Tinnitus-Related Functional and Perceptual Impairments Following COVID-19 Vaccination: An Online Multi-Domain Survey Study
Abstract
1. Introduction
2. Methods
2.1. Survey Design
2.2. Data Collection
2.3. Data Cleaning
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
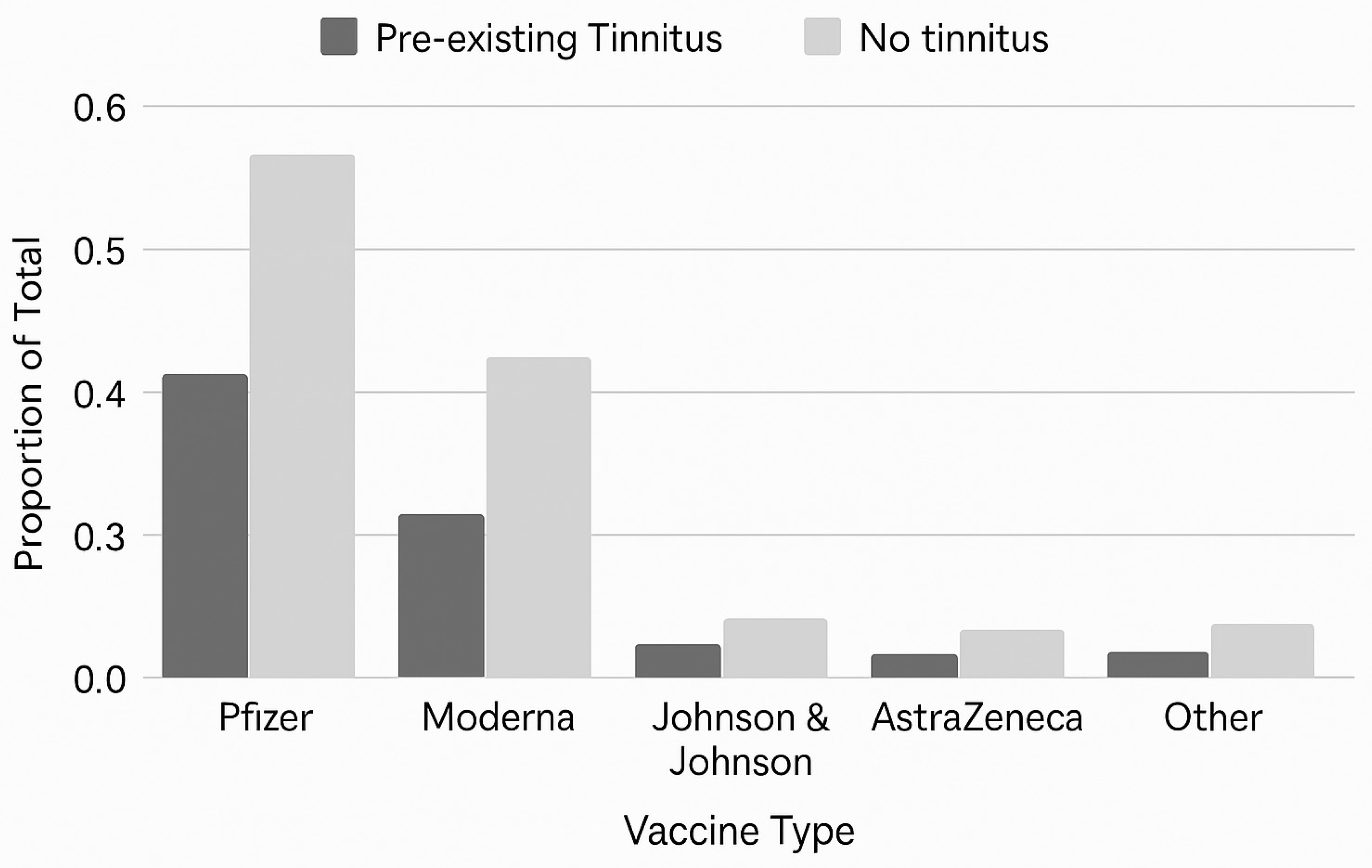
3.2. Vaccination Profiles
3.3. Medical and Audiological Follow-Up
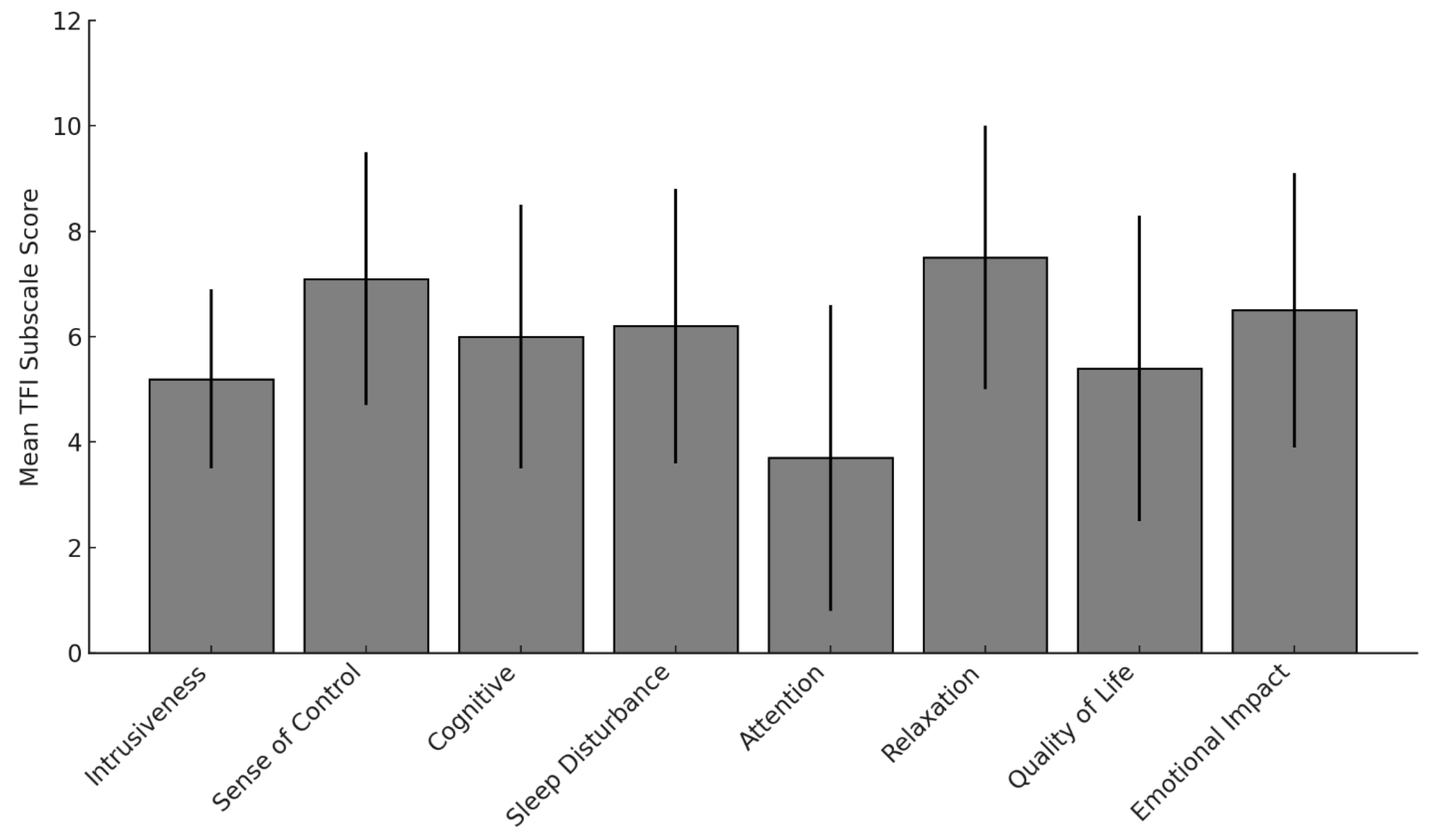
3.3.1. Tinnitus Functional Index (TFI)
3.3.2. Intrusiveness and Sense of Control
3.3.3. Cognitive, Sleep, and Emotional Burden
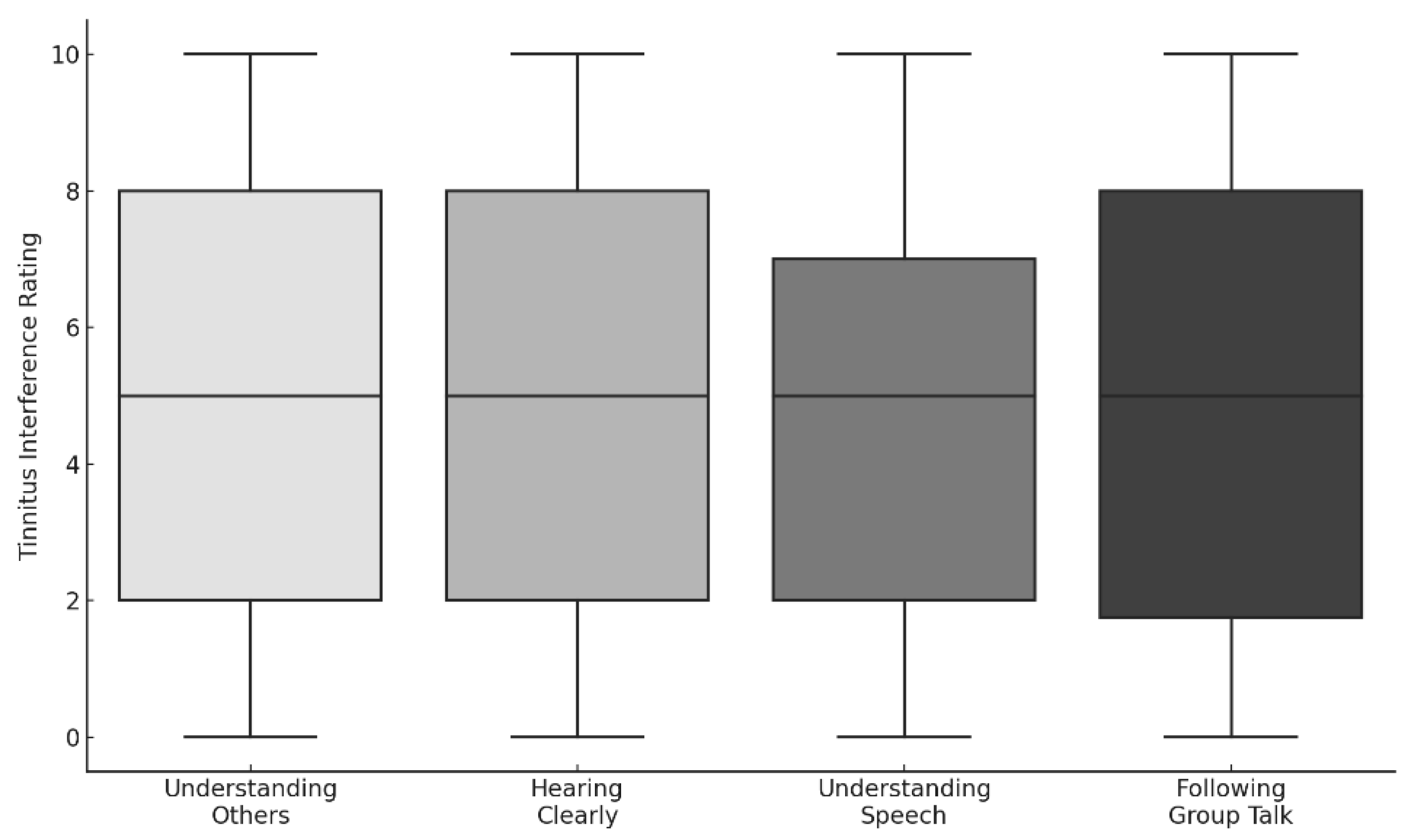
3.3.4. Auditory and Communication Interference
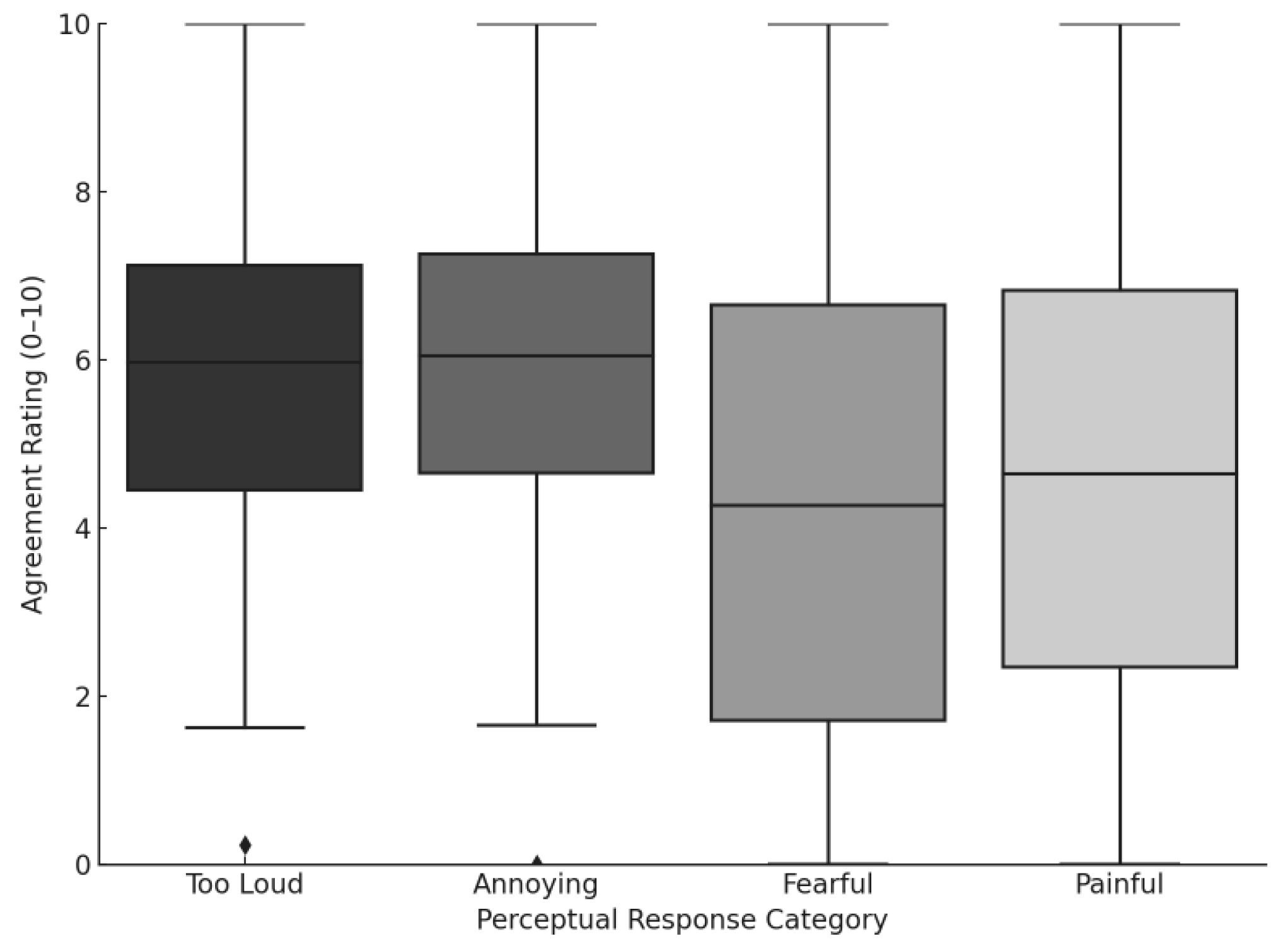
3.3.5. Hyperacusis and Loudness Sensitivity
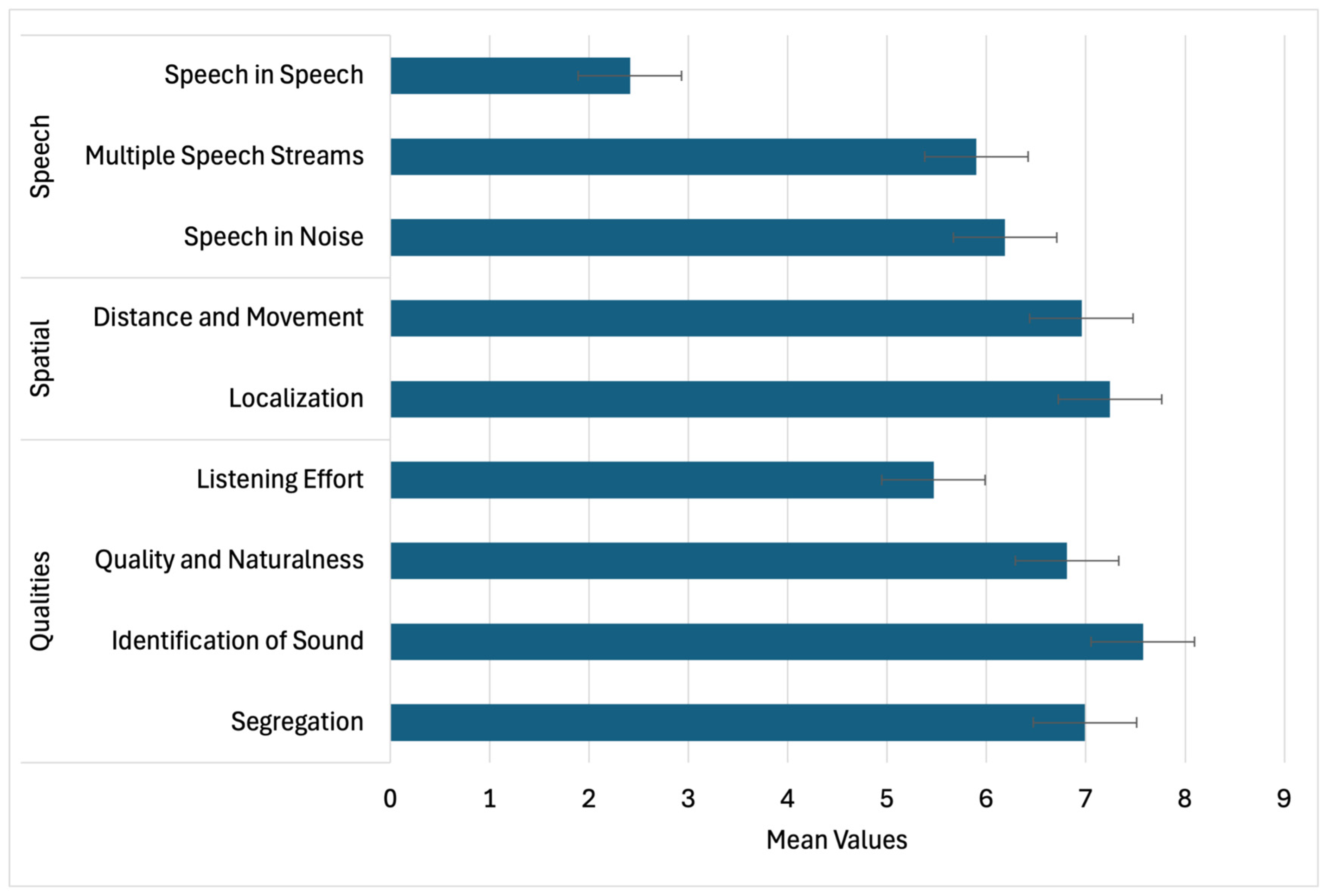
3.3.6. Functional Hearing Outcomes (SSQ)
3.3.7. Loudness Ratings and Lateralization
4. Discussion
4.1. Tinnitus-Related Impairments Across Functional Domains
4.2. Speech Perception and Listening Effort in Complex Environments
4.3. Perceived Loudness and Lateralization
4.4. Mechanisms Underlying COVID-19 Vaccination-Related Tinnitus
4.5. Clinical and Research Implications
4.6. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baguley, D.; McFerran, D.; Hall, D. Tinnitus. Lancet 2013, 382, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Langguth, B.; Kreuzer, P.M.; Kleinjung, T.; De Ridder, D. Tinnitus: Causes and clinical management. Lancet Neurol. 2013, 12, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Rauschecker, J.P.; Leaver, A.M.; Mühlau, M. Tuning out the noise: Limbic–auditory interactions in tinnitus. Neuron 2010, 66, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, Q.; Ding, Y.; Sun, Y. Systematic review and meta-analysis of the correlation between tinnitus and mental health. Am. J. Otolaryngol. 2025, 46, 104611. [Google Scholar] [CrossRef]
- Beukes, E.; Ulep, A.J.; Eubank, T.; Manchaiah, V. The impact of COVID-19 and the pandemic on tinnitus: A systematic review. J. Clin. Med. 2021, 10, 2763. [Google Scholar] [CrossRef]
- Figueiredo, R.R.; Penido, N.D.; Azevedo, A.A.; Oliveira, P.M.; Siqueira, A.G.; Figueiredo, G.D.; Schlee, W.; Langguth, B. Tinnitus emerging in the context of a COVID-19 infection seems not to differ in its characteristics from tinnitus unrelated to COVID-19. Front. Neurol. 2022, 13, 974179. [Google Scholar] [CrossRef]
- Viola, P.; Ralli, M.; Pisani, D.; Malanga, D.; Sculco, D.; Messina, L.; Laria, C.; Aragona, T.; Leopardi, G.; Ursini, F.; et al. Tinnitus and equilibrium disorders in COVID-19 patients: Preliminary results. Eur. Arch. Otorhinolaryngol. 2021, 278, 3725–3730. [Google Scholar] [CrossRef]
- Beukes, E.W.; Baguley, D.M.; Jacquemin, L.; Andersson, G. Changes in tinnitus experiences during the COVID-19 pandemic. Front. Public Health 2020, 8, 592878. [Google Scholar] [CrossRef]
- Yellamsetty, A.; Gonzalez, V. Understanding COVID-19-Related Tinnitus: Emerging Evidence and Implications. Tinnitus Today. 2024. Available online: https://www.researchgate.net/publication/376646284_Understanding_COVID-19_related_tinnitus_Emerging_Evidence_and_Implications (accessed on 20 November 2025). [CrossRef]
- Maihoub, S.; Mavrogeni, P.; Répássy, G.D.; Molnár, A. Exploring how blood cell levels influence subjective tinnitus: A Cross-Sectional Case-Control Study. Audiol. Res. 2025, 15, 72. [Google Scholar] [CrossRef]
- Maihoub, S.; Mavrogeni, P.; Molnár, V.; Molnár, A. Tinnitus and its comorbidities: A comprehensive analysis of Their Relationships. J. Clin. Med. 2025, 14, 1285. [Google Scholar] [CrossRef]
- Tai, Y.; Jain, N.; Kim, G.; Husain, F.T. Tinnitus and COVID-19: Effect of infection, vaccination, and the pandemic. Front. Public Health 2024, 12, 1508607. [Google Scholar] [CrossRef] [PubMed]
- Moon, I.J.; Won, J.H.; Kang, H.W.; Kim, D.H.; An, Y.H.; Shim, H.J. Influence of tinnitus on auditory resolution and speech perception. J. Neurosci. 2015, 35, 14260–14269. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.E.; Eggermont, J.J.; Caspary, D.M.; Shore, S.E.; Melcher, J.R.; Kaltenbach, J.A. Ringing ears: The neuroscience of tinnitus. J. Neurosci. 2010, 30, 14972–14979. [Google Scholar] [CrossRef] [PubMed]
- Meikle, M.B.; Henry, J.A.; Griest, S.E.; Stewart, B.J.; Abrams, H.B.; McArdle, R.; Vernon, J.A. The Tinnitus Functional Index. Ear Hear. 2012, 33, 153–176. [Google Scholar] [CrossRef]
- Gatehouse, S.; Noble, W. The Speech, Spatial and Qualities of Hearing Scale (SSQ). Int. J. Audiol. 2004, 43, 85–99. [Google Scholar] [CrossRef]
- Wilson, P.H.; Henry, J.; Bowen, M.; Haralambous, G. Tinnitus Reaction Questionnaire. J. Speech Hear. Res. 1991, 34, 197–201. [Google Scholar] [CrossRef]
- Yellamsetty, A. COVID-19 vaccination effects on tinnitus and hyperacusis: Longitudinal case study. Int. Tinnitus J. 2023, 27, 253–258. [Google Scholar] [CrossRef]
- Wang, W.; Yellamsetty, A.; Edmonds, R.M.; Barcavage, S.R.; Bao, S. COVID-19 vaccination-related tinnitus is associated with pre-vaccination metabolic disorders. Front. Pharmacol. 2024, 15, 1374320. [Google Scholar] [CrossRef]
- Yellamsetty, A.; Egbe-Etu, E.; Bao, S. Impact of COVID-19 vaccination on tinnitus onset and severity: A comprehensive survey study. Front. Audiol. Otol. 2024, 3, 1509444. [Google Scholar] [CrossRef]
- Hiller, W.; Goebel, G. Factors influencing tinnitus loudness and annoyance. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 1323–1330. [Google Scholar] [CrossRef]
- Erinc, M.; Mutlu, A.; Celik, S.; Kalcioglu, M.T.; Szczepek, A.J. Long-term effects of COVID-19 and the Pandemic on tinnitus patients. Front. Neurol. 2022, 13, 921173. [Google Scholar] [CrossRef] [PubMed]
- Yellamsetty, A.; Shin, M. Trajectory of tinnitus distress across the COVID-19 pandemic: A Cross-Sectional Analysis of Self-Reported Symptoms. Audiol. Res. 2025, 15, 132. [Google Scholar] [CrossRef] [PubMed]
- Tyler, R.S.; Pienkowski, M.; Roncancio, E.R.; Jun, H.J.; Brozoski, T.; Dauman, N.; Coelho, C.B.; Andersson, G.; Keiner, A.J.; Cacace, A.T.; et al. A review of hyperacusis and future directions: Part I. Definitions and manifestations. Am. J. Audiol. 2014, 23, 402–419. [Google Scholar] [CrossRef] [PubMed]
- Taziki Balajelini, M.; Sadoughi, M.; Mirzaei, M. Tinnitus in healthcare workers after COVID-19 vaccination. Noise Health 2022, 24, 17–22. [Google Scholar]
- DeOre, B.J.; Tran, K.A.; Andrews, A.M.; Ramirez, S.H.; Galie, P.A. Spike protein disrupts the blood–brain barrier. J. Neuroimmune Pharmacol. 2021, 16, 722–728. [Google Scholar] [CrossRef]
- Petrovszki, D.; Walter, F.R.; Vigh, J.P.; Kocsis, A.; Valkai, S.; Deli, M.A.; Der, A. Spike protein penetration across the blood–brain barrier. Biomedicines 2022, 10, 188. [Google Scholar]
- Zhang, Q.; Xiang, R.; Huo, S.; Zhou, Y.; Jiang, S.; Wang, Q.; Yu, F. Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. Signal Transduct. Target. Ther. 2021, 6, 233. [Google Scholar] [CrossRef]
- Frank, M.G.; Nguyen, K.H.; Ball, J.B.; Hopkins, S.; Kelley, T.; Baratta, M.V.; Fleshner, M.; Maier, S.F. SARS-CoV-2 spike S1 subunit induces neuroinflammatory, microglial and behavioral sickness responses: Evidence of PAMP-like properties. Brain Behav. Immun. 2022, 100, 267–277. [Google Scholar] [CrossRef]
- Cernera, G.; Gelzo, M.; De Placido, P.; Pietroluongo, E.; Raia, M.; Scalia, G.; Tortora, M.; Formisano, P.; Palmieri, G.; Giuliano, M.; et al. Serum biomarkers of inflammation and vascular damage upon SARS-CoV-2 mRNA vaccine in patients with thymic epithelial tumors. Clin. Chem. Lab. Med. 2024, 62, 1198–1205. [Google Scholar] [CrossRef]
- Terentes-Printzios, D.; Gardikioti, V.; Solomou, E.; Emmanouil, E.; Gourgouli, I.; Xydis, P.; Christopoulou, G.; Georgakopoulos, C.; Dima, I.; Miliou, A.; et al. The effect of an mRNA vaccine against COVID-19 on endothelial function and arterial stiffness. Hypertens. Res. 2022, 45, 846–855. [Google Scholar] [CrossRef]
- Jeong, M.; Ocwieja, K.E.; Han, D.; Wackym, P.A.; Zhang, Y.; Brown, A.; Moncada, C.; Vambutas, A.; Kanne, T.; Crain, R.; et al. Direct SARS-CoV-2 infection of the human inner ear may underlie COVID-19-associated audiovestibular dysfunction. Commun. Med. 2021, 1, 44. [Google Scholar] [CrossRef]
- Mulroney, T.E.; Pöyry, T.; Yam-Puc, J.C.; Rust, M.; Harvey, R.F.; Kalmar, L.; Horner, E.; Booth, L.; Ferreira, A.P.; Stoneley, M.; et al. N1-methylpseudouridylation of mRNA causes +1 ribosomal frameshifting. Nature 2024, 625, 189–194. [Google Scholar] [CrossRef]


| Characteristics | Category | n (%) |
|---|---|---|
| Age (years) | Mean = 54.7 ± 14.4 (SD); Range = 18–85 | — |
| Gender | Female | 450 (58.4%) |
| Male | 303 (39.4%) | |
| Other/Unspecified | 17 (2.2%) | |
| COVID-19 Infection History | Reported SARS-CoV-2 infection | 48 (6.2%) |
| Hospitalized due to infection | 1 (0.1%) |
| Vaccine Manufacturer | All Survey Cases (n = 770) | U.S. Survey Cases (n = 618) |
|---|---|---|
| Pfizer | 427 (55.5%) | 328 (53.1%) |
| Moderna | 256 (33.2%) | 239 (38.7%) |
| Johnson & Johnson | 55 (7.1%) | 48 (7.8%) |
| AstraZeneca | 28 (3.6%) | 2 (0.3%) |
| Other | 4 (0.5%) | 1 (0.2%) |
| Vaccine Manufacturer | 1 Dose | 2 Doses | 3 Doses | 4+ Doses | Total Cases (n = 766) | Pre-Existing Tinnitus (n, %) |
|---|---|---|---|---|---|---|
| Pfizer | 16 (3.7%) | 187 (43.8%) | 177 (41.5%) | 47 (11.0%) | 427 | 113 (26.5%) |
| Moderna | 7 (2.7%) | 94 (36.7%) | 122 (47.7%) | 33 (12.9%) | 256 | 67 (26.2%) |
| Johnson & Johnson | 31 (56.4%) | 10 (18.2%) | 10 (18.2%) | 4 (7.3%) | 55 | 17 (30.9%) |
| AstraZeneca | 18 (64.3%) | 8 (28.6%) | 2 (7.1%) | 0 (0%) | 28 | 4 (14.3%) |
| Type of Evaluation | Total (n = 373) | Male (n = 162) | Female (n = 199) | Other/Unspecified (n = 12) |
|---|---|---|---|---|
| Audiometry (PTA) | 161 (43.2%) | 73 (45.1%) | 85 (42.7%) | 3 (25.0%) |
| ENT Consultation | 105 (28.2%) | 52 (32.1%) | 50 (25.1%) | 3 (25.0%) |
| MRI (Brain) | 89 (23.8%) | 40 (24.7%) | 46 (23.1%) | 3 (25.0%) |
| Blood Tests | 54 (14.5%) | 26 (16.0%) | 27 (13.6%) | 1 (8.3%) |
| Vestibular VNG/caloric Tests | 27 (7.2%) | 12 (7.4%) | 14 (7.0%) | 1 (8.3%) |
| Neurological test | 26 (7.0%) | 13 (8.0%) | 12 (6.0%) | 1 (8.3%) |
| Other (e.g., TMJ, Cardiology) | 18 (4.8%) | 7 (4.3%) | 10 (5.0%) | 1 (8.3%) |
| SSQ Domain | Item Description | χ2 (df) | p-Value |
|---|---|---|---|
| Speech | Speech in Noise (TV on) | 125.61 | <0.001 |
| Multiple Streams (TV & person) | 57.89 | <0.001 | |
| Speech in Speech (Crowd) | 67.16 | <0.001 | |
| Group Speech (Restaurant) | 55.33 | <0.001 | |
| Switching Speakers | 88.26 | <0.001 | |
| Spatial | Localization (Dog barking) | 248.56 | <0.001 |
| Distance Judgement | 168.11 | <0.001 | |
| Directionality (Coming vs. Going) | 188.84 | <0.001 | |
| Qualities | Segregation (Jumbled Sounds) | 272.88 | <0.001 |
| Identifying Instruments | 311.27 | <0.001 | |
| Sound Naturalness (Clarity) | 165.83 | <0.001 | |
| Listening Effort | 46.94 | <0.001 |
| Speech | Spatial | Qualities | |
|---|---|---|---|
| Speech | 1.00 | 0.73 *** | 0.81 *** |
| Spatial | — | 1.00 | 0.77 *** |
| Qualities | — | — | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yellamsetty, A.; Fortuna, G.; Etu, E.-E.; Bao, S. Tinnitus-Related Functional and Perceptual Impairments Following COVID-19 Vaccination: An Online Multi-Domain Survey Study. Audiol. Res. 2025, 15, 164. https://doi.org/10.3390/audiolres15060164
Yellamsetty A, Fortuna G, Etu E-E, Bao S. Tinnitus-Related Functional and Perceptual Impairments Following COVID-19 Vaccination: An Online Multi-Domain Survey Study. Audiology Research. 2025; 15(6):164. https://doi.org/10.3390/audiolres15060164
Chicago/Turabian StyleYellamsetty, Anusha, Gianmaris Fortuna, Egbe-Etu Etu, and Shaowen Bao. 2025. "Tinnitus-Related Functional and Perceptual Impairments Following COVID-19 Vaccination: An Online Multi-Domain Survey Study" Audiology Research 15, no. 6: 164. https://doi.org/10.3390/audiolres15060164
APA StyleYellamsetty, A., Fortuna, G., Etu, E.-E., & Bao, S. (2025). Tinnitus-Related Functional and Perceptual Impairments Following COVID-19 Vaccination: An Online Multi-Domain Survey Study. Audiology Research, 15(6), 164. https://doi.org/10.3390/audiolres15060164






