Vibration-Induced Nystagmus in Patients with Ménière’s Disease: Is There a Correlation to Endolymphatic Hydrops?
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients, Inclusion Criteria and Exclusion Criteria
2.2. Nystagmus Evaluation
2.3. Examination and Complementary Tests
- Audiological Evaluation. Findings were reported in terms of PTA thresholds from 0.5 to 4 kHz (mean between 0.5 Hz, 1 kHz, 2 kHz y 4 kH), expressed in decibels hearing level (dB HL). The audiometric evaluation was performed during the quiescent phase, not during the fluctuating phase.
- Vestibular Evaluation. vHIT was used to analyze the gain of the vestibulo-ocular reflex, establishing a cutoff for normality at ≥0.8, and also to evaluate the presence or absence of corrective saccades, both covert and overt types. For VEMP, both cervical (cVEMP) and ocular (oVEMP) tests were conducted. An abnormal vestibular function was defined as a VEMP response in both ears with an interaural asymmetry ratio (IAAR %) exceeding 40% [21] Burst tones of 500 Hz were used for monaural auditory stimulation using previously calibrated ABR3A inserted hearing aids. The intensity of the acoustic stimulus was 97 dB normalized hearing level. A Blackman envelope was configured (rise/fall time 2 ms, plateau time 0 ms). 100 averages were presented at a rate of 5.1/s. The response evoked by cVEMP describes a positive (p13) and negative (n23) wave. In oVEMP, the response presents a negative (n10) and positive (p16) wave. IAAR was calculated according the following formula:The caloric test was performed using water irrigation at two standard temperatures (44 °C and 30 °C), with each ear tested separately. The resulting responses were analyzed based on the SPV of the induced nystagmus. Jongkees’ formula was applied to calculate canal paresis (considered normal when <21%) [22].
- Imaging studies: All MR studies were performed at 3 Tesla magnets, either a Magnetom Vida or a Magnetom Skyra (Siemens Healthineers, Erlangen, Germany), with 20-channel and 32-channel phased-array receiver coils, respectively. A single dose of intravenous paramagnetic contrast agent gadobutrol (0.1 mmoL/mL, Gadovist, Bayer AG, Zurich, Switzerland) was administrated at a dose of 0.1 mL per kg of body weight. Images were acquired 4 h after the administration of contrast. We performed quantitative and qualitative evaluations of EH.
2.4. Statistical Analysis
3. Results
3.1. Population: Clinical and Demographic Data
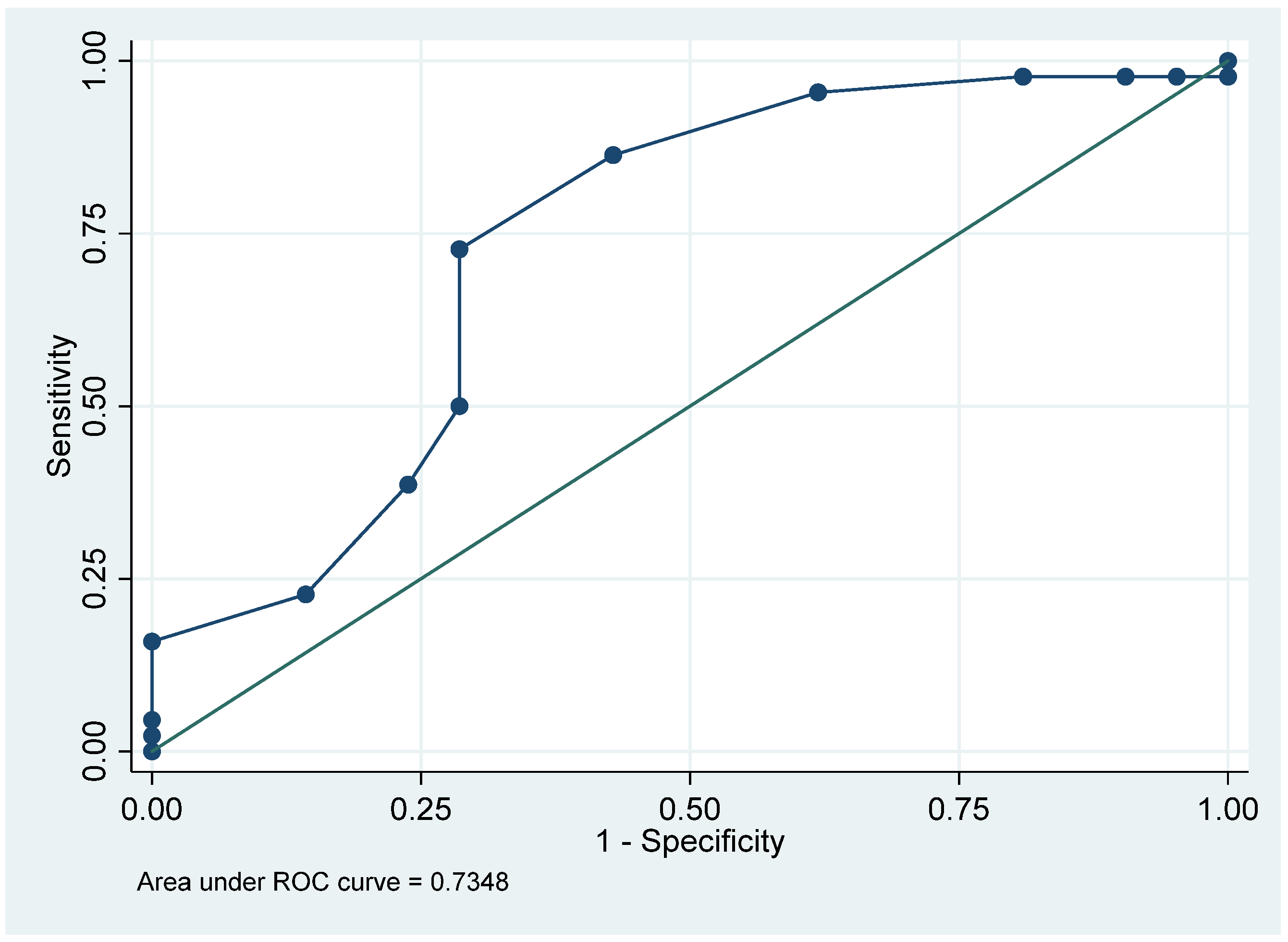
3.2. Analysis of Nystagmus Patterns Neurotological Findings
3.3. Radiological Parameters
3.4. Audiovestibular Outcomes
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dumas, G.; Curthoys, I.S.; Lion, A.; Perrin, P.; Schmerber, S. The Skull Vibration-Induced Nystagmus Test of Vestibular Function—A Review. Front. Neurol. 2017, 8, 41. [Google Scholar] [CrossRef]
- Perez, N. Vibration induced nystagmus in normal subjects and in patients with dizziness. A videonystagmography study. Rev. Laryngol. Otol. Rhinol. 2003, 124, 85–90. [Google Scholar]
- Curthoys, I.S.; Dlugaiczyk, J. Physiology, clinical evidence and diagnostic relevance of sound-induced and vibration-induced vestibular stimulation. Curr. Opin. Neurol. 2020, 33, 126–135. [Google Scholar] [CrossRef]
- Batuecas-Caletrío, A.; Martínez-Carranza, R.; Nuñez, G.M.G.; Nava, M.J.F.; Gómez, H.S.; Ruiz, S.S.; Guillén, V.P.; Pérez-Fernández, N. Skull vibration-induced nystagmus in vestibular neuritis. Acta Otolaryngol. 2020, 140, 995–1000. [Google Scholar] [CrossRef]
- Junet, P.; Karkas, A.; Dumas, G.; Quesada, J.L.; Schmerber, S. Vestibular results after intratympanic gentamicin therapy in disabling Ménière’s disease. Eur. Arch. Otorhinolaryngol. 2016, 273, 3011–3018. [Google Scholar] [CrossRef]
- Martin-Sanz, E.; Esteban-Sánchez, J.; González-Márquez, R.; Larrán-Jiménez, A.; Cuesta, Á.; Batuecas-Caletrio, Á. Vibration-induced nystagmus and head impulse testscreening for vestibular schwannoma. Acta Otolaryngol. 2021, 141, 340–347. [Google Scholar] [CrossRef]
- Dumas, G.; Lion, A.; Karkas, A.; Perrin, P.; Perottino, F.; Schmerber, S. Skull vibration-induced nystagmus test in unilateral superior canal dehiscence and otosclerosis: A vestibular Weber test. Acta Otolaryngol. 2014, 134, 588–600. [Google Scholar] [CrossRef]
- Park, H.J.; Shin, J.E.; Lim, Y.C.; Shin, H.A. Clinical significance of vibration-induced nystagmus. Audiol. Neurotol. 2008, 13, 182–186. [Google Scholar] [CrossRef]
- Hong, S.K.; Koo, J.W.; Kim, J.S.; Park, M.H. Implication of vibration induced nystagmus in Ménière’s disease. Acta Otolaryngol. Suppl. 2007, 127, 128–131. [Google Scholar] [CrossRef]
- Dumas, G.; Karkas, A.; Perrin, P.; Chahine, K.; Schmerber, S. High-frequency skull vibration-induced nystagmus test in partial vestibular lesions. Otol. Neurotol. 2011, 32, 1291–1301. [Google Scholar] [CrossRef]
- Lee, S.-U.; Kee, H.J.; Sheen, S.S.; Choi, B.Y.; Koo, J.W.; Kim, J.-S. Head-shaking and vibration-induced nystagmus duringan between the attacks of unilateral Ménière’s disease. Otol. Neurotol. 2015, 36, 865–872. [Google Scholar] [CrossRef]
- Curthoys, I.S. The Neural Basis of Skull Vibration Induced Nystagmus (SVIN). Audiol. Res. 2021, 11, 557–566. [Google Scholar] [CrossRef]
- Marques, P.S.; Perez-Fernandez, N. Bedside vestibular examination in patients with unilateral definite Ménière’s disease. Acta Otolaryngol. 2012, 132, 498–504. [Google Scholar] [CrossRef]
- Manzari, L.; Burgess, A.M.; MacDougall, H.G.; Bradshaw, A.P.; Curthoys, I.S. Rapid fluctuations in dynamic semicircular canal function in early Meniere’s disease. Eur. Arch. Otorhinolaryngol. 2011, 268, 637–639. [Google Scholar] [CrossRef]
- Gamarra, M.F.V.; Krstulovic, C.; Guillén, V.P.; Pérez-Garrigues, H. Ipsilesional Nystagmus Induced by Vibration in Subjects with Ménière’s Disease or Vestibular Schwannoma. Otol. Neurotol. 2017, 38, e168–e172. [Google Scholar] [CrossRef]
- McClure, J.A.; Copp, J.C.; Lycett, P. Recovery nystagmus in Menieres-disease. Laryngoscope 1981, 91, 1727–1737. [Google Scholar] [CrossRef]
- Nakashima, T.; Naganawa, S.; Sugiura, M.; Teranishi, M.; Sone, M.; Hayashi, H.; Nakata, S.; Katayama, N.; Ishida, I.M. Visualization of endolymphatic hydrops in patients with Meniere’s disease. Laryngoscope 2007, 117, 415–420. [Google Scholar] [CrossRef]
- Naganawa, S.; Yamazaki, M.; Kawai, H.; Bokura, K.; Sone, M.; Nakashima, T. Visualization of endolymphatic hydrops in Ménière’s disease after single-dose intravenous gadolinium-based contrast medium: Timing of optimal enhancement. Magn. Reson. Med. Sci. 2012, 11, 43–51. [Google Scholar] [CrossRef]
- Quatre, R.; Attyé, A.; Karkas, A.; Job, A.; Dumas, G.; Schmerber, S. Relationship between audio-vestibular functional tests and inner ear MRI in Meniere’s disease. Ear Hear. 2019, 40, 168–176. [Google Scholar] [CrossRef]
- Iwasaki, S.; Shojaku, H.; Murofushi, T.; Seo, T.; Kitahara, T.; Origasa, H.; Watanabe, Y.; Suzuki, M.; Takeda, N.; Committee for Clinical Practice Guidelines of Japan Society for Equilibrium Research. Diagnostic and therapeutic strategies for Meniere’s disease of the Japan Society for Equilibrium Research. Auris Nasus Larynx. 2021, 48, 15–22. [Google Scholar] [CrossRef]
- Guajardo-Vergara, C.; Pérez-Fernandez, N. Air and bone stimulation in vestibular evoked myogenic potentials in patients with unilateral Ménière’s disease and in controls. Hear. Balanc. Commun. 2019, 17, 170–178. [Google Scholar] [CrossRef]
- Strupp, M.; Magnusson, M. Acute unilateral vestibulopathy. Neurol. Clin. 2015, 33, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Vega, V.; Manrique-Huarte, R.; Dominguez, P.; Blanco, M.; Alonso-Burgos, A.; Pérez-Fernández, N. Magnetic Resonance Volumetric Quantification of Vestibular Endolymphatic Hydrops in Patients with Unilateral Definite Meniere’s Disease Using 3D Inversion Recovery with Real Reconstruction (3D-REAL-IR) Sequence. J. Clin. Med. 2023, 12, 5965. [Google Scholar] [CrossRef]
- Blanco, M.; Monopoli-Roca, C.; de Linera-Alperi, M.Á.; Fernández-Miranda, P.M.; Molina, B.; Batuecas-Caletrío, A.; Pérez-Fernández, N. Visual Fixation of Skull-Vibration-Induced Nystagmus in Patients with Peripheral Vestibulopathy. Audiol. Res. 2024, 24, 562–571. [Google Scholar] [CrossRef]
- Batuecas-Caletrío, Á.; Jara, A.; Suarez-Vega, V.M.; Marcos-Alonso, S.; Sánchez-Gómez, H.; Pérez-Fernández, N. Skull Vibration-Induced Nystagmus and High Frequency Ocular Vestibular-Evoked Myogenic Potentials in Superior Canal Dehiscence. Audiol. Res. 2022, 12, 202–211. [Google Scholar] [CrossRef]
- Díaz, M.P.G.; Torres-García, L.; Zamora, E.G.; Belén Castilla Jiménez, A.; Pérez Guillén, V. Is Skull Vibration-Induced Nystagmus Useful in Vestibular Neuritis Follow Up? Audiol. Res. 2022, 12, 126–131. Available online: https://pubmed.ncbi.nlm.nih.gov/35314610/ (accessed on 23 September 2025). [CrossRef]
- Park, H.; Shin, J.; Jeong, Y.; Kwak, H.; Lee, Y. Lessons from follow-up examinations in patients with vestibular neuritis: How to interpret findings from vestibular function tests at a compensated stage. Otol. Neurotol. 2009, 30, 806–811. [Google Scholar] [CrossRef]
- Park, H.; Hong, S.-C.; Shin, J. Clinical significance of vibration-induced nystagmus and head-shaking nystagmus through follow-up examinations in patients with vestibular neuritis. Otol. Neurotol. 2008, 29, 375–379. [Google Scholar] [CrossRef]
- Ciacca, G.; Di Giovanni, A.; Califano, L.; Pettorossi, V.E.; Ricci, G.; Pelliccia, C.; Faralli, M. Skull vibration-induced nystagmus test in patients candidates for intratympanic gentamicin injection. Acta Otorhinolaryngol. Ital. 2023, 43, 140–148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bernaerts, A.; Vanspauwen, R.; Blaivie, C.; van Dinther, J.; Zarowski, A.; Wuyts, F.L.; Bossche, S.V.; Offeciers, E.; Casselman, J.W.; De Foer, B. The value of four stage vestibular hydrops grading and asymmetric perilymphatic enhancement in the diagnosis of Ménière’s disease on MRI. Neuroradiology. 2019, 61, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Gürkov, R.; Flatz, W.; Louza, J.; Strupp, M.; Ertl-Wagner, B.; Krause, E. In vivo visualized endolymphatic hydrops and inner ear functions in patients with electrocochleographically confirmed Ménière’s disease. Otol. Neurotol. 2012, 33, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, P.; Han, Z.; Xie, J.; Liu, Y.; Gong, S.; Wang, Z. Evaluation of the relationship between endolymphatic hydrops and hearing loss in Meniere’s disease based on three-dimensional real inversion recovery sequence. Braz. J. Otorhinolaryngol. 2023, 89, 101314. [Google Scholar] [CrossRef]
- Seo, Y.J.; Kim, J.; Choi, J.Y.; Lee, W.S. Visualization of endolymphatic hydrops and correlation with audio-vestibular functional testing in patients with definite Meniere’s disease. Auris Nasus Larynx. 2013, 40, 167–172. [Google Scholar] [CrossRef]
- Lorente-Piera, J.; Suárez-Vega, V.; Blanco-Pareja, M.; Liaño, G.; Garaycochea, O.; Dominguez, P.; Manrique-Huarte, R.; Pérez-Fernández, N. Early and certain Ménière’s disease characterization of predictors of endolymphatic hydrops. Front. Neurology. 2025, 28, 16. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Rajan, G.P.; Shaw, J.; Rohani, S.A.; Ladak, H.M.; Agrawal, S.; Rask-Andersen, H. A synchrotron and Micro-CT study of the human endolymphatic duct system: Is Meniere’s disease caused by an acute endolymph backflow? Front. Surg. 2021, 8, 662530. [Google Scholar] [CrossRef]
- Avan, P.; Djennaoui, I. Auditory biophysics of endolymphatic hydrops. J. Vestib. Res. 2021, 31, 277–281. [Google Scholar] [CrossRef]
- Li, J.; Jin, X.; Kong, X.; Hu, N.; Li, X.; Wang, L.; Liu, M.; Li, C.; Liu, Y.; Sun, L.; et al. Correlation of endolymphatic hydrops and perilymphatic enhancement with the clinical features of Ménière’s disease. Eur. Radiol. 2024, 34, 6036–6046. [Google Scholar] [CrossRef] [PubMed]
| Clinical and Demographic Data | p-Value | |
|---|---|---|
| N (Men: Women) | 50:34 patients | 0.093 |
| Age (Mean ± SD) | 52.60 ± 10.86 years | 0.314 |
| Disease duration (Mean ± SD) | 5.27 ± 6.52 years | 0.026 * |
| Duration < 3 years/>3 years | 42:42 patients | N/A |
| Affected side (Right/Left) | 51:33 ears | 0.462 |
| Days since last dizzy spell (Mean ± SD) | 33.12 ± 64.40 days | 0.018 * |
| Number of dizzy spells last six months (Mean ± SD) | 6.60 ± 4.70 spells | 0.445 |
| Tumarkin crises last six months (Cases, %) | 11 patients (13.95%) | 0.534 |
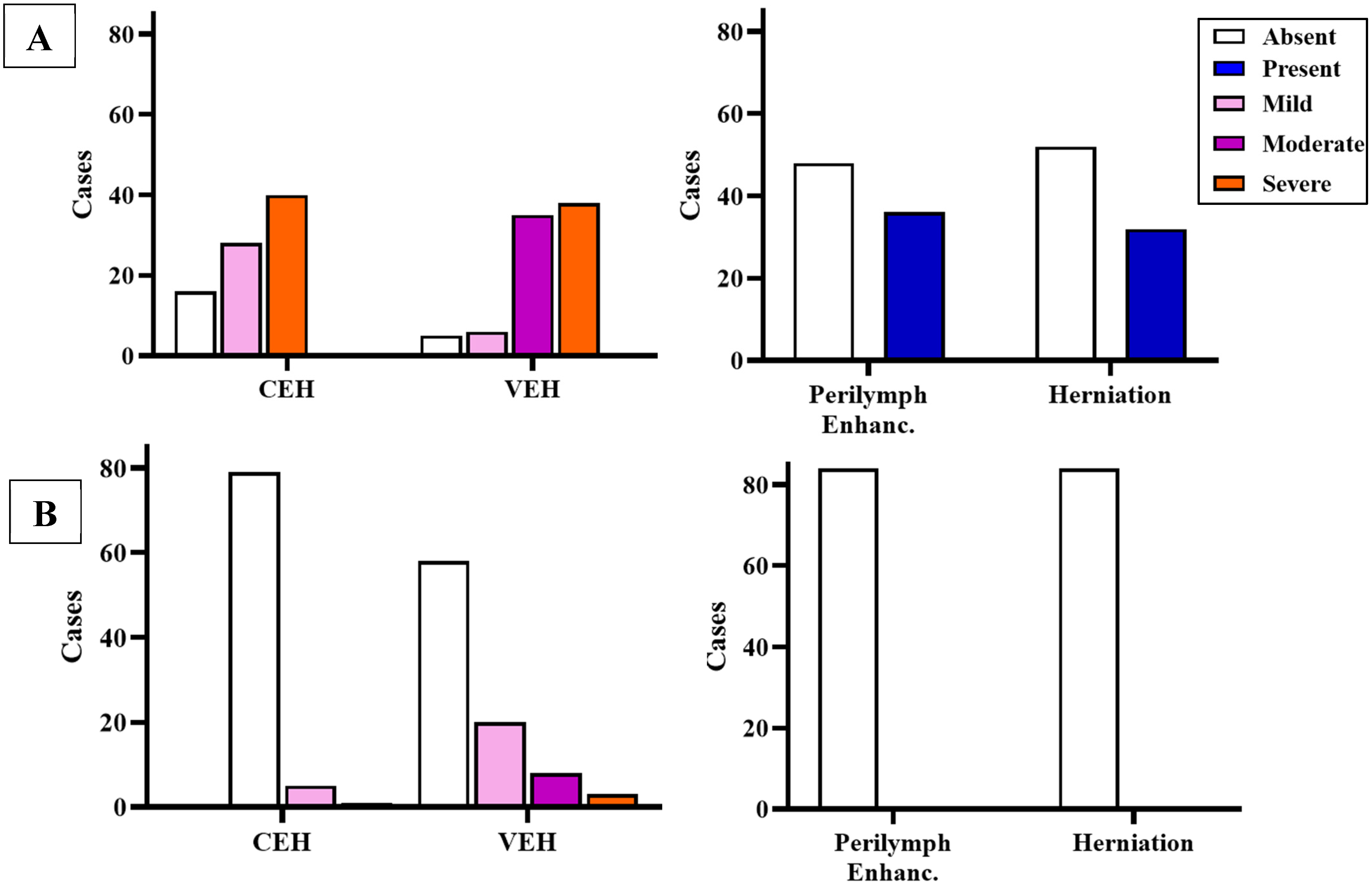
| MRI Evaluation of the Affected Ear | SVIN + (n = 48) | SVIN − (n = 36) | p Value | OR (CI 95%) | |
|---|---|---|---|---|---|
| Cochlear EH (CEH) | None | 8 (16.67%) | 8 (22.22%) | 0.655 | 1.26 (0.52–3.09) |
| Mild | 16 (33.33%) | 11 (30.56%) | |||
| Severe | 24 (50.00%) | 17 (47.22%) | |||
| Vestibular EH (VEH) | None | 2 (4.17%) | 4 (11.11%) | 0.017 * | 3.15 (1.23–8.07) |
| Mild | 1 (2.02%) | 6 (16.67%) | |||
| Moderate | 21 (43.75%) | 13 (36.11%) | |||
| Severe | 24 (50.00%) | 13 (36.11%) | |||
| Vestibular E. Ratio (REL) | Affected (RELAFF) | 80.11 ± 10.27% | 64.13 ± 13.30% | 0.019 * | 7.99 (1.41–45.22) |
| Perilymphatic enhancement (PE) | Absent | 19 (39.58%) | 14 (38.89%) | 0.989 | 0.91 (0.36–2.30) |
| Present | 29 (60.41%) | 22 (61.11%) | |||
| Endolympatic herniation (EHern) | Absent | 29 (60.41%) | 24 (66.67%) | 0.646 | 1.27 (0.50–3.23) |
| Present | 19 (39.58%) | 12 (33.33%) | |||
| MRI Evaluation of the Non-Affected Ear | SVIN + (n = 48) | SVIN − (n = 36) | p Value | OR (CI 95%) | |
|---|---|---|---|---|---|
| Cochlear EH (CEH) | None | 44 (91.67%) | 35 (97.22%) | 0.285 | 0.55 (0.19–1.56) |
| Mild | 4 (8.33%) | 1 (2.78%) | |||
| Severe | 0 (0.00%) | 0 (47.22%) | |||
| Vestibular EH (VEH) | None | 35 (72.92%) | 23 (63.88%) | 0.461 | 0.65 (0.25–1.68) |
| Mild | 10 (20.83%) | 11 (30.56%) | |||
| Moderate | 3 (6.25%) | 2 (5.56%) | |||
| Severe | 0 (0.00%) | 0 (0.00%) | |||
| Vestibular E. Ratio (REL) | Non-affected (RELNAFF) | 38.12 ± 14.36% | 35.13 ± 12.03% | 0.349 | 5.43 (0.16–187.51) |
| Perilymphatic enhancement (PE) | Absent | 48 (100%) | 36 (100%) | - | - |
| Endolympatic herniation (EHern) | Absent | 48 (100%) | 36 (100%) | - | - |
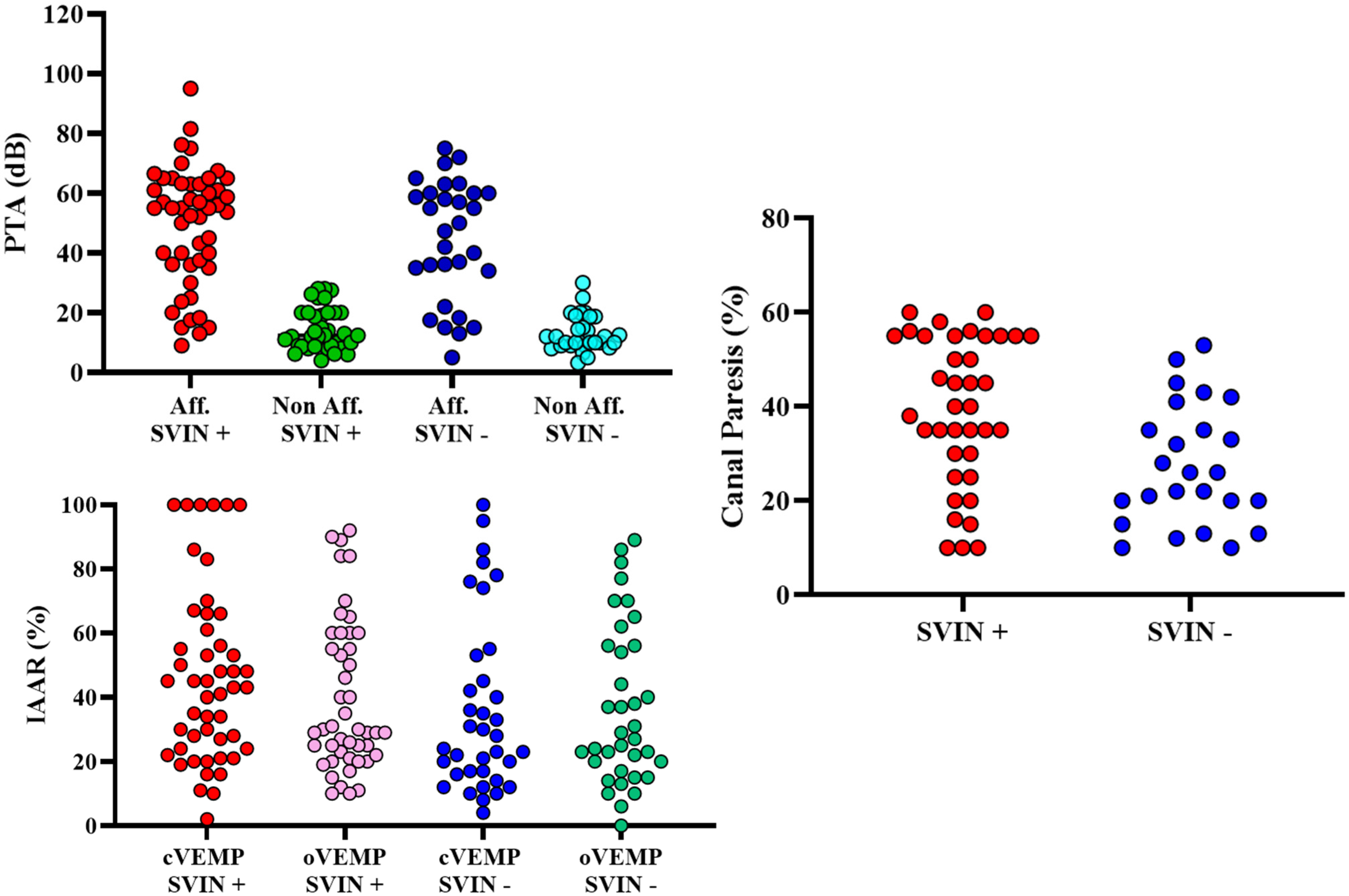
| Audiovestibular Tests | SVIN + (n = 48) | SVIN − (n = 36) | p-Value | OR (CI 95%) | |
|---|---|---|---|---|---|
| Hearing | |||||
| PTA | Affected ear | 47.39 dB ± 19.07 | 44.52 ± 20.01 dB | 0.315 | 1.01 (0.99–1.04) |
| Non-affected ear | 14.47 dB ± 6.98 dB | 13.06 ± 6.02 dB | 0.206 | 1.04 (0.98–1.11) | |
| vHIT | |||||
| Normal | 32 (66.67%) | 33 (91.67%) | 0.122 | 0.38 (0.11–1.25) | |
| Pathologic | 16 (33.33%) | 3 (8.33%) | 0.059 | 3.56 (0.94–13.43) | |
| VEMPs | |||||
| Ocular | Present/Absent | 38/10 | 30/6 | 0.355 | 0.99 (0.98–1.01) |
| IAAR (%) | 37.24 ± 27.18 | 31.40 ± 27.76 | |||
| Cervical | Present/Absent | 42/6 | 31/5 | 0.481 | 1.01 (0.99–1.02) |
| IAAR (%) | 39.40 ± 30.70 | 34.41 ± 31.66 | |||
| Caloric Test | |||||
| Canal Paresis | 36.63 ± 15.85 | 27.32 ± 14.68 | 0.878 | 0.97 (0.93–1.01) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorente-Piera, J.; Blanco, M.; Manrique-Huarte, R.; David, A.; Suarez-Vega, V.; Batuecas-Caletrío, A.; Esteve, G.L.; Dominguez, P.; Pérez-Fernández, N. Vibration-Induced Nystagmus in Patients with Ménière’s Disease: Is There a Correlation to Endolymphatic Hydrops? Audiol. Res. 2025, 15, 125. https://doi.org/10.3390/audiolres15050125
Lorente-Piera J, Blanco M, Manrique-Huarte R, David A, Suarez-Vega V, Batuecas-Caletrío A, Esteve GL, Dominguez P, Pérez-Fernández N. Vibration-Induced Nystagmus in Patients with Ménière’s Disease: Is There a Correlation to Endolymphatic Hydrops? Audiology Research. 2025; 15(5):125. https://doi.org/10.3390/audiolres15050125
Chicago/Turabian StyleLorente-Piera, Joan, Melissa Blanco, Raquel Manrique-Huarte, Adriana David, Victor Suarez-Vega, Angel Batuecas-Caletrío, Gloria Liaño Esteve, Pablo Dominguez, and Nicolás Pérez-Fernández. 2025. "Vibration-Induced Nystagmus in Patients with Ménière’s Disease: Is There a Correlation to Endolymphatic Hydrops?" Audiology Research 15, no. 5: 125. https://doi.org/10.3390/audiolres15050125
APA StyleLorente-Piera, J., Blanco, M., Manrique-Huarte, R., David, A., Suarez-Vega, V., Batuecas-Caletrío, A., Esteve, G. L., Dominguez, P., & Pérez-Fernández, N. (2025). Vibration-Induced Nystagmus in Patients with Ménière’s Disease: Is There a Correlation to Endolymphatic Hydrops? Audiology Research, 15(5), 125. https://doi.org/10.3390/audiolres15050125









