Factor II and Factor V [15,16,17]. Medications, splenectomy, infections, and renal disease are acquired factors that could also be associated with VTE [18,19]. Among the drugs used by the patient, steroids and tacrolimus may increase the thrombotic risk [19,20].

Table 1.
Main risk factor of VTE in the patient.
Renal diseases such as nephrotic syndrome, chronic renal failure, and RT increased the risk of VTE in our patient. Several studies have shown that RT patients have an increased risk of thrombosis, likely caused by a chronic condition of hypercoagulability and inflammatory status. Pulmonary embolism and deep vein thrombosis occur in 6–8% of patients with RT within a median of 6 months post-transplantation [21,22,23,24]. Recurrence rate of VTE in a patient with RT, after the first event, is between 48 and 50%, which is higher in comparison with patients without RT [21,25]. To our knowledge, there are no studies in the literature on the recurrence rate of superficial vein thrombosis in both RT and SCD patients. However, in patients without cancer and with superficial vein thrombosis, the risk of recurrence of VTE is similar to that of patients with previous deep vein thrombosis [26]. Furthermore, 50% of relapses in a patient with a previous superficial vein thrombosis are deep vein thrombosis [26,27,28]. Regardless, no clear guidelines have been published to recommend the duration of anticoagulant treatment for patients with RT and recurrent episodes of VTE.
Todeschini et al. [24] attempted to evaluate which factors may increase the risk of VTE in RT patients. These included the type of pathology causing the transplant, the type of pre-transplant dialysis, and the immunosuppressive therapy administered. In this study, nephrotic syndrome and immunosuppressive treatment based on steroids, mycophenolate, and tacrolimus were likely associated with a higher risk of VTE, while infections post-transplantation and the type of dialysis did not show a correlation with VTE. Thus, the recurrence of VTE events in our patient, besides the duration of anticoagulant treatment, may be due to the presence of pre-transplant nephrotic syndrome, the type of immunosuppressive treatment, and the risk related to SCD.
4. Conclusions
In conclusion, this case report may suggest that RT may increase the already high thrombotic risk in SCD patients, and the use of long-term anticoagulant treatment may be recommended in individuals who develop recurrent VTE following RT.
Author Contributions
Case management: R.D.M. Manuscript drafting: A.G. Manuscript review and editing for important intellectual content: R.D.M., A.G., A.M., D.R., G.C., S.R., L.P. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data can be made available upon request to the corresponding author.
Acknowledgments
The authors thank the Foundation Franco and Piera Cutino for the support with conducting research on thalassemia. The help of Eurobloodnet (ERN) with spreading the research on rare diseases is also fully appreciated.
Conflicts of Interest
Aurelio Maggio has been or is a member of advisory boards for Novartis, Celgene Corp (Bristol Meyers Squibb), Vertex, and Bluebird Bio. The remaining authors have no conflicts of interest to disclose.
References
- Weatherall, D.J.; Clegg, J.B. Inherited haemoglobin disorders: An increasing global health problem. Bull. World Health Organ. 2001, 79, 704–712. [Google Scholar] [PubMed]
- Aygun, B.; Odame, I. A global perspective on sickle cell disease. Pediatr. Blood Cancer. 2012, 59, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Modell, B.; Darlison, M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull. World Health Organ. 2008, 86, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Liem, R.I.; Lanzkron, S.; Coates, T.D.; DeCastro, L.; Desai, A.A.; Ataga, K.I.; Cohen, R.T.; Haynes, J.; Osunkwo, I.; Lebensburger, J.D.; et al. American Society of Hematology 2019 guidelines for sickle disease: Cardiopulmonary and kidney disease. Blood Adv. 2019, 3, 3867–3897. [Google Scholar] [CrossRef]
- Guasch, A.; Navarrete, J.; Nass, K.; Zayas, C.F. Glomerular involvement in adults with sickle cell hemoglobinopathies: Prevalence and clinical correlates of progressive renal failure. J. Am. Soc. Nephrol. 2006, 17, 2228–2235. [Google Scholar] [CrossRef] [Green Version]
- Yee, M.M.; Jabbar, S.F.; Osunkwo, I.; Clement, L.; Lane, P.A.; Eckman, J.R.; Guasch, A. Chronic kidney disease and albuminuria in children with sickle cell disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 2628–2633. [Google Scholar]
- McClellan, A.C.; Luthi, J.C.; Lynch, J.R.; Soucie, J.M.; Kulkarni, R.; Guasch, A.; Huff, E.D.; Gilbertson, D.; McClellan, W.M.; DeBaun, M.R. High one year mortality in adults with sickle cell disease and end-stage renal disease. Br. J. Haematol. 2012, 159, 360–367. [Google Scholar] [CrossRef]
- Montgomery, R.; Zibari, G.; Hill, G.S.; Ratner, L.E. Renal transplantation in patients with sickle cell nephropathy. Transplantation 1994, 58, 618–620. [Google Scholar] [CrossRef]
- Naik, R.P.; Streiff, M.B.; Haywood, C., Jr.; Segal, J.B.; Lanzkron, S. Venous thromboembolism incidence in the Cooperative Study of Sickle Cell Disease. J. Thromb. Haemost. 2014, 12, 2010–2016. [Google Scholar] [CrossRef] [Green Version]
- Noubiap, J.J.; Temgoua, M.N.; Tankeu, R.; Tochie, J.N.; Wonkam, A.; Bigna, J.J. Sickle cell disease, sickle trait and the risk for venous thromboembolism: A systematic review and meta-analysis. Thromb. J. 2018, 16, 27. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart. J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [Green Version]
- Witt, D.M.; Nieuwlaat, R.; Clark, N.P.; Ansell, J.; Holbrook, A.; Skov, J.; Shehab, N.; Mock, J.; Myers, T.; Dentali, F.; et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Optimal management of anticoagulation therapy. Blood Adv. 2018, 2, 3226–3256. [Google Scholar] [CrossRef]
- Kumar, R.; Stanek, J.; Creary, S.; Dunn, A.; O’Brien, S.H. Prevalence and risk factors for venous thromboembolism in children with sickle cell disease: An administrative database study. Blood Adv. 2018, 2, 285–291. [Google Scholar] [CrossRef] [Green Version]
- Brunson, A.; Lei, A.; Rosenberg, A.S.; White, R.H.; Keegan, T.; Wun, T. Increased incidence of VTE in sickle cell disease patients: Risk factors, recurrence and impact on mortality. Br. J. Haematol. 2017, 178, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Makris, M.; Preston, F.E.; Beauchamp, N.J.; Cooper, P.C.; Daly, M.E.; Hampton, K.K.; Bayliss, P.; Peake, I.R.; Miller, G.J. Co-inheritance of the 20210A allele of the prothrombin gene increases the risk of thrombosis in subjects with familial thrombophilia. Thromb. Haemost. 1997, 78, 1426–1429. [Google Scholar] [CrossRef]
- Ridker, P.M.; Hennekens, C.H.; Lindpaintner, K.; Stampfer, M.J.; Eisenberg, P.R.; Miletich, J.P. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N. Engl. J. Med. 1995, 332, 912–917. [Google Scholar] [CrossRef]
- De Moerloose, P.; Wutschert, R.; Heinzmann, M.; Perneger, T.; Reber, G.; Bounameaux, H. Superficial vein thrombosis of lower limbs: Influence of factor V Leiden, factor II G20210A and overweight. Thromb. Haemost. 1998, 80, 239–241. [Google Scholar]
- Ocak, G.; Vossen, C.Y.; Verduijn, M.; Dekker, F.W.; Rosendaal, F.R.; Cannegieter, S.C.; Lijfering, W.M. Risk of venous thrombosis in patients with major illnesses: Results from the MEGA study. J. Thromb. Haemost. 2013, 11, 116–123. [Google Scholar] [CrossRef]
- Huerta, C.; Johansson, S.; Wallander, M.A.; García Rodríguez, L.A. Risk factors and short-term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch. Intern. Med. 2007, 167, 935–943. [Google Scholar] [CrossRef] [Green Version]
- Johannesdottir, S.A.; Horváth-Puhó, E.; Dekkers, O.M.; Cannegieter, S.C.; Jørgensen, J.O.; Ehrenstein, V.; Vandenbroucke, J.P.; Pedersen, L.; Sørensen, H.T. Use of glucocorticoids and risk of venous thromboembolism: A nationwide population-based case-control study. JAMA Intern. Med. 2013, 173, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Poli, D.; Zanazzi, M.; Antonucci, E.; Marcucci, R.; Rosati, A.; Bertoni, E.; Salvadori, M.; Liotta, A.A.; Abbate, R.; Prisco, D.; et al. High rate of recurrence in renal transplant recipients after a first episode of venous thromboembolism. Transplantation 2005, 80, 789–793. [Google Scholar] [CrossRef]
- Andrassy, J.; Zeier, M.; Andrassy, K. Do we need screening for thrombophilia prior to kidney transplantation? Nephrol. Dial. Transplant. 2004, 4, 64–68. [Google Scholar] [CrossRef]
- Allen, R.D.; Michie, C.A.; Murie, J.A.; Morris, P.J. Deep venous thrombosis after renal transplantation. Surg. Gynecol. Obstet. 1987, 164, 137–142. [Google Scholar]
- Todeschini, P.; La Manna, G.; Dalmastri, V.; Feliciangeli, G.; Cuna, V.; Montanari, M.; Angelini, M.L.; Scolari, M.P.; Stefoni, S. Incidence of late deep venous thrombosis among renal transplant patients. Transplant Proc. 2013, 45, 2666–2668. [Google Scholar] [CrossRef]
- Poli, D.; Zanazzi, M.; Antonucci, E.; Bertoni, E.; Salvadori, M.; Abbate, R.; Prisco, D. Renal transplant recipients are at high risk for both symptomatic and asymptomatic deep vein thrombosis. J. Thromb. Haemost. 2006, 4, 988–992. [Google Scholar] [CrossRef]
- Galanaud, J.P.; Sevestre, M.A.; Pernod, G.; Kahn, S.R.; Genty, C.; Terrisse, H.; Brisot, D.; Gillet, J.L.; Quéré, I.; Bosson, J.L. Long-term risk of venous thromboembolism recurrence after isolated superficial vein thrombosis. J. Thromb. Haemost. 2017, 15, 1123–1131. [Google Scholar] [CrossRef] [Green Version]
- Van Langevelde, K.; Lijfering, W.M.; Rosendaal, F.R.; Cannegieter, S.C. Increased risk of venous thrombosis in persons with clinically diagnosed superficial vein thrombosis: Results from the MEGA study. Blood 2011, 118, 4239–4241. [Google Scholar] [CrossRef]
- Roach, R.E.J.; Lijfering, W.M.; van Hylckama Vlieg, A.; Helmerhorst, F.M.; Rosendaal, F.R.; Cannegieter, S.C. The risk of venous thrombosis in individuals with a history of superficial vein thrombosis and acquired venous thrombotic risk factors. Blood 2013, 122, 4264–4269. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).