Hearing Aid Amplification Schemes Adjusted to Tinnitus Pitch: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Questionnaires
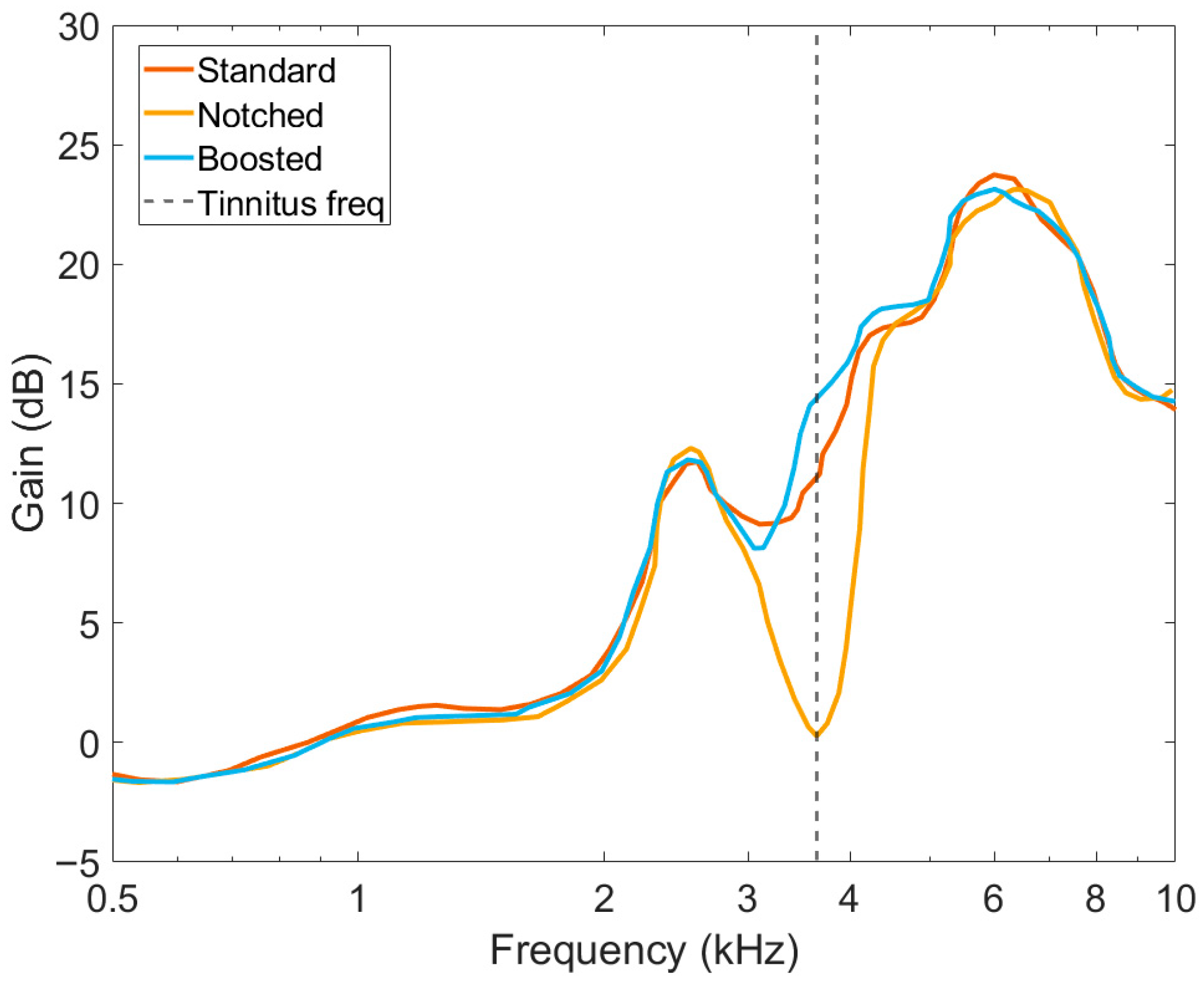
2.3. Hearing Aids and Amplification Schemes
- Standard amplification. In this setting, all available frequencies are amplified according to the standard clinical fitting using the formula NAL-NL2 [29]. This approach is consistent with the recommendation for patients with tinnitus and hearing loss in the ENT clinic of the UMCG.
- Notch amplification. This setting uses the previous fitting formula and, additionally, applies a notch filter centered at the participant’s tinnitus frequency. The rationale of this approach is based on lateral inhibition, by which excitation of neurons with a characteristic frequency close to the tinnitus frequency might suppress the tinnitus. The notch filter has a depth of 60 dB and a bandwidth of 0.5 octaves. The filter can be placed at each of 31 logarithmically distributed frequencies in the range of 0.250 to 8 kHz.
- Boosted amplification. The same fitting formula is applied. Additionally, the closest frequency band to the participant’s tinnitus frequency is amplified by 5 dB.
2.4. Audiological Assessment and Hearing Aid Fitting
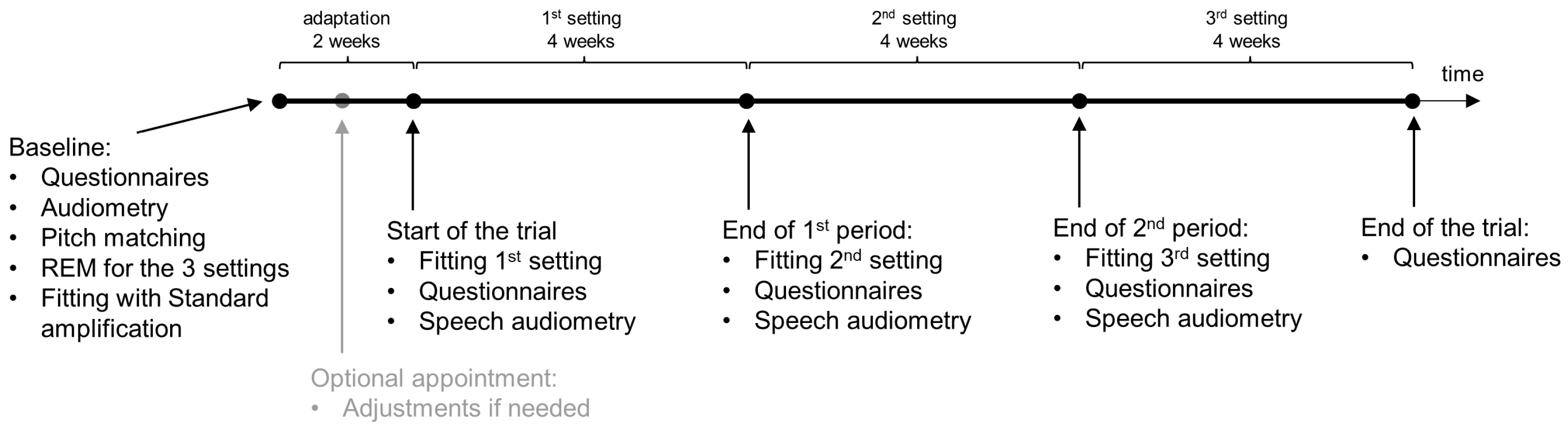
2.5. Study Design and Blinding
2.6. Procedure
2.7. Statistical Analysis
3. Results
3.1. Demographics
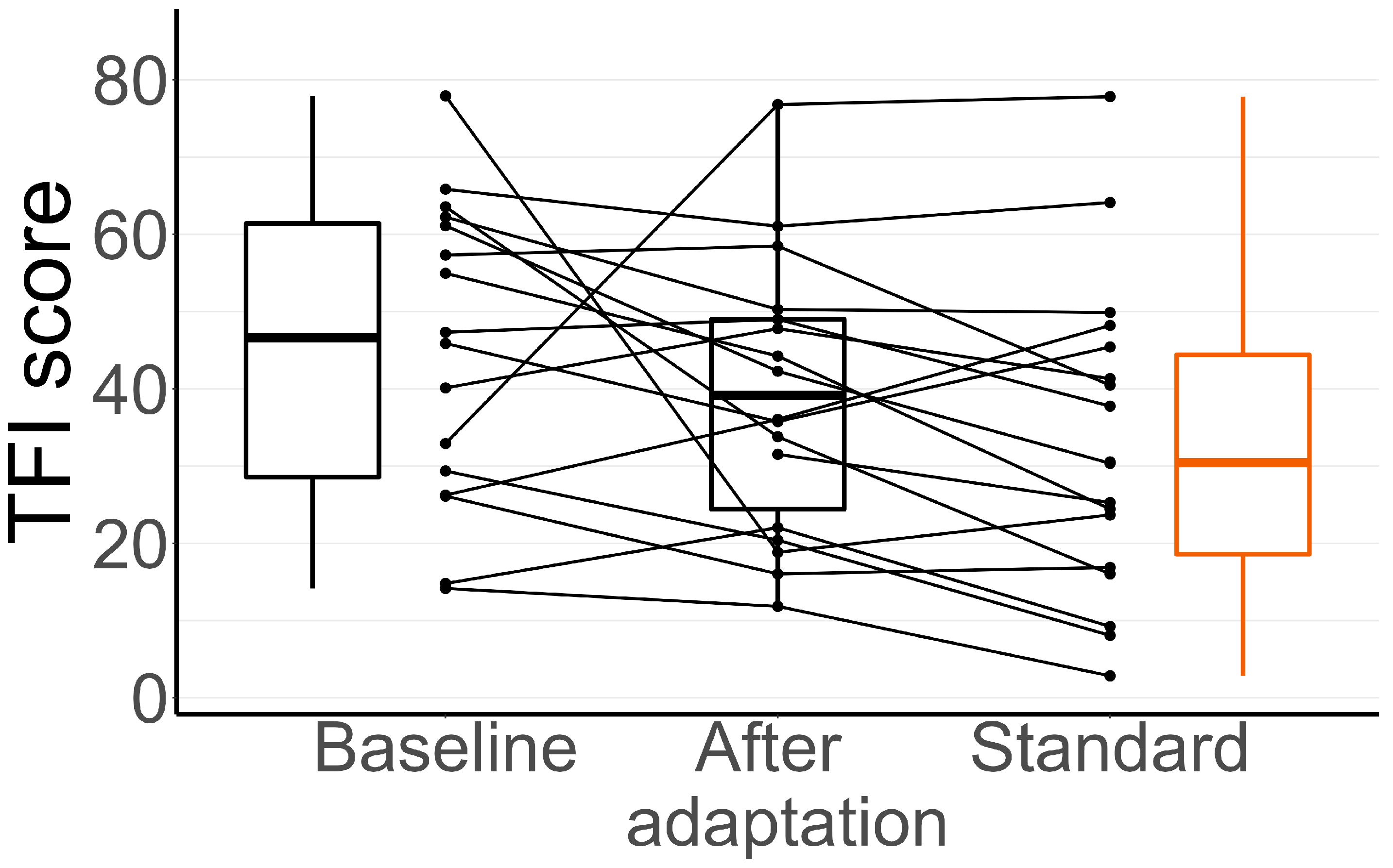
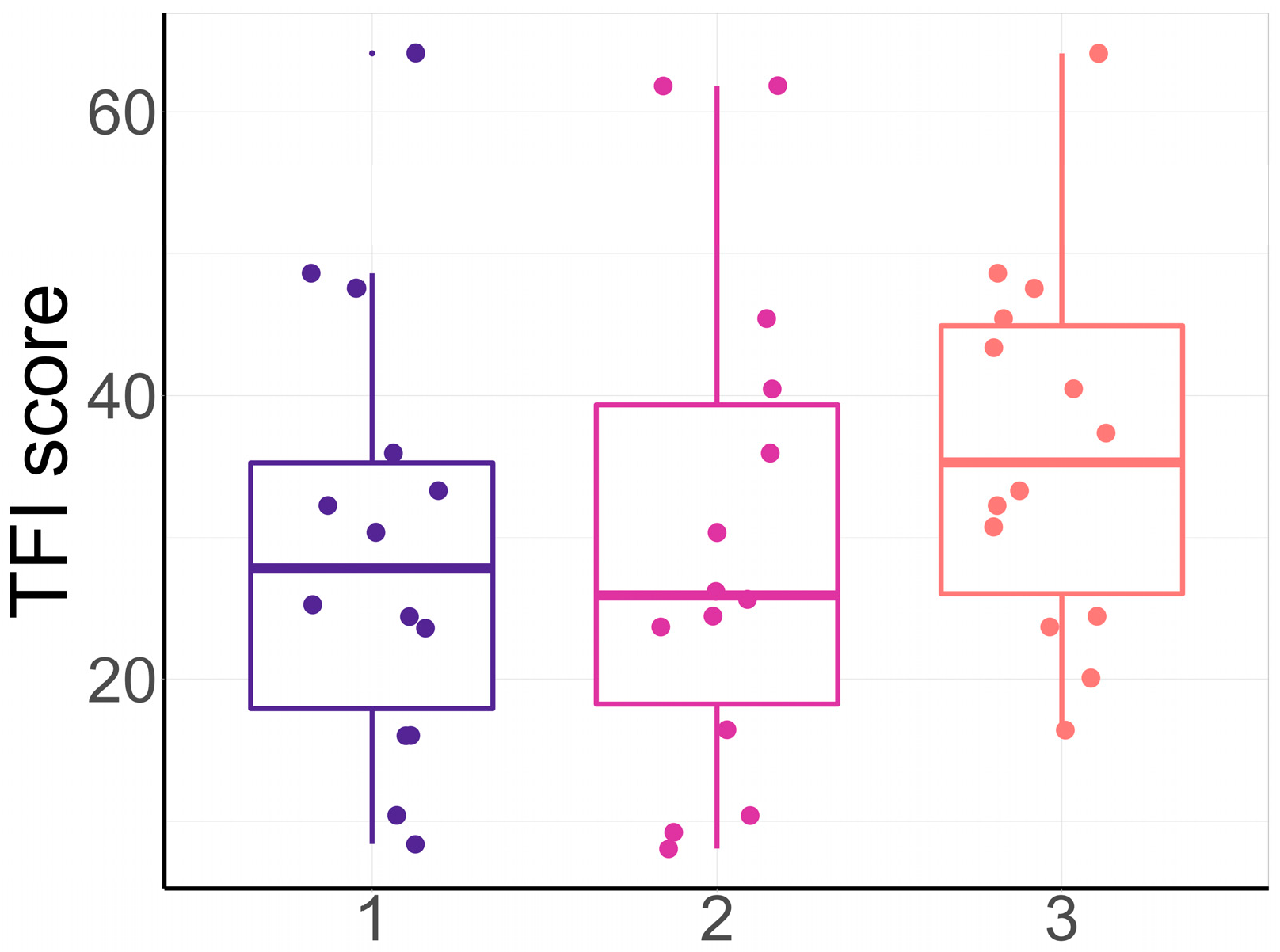
3.2. TFI During the Adaptation Period
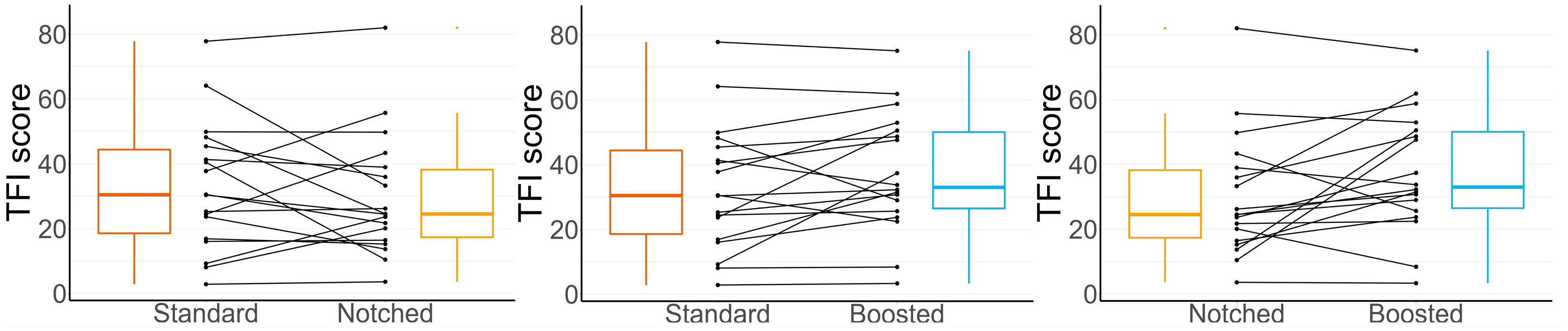
3.3. TFI Results After Using Each Setting
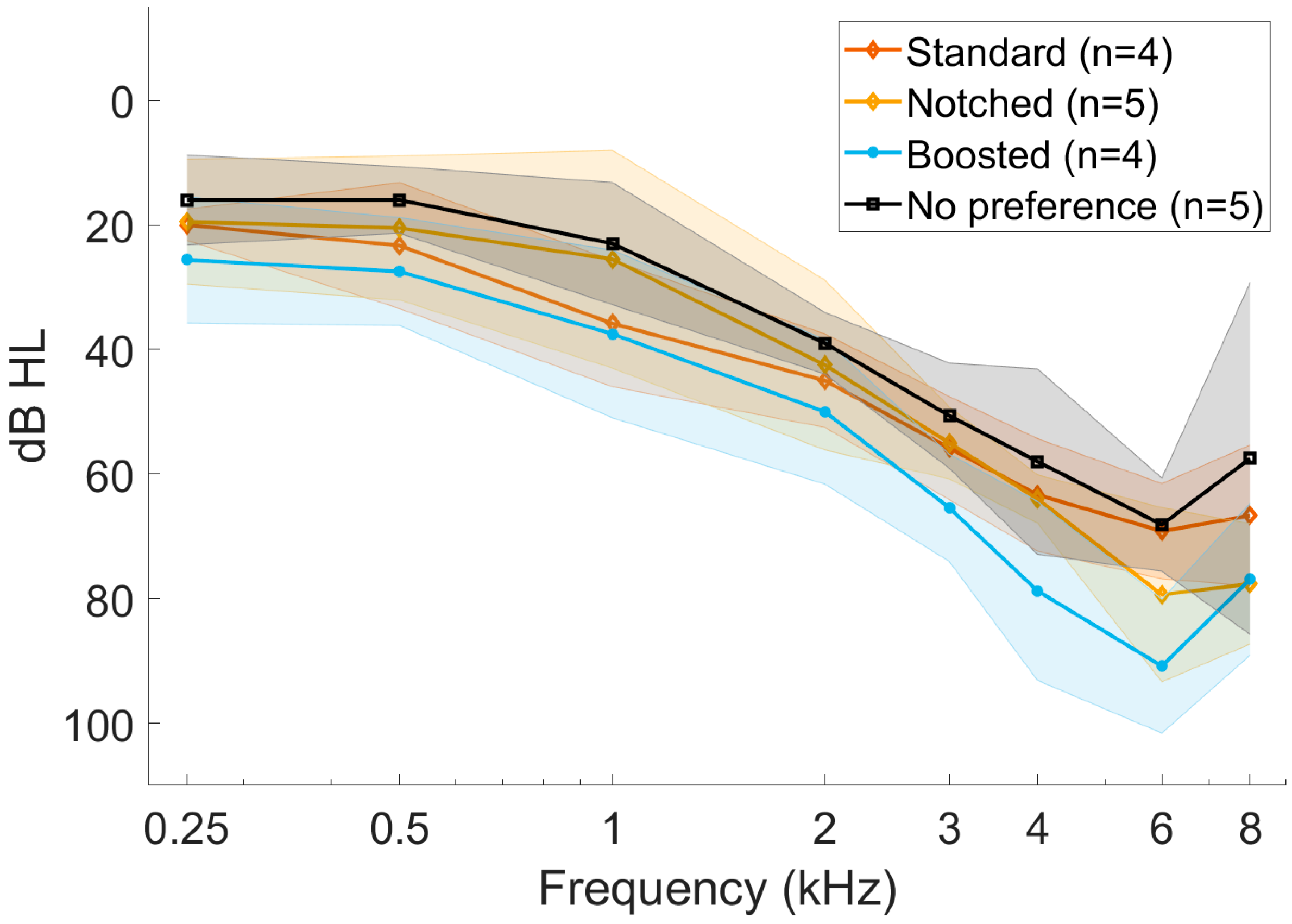
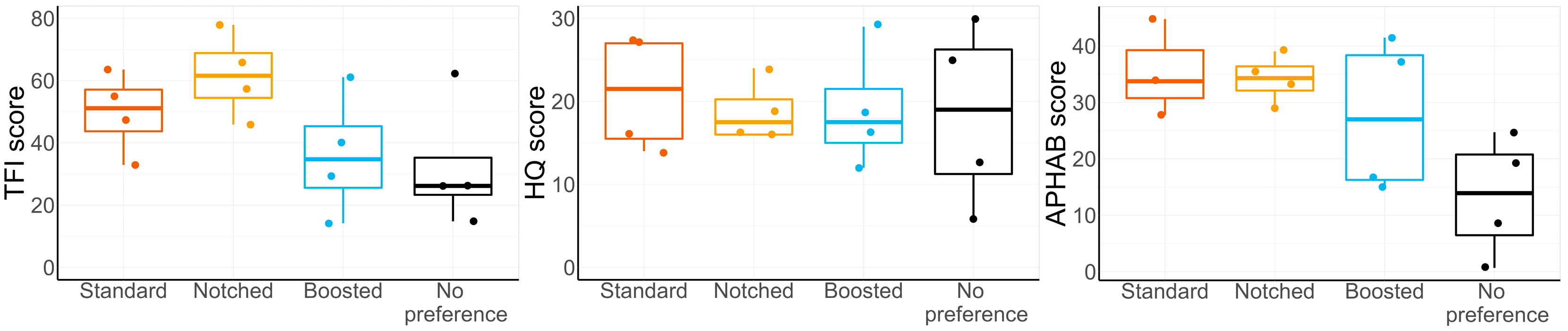
3.4. Preferred Setting
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TMNMT | Tailor-Made Notched Music Training |
| UMCG | University Medical Center Groningen |
| CCMO | Central Committee of Research Involving Human Subjects |
| REM | Real-Ear Measurement |
| RCT | Randomized Controlled Trial |
| PTA | Pure Tone Audiometry |
| RIC | Receiver In Canal |
| TFI | Tinnitus Functional |
| APHAB | Abbreviated Profile of Hearing Aid Benefit |
| HQ | Hyperacusis Questionnaire |
| ISTS | International Speech Test Signal |
References
- Biswas, R.; Lugo, A.; Akeroyd, M.A.; Schlee, W.; Gallus, S.; Hall, D.A. Tinnitus prevalence in Europe: A multi-country cross-sectional population study. Lancet Reg. Health-Eur. 2022, 12, 100250. [Google Scholar] [CrossRef]
- Schubert, N.M.; Rosmalen, J.G.; van Dijk, P.; Pyott, S.J. A retrospective cross-sectional study on tinnitus prevalence and disease associations in the Dutch population-based cohort Lifelines. Hear. Res. 2021, 411, 108355. [Google Scholar] [CrossRef]
- Eggermont, J.J.; Roberts, L.E. The neuroscience of tinnitus. Trends Neurosci. 2004, 27, 676–682. [Google Scholar] [CrossRef]
- Knipper, M.; Van Dijk, P.; Nunes, I.; Rüttiger, L.; Zimmermann, U. Advances in the neurobiology of hearing disorders: Recent developments regarding the basis of tinnitus and hyperacusis. Prog. Neurobiol. 2013, 111, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Koops, E.A.; Renken, R.J.; Lanting, C.P.; van Dijk, P. Cortical tonotopic map changes in humans are larger in hearing loss than in additional tinnitus. J. Neurosci. 2020, 40, 3178–3185. [Google Scholar] [CrossRef]
- Schaette, R.; Kempter, R. Development of tinnitus-related neuronal hyperactivity through homeostatic plasticity after hearing loss: A computational model. Eur. J. Neurosci. 2006, 23, 3124–3138. [Google Scholar] [CrossRef] [PubMed]
- Norena, A. An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neurosci. Biobehav. Rev. 2011, 35, 1089–1109. [Google Scholar] [CrossRef] [PubMed]
- Boyen, K.; de Kleine, E.; van Dijk, P.; Langers, D.R. Tinnitus-related dissociation between cortical and subcortical neural activity in humans with mild to moderate sensorineural hearing loss. Hear. Res. 2014, 312, 48–59. [Google Scholar] [CrossRef]
- Hofmeier, B.; Wolpert, S.; Aldamer, E.S.; Walter, M.; Thiericke, J.; Braun, C.; Zelle, D.; Rüttiger, L.; Klose, U.; Knipper, M. Reduced sound-evoked and resting-state BOLD fMRI connectivity in tinnitus. NeuroImage Clin. 2018, 20, 637–649. [Google Scholar] [CrossRef]
- Pantev, C.; Okamoto, H.; Teismann, H. Music-induced cortical plasticity and lateral inhibition in the human auditory cortex as foundations for tonal tinnitus treatment. Front. Syst. Neurosci. 2012, 6, 50. [Google Scholar] [CrossRef]
- Pape, J.; Paraskevopoulos, E.; Bruchmann, M.; Wollbrink, A.; Rudack, C.; Pantev, C. Playing and listening to tailor-made notched music: Cortical plasticity induced by unimodal and multimodal training in tinnitus patients. Neural Plast. 2014, 2014, 516163. [Google Scholar] [CrossRef] [PubMed]
- Marcrum, S.C.; Picou, E.M.; Steffens, T.; Hannemann, R.; Vielsmeier, V.; Schecklmann, M.; Langguth, B.; Schlee, W. Conventional versus notch filter amplification for the treatment of tinnitus in adults with mild-to-moderate hearing loss. Prog. Brain Res. 2021, 260, 235–252. [Google Scholar]
- Sereda, M.; Hoare, D.J.; Nicholson, R.; Smith, S.; Hall, D. Consensus on hearing aid candidature and fitting for mild hearing loss, with and without tinnitus. Ear Hear. 2015, 36, 417–429. [Google Scholar] [CrossRef]
- Panayiota, M.; Maihoub, S.; Molnár, A. Speech recognition thresholds correlate with tinnitus intensity in individuals with primary subjective tinnitus. Front. Neurol. 2025, 16, 1672762. [Google Scholar]
- König, O.; Schaette, R.; Kempter, R.; Gross, M. Course of hearing loss and occurrence of tinnitus. Hear. Res. 2006, 221, 59–64. [Google Scholar] [CrossRef]
- Norena, A.; Micheyl, C.; Chéry-Croze, S.; Collet, L. Psychoacoustic characterization of the tinnitus spectrum: Implications for the underlying mechanisms of tinnitus. Audiol. Neurotol. 2002, 7, 358–369. [Google Scholar] [CrossRef]
- Roberts, L.E.; Moffat, G.; Baumann, M.; Ward, L.M.; Bosnyak, D.J. Residual inhibition functions overlap tinnitus spectra and the region of auditory threshold shift. J. Assoc. Res. Otolaryngol. 2008, 9, 417–435. [Google Scholar] [CrossRef]
- Joergensen, M.L.; Hyvärinen, P.; Caporali, S.; Dau, T. Broadband Amplification as Tinnitus Treatment. Brain Sci. 2022, 12, 719. [Google Scholar] [CrossRef]
- McNeill, C.; Távora-Vieira, D.; Alnafjan, F.; Searchfield, G.D.; Welch, D. Tinnitus pitch, masking, and the effectiveness of hearing aids for tinnitus therapy. Int. J. Audiol. 2012, 51, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Schaette, R.; König, O.; Hornig, D.; Gross, M.; Kempter, R. Acoustic stimulation treatments against tinnitus could be most effective when tinnitus pitch is within the stimulated frequency range. Hear. Res. 2010, 269, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Hoare, D.J.; Edmondson-Jones, M.; Sereda, M.; Akeroyd, M.; Hall, D. Amplification with hearing aids for patients with tinnitus and co-existing hearing loss (Review). Cochrane Database Syst. Rev. 2014, 2014, CD010151. [Google Scholar]
- Shekhawat, G.; Searchfield, G.D.; Stinear, C.M. Role of hearing aids in tinnitus intervention: A scoping review. J. Am. Acad. Audiol. 2013, 24, 747–762. [Google Scholar] [CrossRef]
- Jacquemin, L.; Gilles, A.; Shekhawat, G.S. Hearing more to hear less: A scoping review of hearing aids for tinnitus relief. Int. J. Audiol. 2022, 61, 887–895. [Google Scholar] [CrossRef]
- Tunkel, D.E.; Bauer, C.A.; Sun, G.H.; Rosenfeld, R.M.; Chandrasekhar, S.S.; Cunningham, E.R.; Waguespack, R. Clinical practice guideline. Otolaryngol.-Head Neck Surg. 2014, 151, 533–541. [Google Scholar] [CrossRef]
- Meikle, M.B.; Henry, J.A.; Griest, S.E.; Stewart, B.J.; Abrams, H.B.; McArdle, R.; Myers, P.J.; Newman, C.W.; Sandridge, S.; Turk, D.C.; et al. The tinnitus functional index: Development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear. 2012, 33, 153–176. [Google Scholar] [CrossRef]
- Cox, R.M.; Alexander, G.C. The abbreviated profile of hearing aid benefit. Ear Hear. 1995, 16, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Khalfa, S.; Dubal, S.; Veuillet, E.; Perez-Diaz, F.; Jouvent, R.; Collet, L. Psychometric normalization of a hyperacusis questionnaire. Orl 2002, 64, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Sivantos, Erlangen, Germany. Available online: https://sivantos.com/ (accessed on 19 October 2025).
- Keidser, G.; Dillon, H.; Carter, L.; O’Brien, A. NAL-NL2 Empirical Adjustments. Trends Amplif. 2012, 16, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Santacruz, J.L.; De Kleine, E.; Van Dijk, P. Comparison between two self-guided tinnitus pitch matching methods. Front. Aging Neurosci. 2023, 15, 8. [Google Scholar] [CrossRef]
- Interacoustics, Denmark. Available online: https://www.interacoustics.com/ (accessed on 19 October 2025).
- Holube, I.; Fredelake, S.; Vlaming, M.; Kollmeier, B. Development and analysis of an international speech test signal (ISTS). Int. J. Audiol. 2010, 49, 891–903. [Google Scholar] [CrossRef]
- Sibbald, B.; Roberts, C. Understanding controlled trials crossover trials. BMJ 1998, 316, 1719–1720. [Google Scholar] [CrossRef] [PubMed]
- Amirah Fatin, I.; Khatijah, L.A.; Zilany, M.S.A.; Zuheir, A.Z.; Ong, S.H.; Tan, M.P. The effect of hearing augmentation on cognitive assessment scores: A pilot crossover randomized controlled trial. In Proceedings of the International Conference for Innovation in Biomedical Engineering and Life Sciences: ICIBEL2015, Putrajaya, Malaysia, 6–8 December 2015; Springer: Singapore, 2016; Volume 1, pp. 24–29. [Google Scholar]
- Edwards, A.L. Balanced latin-square designs in psychological research. Am. J. Psychol. 1951, 64, 598–603. [Google Scholar] [CrossRef]
- Fackrell, K.; Hall, D.A.; Barry, J.; Hoare, D.J. Integrating Distribution-Based and Anchor-Based Techniques to Identify Minimal Important Change for the Tinnitus Functional Index (TFI) Questionnaire. Brain Sci. 2022, 12, 726. [Google Scholar] [CrossRef]
- Nakagawa, S.; Johnson, P.C.; Schielzeth, H. The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 2017, 14, 20170213. [Google Scholar] [CrossRef]
- Henry, J.A.; Griest, S.; Thielman, E.; McMillan, G.; Kaelin, C.; Carlson, K.F. Tinnitus Functional Index: Development, validation, outcomes research, and clinical application. Hear. Res. 2016, 334, 58–64. [Google Scholar] [CrossRef]
- Aazh, H.; Moore, B.C. Factors related to uncomfortable loudness levels for patients seen in a tinnitus and hyperacusis clinic. Int. J. Audiol. 2017, 56, 793–800. [Google Scholar] [CrossRef]
- Cox, R.M. Administration and application of the APHAB. Hear. J. 1997, 50, 32–35. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Multiple significance tests: The Bonferroni method. BMJ 1995, 310, 170. [Google Scholar] [CrossRef]
- Dawes, P.; Hopkins, R.; Munro, K.J. Placebo effects in hearing-aid trials are reliable. Int. J. Audiol. 2013, 52, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Humes, L.E.; Rogers, S.E.; Quigley, T.M.; Main, A.K.; Kinney, D.L.; Herring, C. The effects of service-delivery model and purchase price on hearing-aid outcomes in older adults: A randomized double-blind placebo-controlled clinical trial. Am. J. Audiol. 2017, 26, 53–79. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, B.; Boecking, B.; Brueggemann, P. Association between stress and tinnitus—New aspects. Otol. Neurotol. 2019, 40, e467–e473. [Google Scholar] [CrossRef] [PubMed]
- Maihoub, S.; Mavrogeni, P.; Répássy, G.D.; Molnár, A. Exploring How Blood Cell Levels Influence Subjective Tinnitus: A Cross-Sectional Case-Control Study. Audiol. Res. 2025, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, M.; Del Bo, L.; Jastreboff, M.; Tognola, G.; Ravazzani, P. Open ear hearing aids in tinnitus therapy: An efficacy comparison with sound generators. Int. J. Audiol. 2011, 50, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Wellek, S.; Blettner, M. On the proper use of the crossover design in clinical trials: Part 18 of a series on evaluation of scientific publications. Dtsch. Ärzteblatt Int. 2012, 109, 276–281. [Google Scholar]
| Subjects | Treatment Order | ||
|---|---|---|---|
| 3 | Standard | Notched | Boosted |
| 3 | Notched | Boosted | Standard |
| 3 | Boosted | Standard | Notched |
| 3 | Standard | Boosted | Notched |
| 3 | Notched | Standard | Boosted |
| 3 | Boosted | Notched | Standard |
| Demographic Data | |||
|---|---|---|---|
| Number of subjects (n) | 18 | ||
| Sex—n | |||
| male | 12 | ||
| female | 6 | ||
| Age (years) | 60.7 ± 12.7 | ||
| Speech audiometry with hearing aids (% correct) | |||
| at 60 dB SPL | 87.7 ± 7.9 | ||
| at 70 dB SPL | 95.5 ± 5.5 | ||
| PTA at 2, 4, and 8 kHz (dB HL) | 57.3 ± 9.0 | ||
| Questionnaires | |||
| TFI score | 46.1 ± 13.2 | ||
| Intrusiveness | 44.9 ± 19.4 | ||
| Sense of control | 52.7 ± 24.5 | ||
| Cognition | 38.3 ± 25.3 | ||
| Sleep | 40.4 ± 31.4 | ||
| Auditory | 56.2 ± 27.2 | ||
| Relaxation | 58.3 ± 28.3 | ||
| Quality of life | 40.7 ± 21.5 | ||
| Emotional distress | 28.3 ± 22.3 | ||
| HQ score | 19.3 ± 6.9 | ||
| APHAB score * | 27.1 ± 12.7 | ||
| Ease of communication | 30.3 ± 16.1 | ||
| Reverberation | 23.3 ± 14.6 | ||
| Background noise | 27.1 ± 14.7 | ||
| Aversiveness | −11.9 ± 16.6 | ||
| Tinnitus | |||
| Frequency (kHz) | 5.1 ± 3.0 | ||
| Type—n (pure tone/noise-like) | 6/12 | ||
| Hours of use per day | |||
| Standard setting | 10.5 ± 4.5 | ||
| Notched setting | 8.5 ± 5.5 | ||
| Boosted setting | 9.8 ± 4.9 | ||
| Comparison | Mean Reduction in the TFI Score (Points) |
|---|---|
| Baseline—Adaptation | 6.9 ± 2.0 |
| Baseline—Standard | 12.0 ± 0.4 |
| Adaptation—Standard | 5.1 ± 2.4 |
| Parameter | Comparison | df | p Value | Adjusted p Value |
|---|---|---|---|---|
| TFI score | Baseline—Adaptation | 30.4 | 0.28 | 0.84 |
| Baseline—Standard | 31.6 | 0.08 | 0.24 | |
| Adaptation—Standard | 33.4 | 0.41 | 1 | |
| HQ score | Baseline—Adaptation | 31.5 | 0.89 | 1 |
| Baseline—Standard | 32 | 0.83 | 1 | |
| Adaptation—Standard | 33.7 | 0.92 | 1 | |
| APHAB score | Baseline—Adaptation | 44.6 | 0.52 | 1 |
| Baseline—Standard | 25.40 | 0.31 | 1 | |
| Adaptation—Standard | 26.5 | 0.11 | 1 |
| Predictor | numDF | Estimate | Std. Error | p Value |
|---|---|---|---|---|
| setting—notched | 2 | −3.2 | 9.8 | 0.28 |
| setting—boosted | 2 | 3.7 | 3.0 | 0.21 |
| tinnitus type—noise-like | 1 | 0.4 | 8.0 | 0.92 |
| tinnitus frequency | 1 | −21.0 | 9.9 | <0.05 |
| tinnitus frequency × tinnitus type | 1 | 20.8 | 10.5 | 0.06 |
| HQ score | 1 | 1.2 | 0.3 | <0.001 |
| R2 conditional | R2 marginal | |||
| Model performance | 0.78 | 0.39 |
| Comparison | Estimate | Std. Error | Adjusted p Value |
|---|---|---|---|
| Notched—Standard | −3.2 | 3.0 | 0.516 |
| Boosted—Standard | 3.7 | 3.0 | 0.414 |
| Boosted—Notched | 7.0 | 3.0 | 0.047 |
| Comparison | Adjusted p Value |
|---|---|
| Notched—Standard | 1.0 |
| Boosted—Standard | 0.34 |
| Boosted—Notched | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santacruz, J.L.; de Kleine, E.; van Dijk, P. Hearing Aid Amplification Schemes Adjusted to Tinnitus Pitch: A Randomized Controlled Trial. Audiol. Res. 2025, 15, 143. https://doi.org/10.3390/audiolres15060143
Santacruz JL, de Kleine E, van Dijk P. Hearing Aid Amplification Schemes Adjusted to Tinnitus Pitch: A Randomized Controlled Trial. Audiology Research. 2025; 15(6):143. https://doi.org/10.3390/audiolres15060143
Chicago/Turabian StyleSantacruz, Jose L., Emile de Kleine, and Pim van Dijk. 2025. "Hearing Aid Amplification Schemes Adjusted to Tinnitus Pitch: A Randomized Controlled Trial" Audiology Research 15, no. 6: 143. https://doi.org/10.3390/audiolres15060143
APA StyleSantacruz, J. L., de Kleine, E., & van Dijk, P. (2025). Hearing Aid Amplification Schemes Adjusted to Tinnitus Pitch: A Randomized Controlled Trial. Audiology Research, 15(6), 143. https://doi.org/10.3390/audiolres15060143







